Abstract
Purpose
We analyzed the trends and predictors of multidrug-resistant (MDR) or rifampicin-resistant (RR) tuberculosis (TB) in culture-positive cases in Shenzhen during 2012–2020, after the implementation of improved strategies (scale-up molecular drug susceptibility testing [mDST], expansion of DST eligibility, and generous reimbursement of MDR-TB outpatient care costs).
Materials and Methods
We retrospectively extracted and analyzed data from the TB Information System on drug-resistant pulmonary tuberculosis diagnosed in Shenzhen during the 2012–2020 period. We analyzed trends in RR- and MDR-TB rates in new cases during 2012–2018 and 2018–2020 periods, and among previously-treated cases during 2012–2017 and 2017–2020 periods, using Cochran-Armitage tests. We generated multivariate logistic regression models to analyze demographic predictors of MDR/RR-TB rates.
Results
We found 21,367 positive mycobacterial cultures in Shenzhen during the 2012–2020 period, and 19,951 (93.4%) were identified as Mycobacterium tuberculosis and had DST results (92.0% of those were mDST-based). Of these patients with DST results, 1630 (8.2%) were RR-TB, and 1142 (5.7%) were MDR-TB. Of the RR-TB, 70% were MDR-TB. The MDR/RR-TB rate in new TB cases increased significantly during the 2012–2018 period (Ptrend < 0.05), but it decreased in the 2018–2020 period (Ptrend > 0.05, with a significant trend for MDR-TB). Among previously treated cases, the temporal MDR/RR-TB rate trends did not differ significantly (Ptrend > 0.05). Our multivariate analysis showed that age younger than 30 years, housework service/unemployment, local residency, and previous TB treatment were all predictors of MDR/RR-TB. The percentage of patients with MDR-TB on treatment increased from 49.4% in 2012 to 70.5% in 2020. The treatment success rate of patients with MDR-TB during the 2012–2018 period was 71%.
Conclusion
During the study period in Shenzhen, the cases of MDR/RR-TB were detected, and the treatment enrollment increased and the MDR-TB rates decreased gradually after 2017. Decreasing trends may reflect the efficacy of improved strategies; however, their long-term impact on the MDR-TB burden remains to be investigated. The predictors of MDR-TB identified in our study should be considered when developing targeted MDR-TB control strategies.
Introduction
Since the widespread implementation of the directly-observed therapy short course (DOTS) program, the tuberculosis (TB) incidence and mortality have decreased worldwide; however, drug-resistant TB (DR-TB) continues to be a public health problem threatening TB control efforts in many countries.Citation1 Multidrug resistant TB (MDR-TB), defined as TB resistant to at least rifampicin (RMP) and isoniazid (INH), is not cured by the standard short-course chemotherapy with first-line anti-TB drugs. If MDR-TB is not detected and adequately treated, it leads to treatment failures, high mortality, and long periods of community transmission.Citation2 Rifampicin-resistant TB (RR-TB), defined as TB resistant to at least RMP, is often used as a surrogate marker for MDR-TB because up to 90% of RR-TB isolates are also resistant to isoniazid. Therefore, these categories are often grouped together as MDR/RR-TB and treated with MDR-TB regimens.Citation3 Global estimates indicated an incidence of 465000 MDR/RR-TB cases in 2019, and nearly 50% of these occurred in just three countries: India (27%), China (14%), and the Russian Federation (8%). In 2019, 7.1% of new and 23.0% of previously-treated TB cases had MDR/RR-TB in China, these percentages are higher than the average global counterparts at 3.3% and 17.7%, respectively.Citation4 However, there is a large geographic disparity in the MDR/RR-TB burden in China. Studies have found MDR/RR-TB rates among the new TB cases ranging from 4.1% in ShanghaiCitation5 to 9.5% in JiangsuCitation6 and among the previously-treated TB cases ranging from 17.9% in ShanghaiCitation5 to 41.5% in Chongqing.Citation7
Shenzhen is one of the most developed cities in China. It was established as China’s first special economic zone in 1980 and since then has become one of the fastest growing cities in China and has attracted a large number of internal migrant workers from across the county. In 2017, 66% of the population of Shenzhen was comprised of internal migrantsCitation8 and studies have found that more than 90% of TB and MDR-TB cases in the city are diagnosed in internal migrants;Citation9,Citation10 thus, the control of TB and MDR-TB in this city is difficult. The government of Shenzhen launched the DOTS strategy in 1993, and the incidence and mortality of TB have decreased over the last two decades.Citation9 Additionally, progress was achieved in the screening, diagnosis accuracy, treatment standardization, and MDR-TB management with the help of the Global Fund Program for programmatic management of DR-TB during the 2008–2014 period.Citation11 A study analyzing MDR-TB trends in Shenzhen found that the MDR-TB incidence declined in new cases between 2000 and 2013 (probably due to the implementation of the DOTS and the Global Fund Program strategies).Citation12 Thereafter, multiple updated DR-TB screening and diagnosis strategies were implemented (such as the introduction of molecular drug-susceptibility testing [mDST] and the expansion of the eligibility for DSTCitation13) and likely helped decrease the MDR-TB burden in Shenzhen. In this study, we aimed to evaluate the impact of these updated control strategies on the trends and predictors of MDR/RR-TB rates among patients with culture-positive TB in Shenzhen during the 2012–2020 period.
Materials and Methods
Study Setting
The city of Shenzhen lies on the southern tip of the Guangdong province, just across the bay from Hong Kong. Its total area is 1997.47 square kilometers and its population was estimated at 13 million in 2019 (63.2% of those were internal migrants). Shenzhen is one of the most developed cities in the province with a GDP of 400 billion dollars in 2019. It is a sub-provincial city with direct jurisdiction over 9 districts (Futian, Luohu, Yantian, Nanshan, Bao’an, Longgang, Longhua, Pingshan, and Guangming, and the New Area of Dapeng).
Study Design and Data Source
This was a retrospective analysis of DR-TB data from the National Tuberculosis Information System (TBIMS), which regularly collects information on DR-TB surveillance and management. The information in the TBIMS includes patient demographics, drug resistance profiles, treatment enrollment, and outcomes. We extracted case records from the TBIMS for all patients with positive TB cultures in Shenzhen between January 2012 and December 2020. We only extracted non-identifying information from the database.
DST Procedure, MDR-TB Diagnosis and Treatment
In Shenzhen, all suspected TB cases presenting to general hospitals or primary healthcare centers are referred to the district-level Center for Chronic Disease Control (CCDC) for diagnosis by sputum smear, culture, chest radiography, and/or molecular tests. Positive cultures from TB patients are sent to the Shenzhen Municipal CCDC or the Third People’s Hospital for definitive identification of M. tuberculosis and DST for rifampicin (RMP) and isoniazid (INH). Drug-susceptible TB is generally diagnosed and treated at the district-level Centers for Chronic Disease Control (CCDC), while DR-TB is diagnosed and treated at the municipal Shenzhen City CCDC. Patients with TB requiring hospitalization are treated at the Third People’s Hospital.Citation12,Citation14
Prior to 2017, only sputum samples from patients with positive smears were cultured, and all positive cultures were referred for DST. Since 2017, sputa from both smear-positive and -negative patients get cultured and DST is performed on all cultured Mycobacterium tuberculosis (MTB) isolates. During the 2012–2018 period, all mDST in Shenzhen was performed using the GenoType MTBDRplus (Hain Lifescience, Nehren, Germany) assay. In 2019 and 2020, 10% of the mDST was performed using Xpert MTB/RIF or PCR melting curve assays. In this report, mDST refers to GenoType MTBDRplus, Xpert MTB/RIF, or PCR melting curve assay tests. Phenotypic DST was performed in cases in which mDST was unavailable, but the proportion of all DSTs that were phenotypic was less than 10%. Phenotypic DST was performed using the proportion method on Lowenstein-Jensen medium impregnated with INH and RIF. The concentrations of anti-TB drugs used were 0.2 μg/mL for INH and 40 μg/mL for RIF. After completion of all DSTs, 10% of strains were randomly selected for retesting.
Most of the patients with MDR-TB in Shenzhen were internal migrants who could choose to receive their TB treatment in Shenzhen or to return to their hometowns for treatment. The costs of MDR-TB care were partially reimbursed by basic medical insurance, but coverage for MDR-TB outpatient care was limited prior to 2017. In 2017, the Shenzhen government began covering the costs of MDR-TB outpatient care through the Critical Illness Insurance Program (CIIP). This program reimbursed 60–90% of MDR-TB outpatient costs and was available for internal migrant workers with basic medical insurance. Some expenses beyond CIIP coverage were further reimbursed with funds from the Shenzhen government. Patients staying in Shenzhen were enrolled in long-term standard or individualized MDR-TB treatment regimens based on their treatment histories and DST results. The standard regimen consisted of intensive and continuation phases lasting from 18 months to 24 months depending on the length of the intensive phase. The anti-TB drugs used to treat MDR-TB included pyrazinamide, ethambutol, amikacin, capreomycin, levofloxacin, moxifloxacin, para-aminosalicylic acid, and protionamide. The selection of anti-TB drugs was based on the China Drug-resistant Tuberculosis Treatment Guidelines (2015).Citation15
Definitions
MDR-TB is defined as TB resistant to at least RMP and INH. RR-TB is defined as TB resistant to at least RMP, with or without resistance to other anti-TB drugs; therefore, the term RR-TB includes mono-RMP resistant-TB and MDR-TB. RR-TB is often grouped together with MDR-TB as MDR/RR-TB (due to the high prevalence of resistance to INH in these patients) and is treated with the same regimens used for MDR-TB.Citation3 We defined a “new case” as a newly registered episode of TB in a patient who had never been diagnosed as having TB or in one who had initiated anti-TB drugs less than one month before. We defined a “previously treated case” as a newly registered episode of TB in a patient who had received at least one month of anti-TB drugs in the past.Citation3 We classified people who reside in Shenzhen but whose “Hukou” or official residence is registered elsewhere as “internal migrants”.Citation12 The city center refers to the Futian, Yantian, Luohu, and Nanshan districts in Shenzhen, where most of the business services and urban residential communities are located. Factories and rural residential communities are predominantly located outside of the city center.Citation14 We considered cured patients or those who completed their treatment as treatment success. For a cure to be declared the treatment had to be completed as recommended by the national TB policy without evidence of failure and three or more consecutive negative cultures taken at least 30 days apart after the intensive phase. Treatment completion required a treatment completed as recommended by the national TB policy without evidence of failure but without record of three or more consecutive cultures taken at least 30 days apart after the intensive phase. Treatment failed was defined as a patient whose treatment terminated or need for permanent regimen change of at least two anti-TB drugs because of lack of conversion at the end of the intensive phase, or bacteriological reversion in the continuation phase after conversion to negative.Citation15
Data Quality Controls
One investigator collected data from the TBIMS using a structured data collection form, we verified all DST results from the TBIMS with DST records from the municipal CCDC reference laboratory or the Third People’s Hospital laboratory. We used DST results from laboratory records when the results from TBIMS and laboratory records were discordant. The principal investigator oversaw the overall data extraction process, and we excluded incomplete data on DST results or demographic information from the analysis.
Statistical Analysis
We used the Stata software (version 14.0) to analyze the data. We calculated RR‑TB and MDR-TB rates by dividing the number of RR‑TB and MDR-TB cases by the total number of MTB cases with DST results. We used the Cochran-Armitage test to determine trends in the RR‑TB and MDR-TB rates for the 2012–2018 and 2018–2020 periods among new cases and for the 2012–2017 and 2017–2020 periods among previously-treated patients. We looked for significant differences in gender, age-group, occupation, migrant status, treatment history, and city center residence proportions using either the Chi-squared or Wilcoxon rank sum tests to identify predisposing factors for RR‑TB and MDR-TB. Next, we generated multivariate logistic regression models and estimated adjusted odds ratios (ORs) and 95% confidence intervals (CI) to detect possible predictors of RR‑TB and MDR-TB. In addition, we analyzed results for possible interactions between variables such as age-group, migrant status, treatment history, and city center residence. We considered P-values < 0.05 as statistically significant.
Results
MDR/RR-TB Diagnosis, Treatment Enrollment and Outcomes
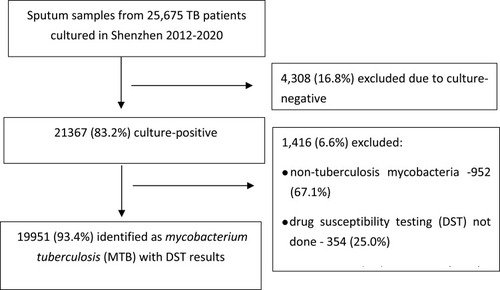
During the 2012–2020 study period, 21,367 culture-positive isolates were sent to the Shenzhen Municipal CCDC laboratory for MTB confirmation and DST for RMP and INH. After excluding non-tuberculous mycobacteria (NTM), specimens contaminated in the laboratory, and those without DST results, 19,951 (93.4%) were identified as MTB and with available DST results (). During the 2012–2020 period, 92.0% of DST in Shenzhen were performed by mDST and 8.0% by phenotypic DST. Our analysis suggests a marked improvement in the ability of the health care system to detect both RR- and MDR-TB cases during the study period. The annual number of MTB isolates with DST increased from 1747 in 2012 to 3049 in 2020; and, the number of RR- and MDR-TB cases detected increased gradually starting in 2012 and had almost doubled by 2020 (RR-TB, 106 vs 214; MDR-TB, 77 vs 122). The largest increase occurred from 2016 to 2017, coinciding with the expansion of the DST program to include cultures from patients with smear-negative TB (). Additionally, we observed an increase in treatment enrollment after the 2017 implementation of increased reimbursement for MDR-TB outpatient care and the use of local government funds to cover out-of-pocket patient expenses. Overall, 674 (59.0%) patients were enrolled in MDR treatment in Shenzhen between 2012 and 2020. The proportion of patients diagnosed as having MDR-TB enrolled on an MDR treatment regimen increased gradually from 49.4% in 2012 to 70.5% in 2020, with the largest increase observed between 2016 and 2017 (). The average treatment success rate was 71% (ranging from 63–74%), but a differing trend in treatment success rates from 2012 to 2018 was not statistically significant (Ptrend > 0.05). The treatment outcomes of patients diagnosed in 2019 and 2020 were unavailable because their 24-month-long treatment regimens were ongoing at the time of data collection.
Table 1 Diagnosis and Treatment Enrollment Cascade for MDR/RR-TB in Shenzhen, 2012–2020 [n (%)]
Drug Resistance Patterns
Of the 19951 MTB isolates that underwent DST in the 2012–2020 period, 17,302 (86.7%) were susceptible to both RMP and INH, 1019 (5.1%) were mono-INH resistant, 488 (2.5%) were mono-RMP resistant, and 1142 (5.7%) were resistant to at least RMP and INH and therefore MDR. shows the differences in drug resistance patterns between new and previously treated cases. Within the new cases, the incidences of MDR-TB and RR-TB were higher among local residents than among internal migrants (P values < 0.05); but, within the previously treated cases, the drug resistance patterns were similar in the two groups (P values > 0.05).
Table 2 Drug Resistance Patterns of 19,951 MTB Cases Stratified by Residential Status [n (%)]
RR-TB and MDR-TB Rates and Their Predictors in Shenzhen
show the characteristics of the patients with TB whose isolates had DST results. More than 60% were men younger than 40 years. The most common occupations were housework service/unemployment and construction/factory workers (76.6%). Internal migrants accounted for 88.9% of patients and 91.7% were new cases. The pooled proportions of RR-TB and MDR-TB among those with bacteriologically-positive TB results in Shenzhen through the 2012–2020 period were 8.2% and 5.7%, respectively.
Table 3 RR-TB and MDR-TB Rates Among 19,951 MTB Patients in Shenzhen, 2012–2020
Our univariate analysis results revealed that the pooled proportion of MDR/RR-TB among patients with bacteriologically-positive TB was higher in men, individuals between 31 and 60 years of age, staff officers, teachers/students, local residents, and patients previously treated for TB (P values < 0.05). The distribution of the pooled proportion of MDR-TB was similar to that of RR-TB (). The multivariate analysis results showed significant differences in the proportions of RR-TB in different age-groups (≥ 61 years vs ≤ 30 years; adjusted OR, 0.67; 95% CI, 0.53–0.84), occupation (construction/factory workers vs housework service/unemployment; adjusted OR, 0.82; 95% CI, 0.72–0.94), residential status (local residents vs internal migrants; adjusted OR, 0.83; 95CI%, 0.71–0.98) and treatment history (previously treated cases vs new cases; adjusted OR, 9.04; 95% CI, 7.98–10.25) (). The proportions of MDR-TB were similar among the patient subgroups, except within the occupations (teachers/students vs housework service/unemployment, adjusted OR, 1.47; 95% CI, 1.06–2.04) where belonging to the housework service/unemployment class was an additional predictor of MDR-TB ().
Table 4 Multivariate Analysis on Predictors of MDR-TB and RR-TB
Trends in RR-TB and MDR-TB in Shenzhen During the 2012–2020 Period
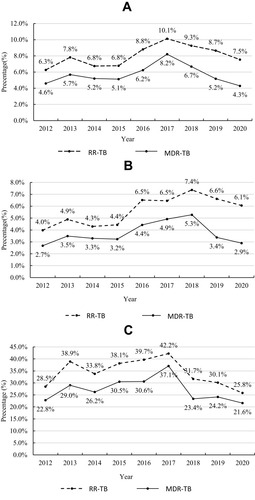
shows the RR-TB and MDR-TB trends in patients with positive TB cultures in Shenzhen during the 2012–2020 period. Among the new cases, the proportion of RR-TB increased gradually, peaking at 7.4% in 2018 (Ptrend = 0.02), and then decreasing to 6.1% in 2020 (Ptrend =0.50). Among the previously treated cases, the proportion of RR-TB was stable during both the 2012–2017 and 2017–2020 periods (Ptrend > 0.05). MDR-TB incidence trends were similar to those of RR-TB. Among the new cases (), both the increase in MDR-TB during the 2012–2018 period and the decrease during the 2018–2020 period were statistically significant. However, among the previously treated patients (), the decrease in MDR-TB during the 2017–2020 period was significant, but the increase in the 2012–2017 period was not.
Figure 2 Trends in RR-TB and MDR-TB in Shenzhen during the 2012–2020 period.
Discussion
In this study, we analyzed the numbers and proportions of MDR/RR-TB and MDR-TB among patients with culture-positive TB in Shenzhen between 2012 and 2020 to identify the trends that should reflect the effect of the improved strategies for MDR-TB control that were introduced during this period. We found that the proportions of both MDR/RR-TB and MDR-TB increased during the 2012–2018 period among the new cases and decreased during the 2018–2020 period among both the new and previously treated cases (although the decrease was not statistically significant for MDR/RR-TB). We also found that the proportions of MDR/RR-TB and MDR-TB to be higher in patients younger than 30 years, in those employed in housework service or unemployed, in local residents, and in patients previously treated for TB.
Other studies in Shenzhen have shown that MDR-TB decreased from 2000 to 2009 among the new cases, and then began to increase again;Citation12 we found that this increasing trend continued until 2017. During the 2009–2017 period there were concerted efforts to improve detection of MDR-TB in Shenzhen, aided by the Global Fund Program (2008–2014). Routine DST, which had been limited to high-risk groups, was expanded in 2009 to include cultures from all patients with positive smears, and expanded again in 2017 to include sputum cultures from both smear-positive and -negative patients. In addition, by 2012 mDST covered 92% of all DST. Thus, the increase in MDR-TB cases from 2009 to 2017 may be the result of enhanced case detection measures,Citation16–Citation18 although it could also reflect a true increase in the MDR/RR-TB and MDR-TB infection rates in Shenzhen during this period, a possibility that should be investigated.
Notably, the percentage of MDR-TB among culture-positive patients with TB has been decreasing since 2018 in both new and previously treated patients; these trends could be the result of years of improved case detection and treatment enrollment programs. The financial costs of TB can force patients (particularly internal migrants) to remain undiagnosed and untreated, leading to prolonged transmission of MDR-TB.Citation19–Citation22 Changes in coverage and reimbursement policies have helped alleviate these financial difficulties, which may partially explain the increase in the proportion of patients enrolled on treatments (from 49.4% in 2012 to 70% in 2017). This financing policy may further improve MDR-TB treatment compliance and outcomes, leading to lower ongoing MDR-TB transmissions in Shenzhen.Citation23 However, this decreasing trend in the incidence of MDR-TB has only been observed during the past three years, and the long-term impact of the implemented strategies on the MDR-TB burden in Shenzhen should be evaluated again in the future.
We found that local residents had a higher proportion of RR-TB and MDR-TB than internal migrants after adjusting for potential confounders, but only among newly diagnosed cases, indicating that primary drug resistance is mostly transmitted among local MDR-TB patients. Our previous molecular epidemiological study of MDR-TB strains collected in Shenzhen during the 2013–2017 period showed that being a local resident was independently associated with having a clustered strain, again indicating transmission of MDR-TB among local residents.Citation14 In this study, we also found that students/teachers presented a higher rate of MDR-TB than other groups of patients, which is consistent with a published finding indicating a high likelihood for students/teachers of having clustered strains.Citation14 As in other studies,Citation24,Citation25 we found that previous TB treatment and younger age were both risk factors for RR-TB and MDR-TB, but we found no gender differences. MDR-TB can develop due to the selection of resistant M. tuberculosis bacilli during poorly managed anti-TB treatment. Inappropriate chemotherapy regimens, inadequate or irregular drug supply, and poor compliance by TB patients have been common among previously treated patients, which helps explain their high MDR-TB rates.Citation26 The positive association of younger age with MDR-TB could be due to a tendency of these patients to not adhere to anti-TB medication regimens because of working schedule conflicts, the need to travel to distant cities to find employment, or alcohol addiction. Older people, by comparison, have a more sedentary lifestyle.Citation27 The associations or predictors identified in our study should be considered when developing targeted MDR-TB control strategies in the future.
RMP-resistance has been considered a useful surrogate marker for MDR-TB,Citation28 but a recent meta-analysis reported that RMP-resistance is an unreliable predictor of MDR-TB in China.Citation29 In this study, we found that only 70% of RR-TB cases in Shenzhen were MDR, and the statistical significance of the trends and occupational associations differed between the patients with MDR- and RR-TB. Therefore, using RMP-resistance as a surrogate marker for MDR in Shenzhen could lead to overdiagnosis and overtreatment of MDR-TB. We also found that the proportion of RR-TB that was MDR-TB decreased to about 60% in 2019 and 2020. However, during the 2019–2020 period there was an increased use of the Xpert MTB/RIF, which detects only RMP-resistance and not INH-resistance. Therefore, some cases of MDR-TB could have been missed although the proportion of RR-TB should be accurate. As the use of Xpert MTB/RIF increases in China, the detection of RIF-R should be confirmed with conventional DST or a mDST assay that also detects INH-R. This has implications for treatment, as current recommendations stipulate that INH should be included in the treatment regimen of RR-TB until INH resistance is demonstrated.Citation30
Our study had some limitations. First, DST results for second-line anti-tuberculosis drugs were unavailable and we could not determine the prevalence of pre-XDR-TB and XDR-TB among the cases diagnosed as MDR-TB in Shenzhen. Second, patient data such as treatment adherence, HIV infection status, diabetes, smoking, alcohol consumption, and history of contact with MDR/RR-TB patients were not routinely collected; therefore, we could not assess possible associations with MDR/RR-TB. Finally, we cannot exclude the possibility that the covid-19 pandemic resulted in decreased MDR/RR-TB diagnoses in 2020 and that the actual MDR/RR-TB incidence in Shenzhen was higher than the one recorded.Citation31
Conclusion
We found that age younger than 30 years, housework service/unemployment, local residency, and previous TB treatment were all associated with high RR-TB and MDR-TB rates. These risk factors should be considered when developing targeted MDR-TB control strategies in the future. During our study period, the MDR-TB detection in Shenzhen improved, the percentage of MDR-TB-diagnosed patients receiving treatment increased, and MDR-TB rates started to decrease gradually after 2017. These positive trends may reflect the efficacy of implemented strategies (increase in sputum culturing, expansion of DST testing, increased use of mDST, and generous reimbursement of MDR-TB outpatient care costs). However, the observation period for our study was relatively short and the long-term impact of these improved strategies remains to be evaluated.
Abbreviations
TB, tuberculosis; DST, drug sensitivity testing; mDST, molecular drug sensitivity testing; INH, isoniazid; RMP, rifampicin; MDR-TB, multidrug-resistant TB; MDR/RR-TB multidrug-resistant TB or rifampicin resistant TB; MTB, Mycobacterium tuberculosis; CCDC, center for chronic disease control; aOR, adjusted odds ratios.
Data Sharing Statement
The dataset used to support the findings of this study will be made available by the corresponding author upon reasonable request.
Ethical Approval and Informed Consent
The Ethics Committee of Shenzhen Center for Chronic Disease Control approved this study (Approval number: SZCCC-2020-021-01-YJ). We ensured the confidentiality of patient data and complied with the Helsinki statement tenets. Due to the retrospective nature of the study, the Ethics Committee waived the requirement for patient consents.
Author Contributions
All authors made a significant contribution to the work reported during the conception, study design, execution, data acquisition, analysis, interpretation, drafting, and revising, or critically reviewing the article. All authors gave their final approval of the version to be published and the chosen journal for submission, and they agreed to be accountable for all aspects of the work.
Acknowledgments
We express our thanks and appreciation to all of the staff involved in data recording and data management in the Shenzhen CCDC. Special thanks to Prof. Howard Takiff for the critical review and English language editing of this manuscript.
Disclosure
The authors declare having no conflicts of interest in this work.
References
- Lange C, Dheda K, Chesov D, et al. Management of drug-resistant tuberculosis. Lancet. 2019;394(10202):953–966. doi:10.1016/S0140-6736(19)31882-331526739
- Millard J, Ugarte-Gil C, Moore DA. Multidrug resistant tuberculosis. BMJ. 2015;350:h882. doi:10.1136/bmj.h88225721508
- World Health Organization. Guidance for the Surveillance of Drug Resistance in Tuberculosis. 6th ed. Geneva: World Health Organization; 2020.
- World Health Organization. Global tuberculosis report 2020. Geneva: World Health Organization; 2020.
- Shen X, DeRiemer K, Yuan ZA, et al. Drug-resistant tuberculosis in Shanghai, China, 2000–2006: prevalence, trends and risk factors. Int J Tuberc Lung Dis. 2009;13(2):253–259.19146756
- Shao Y, Yang D, Xu W, et al. Epidemiology of anti-tuberculosis drug resistance in a Chinese population: current situation and challenges ahead. BMC Public Health. 2011;11(1):110. doi:10.1186/1471-2458-11-11021324205
- Wu B, Yu Y, Du C, Liu Y, Hu D. Epidemiology of drug-resistant tuberculosis in Chongqing, China: a retrospective observational study from 2010 to 2017. PLoS One. 2019;14(12):e0216018. doi:10.1371/journal.pone.021601831821321
- Shenzhen Statistics Bureau, NBS Survey Office in Shenzhen. Shenzhen Statistical Yearbook 2017. Beijing: China Statistics Press; 2017.
- Wu AF, Lu DL, Guang HY, et al. Analysis of epidemiological characteristics of pulmonary tuberculosis in Shenzhen from 2007 to 2016. J Trop Med. 2018;18(1):86–89. Chinese.
- Che XL, Wu QF, Li MZ, Guan HY, Tan WG. Detection and treatment of multidrug-resistant tuberculosis in migrating population in Shenzhen, 2012–2016. Chin J Trop Med. 2018;18(6):553–554. Chinese.
- Long Q, Qu Y, Lucas H. Drug-resistant tuberculosis control in China: progress and challenges. Infect Dis Poverty. 2016;5(1):9. doi:10.1186/s40249-016-0103-326822738
- Zhu L, Yang YZ, Guan HY, et al. Trends in drug-resistant tuberculosis after the implementation of the DOTS strategy in Shenzhen, China, 2000–2013. Int J Tuberc Lung Dis. 2017;21(7):759–765. doi:10.5588/ijtld.16.075928633700
- Tang WG, Guan HY, Wu QF, et al. The implement effects and innovative strategies for tuberculosis controlling among migrants. Elec J Emerg Infect Dis. 2020;5(3):145–149. Chinese.
- Jiang Q, Liu Q, Ji L, et al. Citywide transmission of multidrug-resistant tuberculosis under China’s rapid urbanization: a retrospective population-based genomic spatial epidemiological study. Clin Infect Dis. 2020;71(1):142–151. doi:10.1093/cid/ciz79031504306
- Chinese Antituberculosis Association. China drug-resistant tuberculosis chemotherapy guidelines (2015). Chin J Antituberc. 2015;37(5):421–469. Chinese.
- Ding XY, Mao WH, Lu W, et al. Impact of multiple policy interventions on the screening and diagnosis of drug-resistant tuberculosis patients: a cascade analysis on six prefectures in China. Infect Dis Poverty. 2021;10(1):8. doi:10.1186/s40249-021-00793-933468247
- Huang F, van den Hof S, Qu Y, et al. Added value of comprehensive program to provide universal access to care for sputum smear-negative drug-resistant tuberculosis, China. Emerg Infect Dis. 2019;25(7):1289–1296. doi:10.3201/eid2507.18141731211666
- Wang L, Li R, Xu C, et al. The global fund in China: multidrug-resistant tuberculosis nationwide programmatic scale-up and challenges to transition to full country ownership. PLoS One. 2017;12(6):e0177536. doi:10.1371/journal.pone.017753628628669
- Benatar SR, Upshur R. Tuberculosis and poverty: what could (and should) be done? Int J Tuberc Lung Dis. 2010;14(10):1215–1221.20843410
- Zhang P, Xu G, Song Y, Tan J, Chen T, Deng G. Challenges faced by multidrug-resistant tuberculosis patients in three financially affluent Chinese cities. Risk Manag Health Policy. 2020;13:2387–2394. doi:10.2147/RMHP.S275400
- Long Q, Jiang WX, Zhang H, et al. Multi-source financing for tuberculosis treatment in China: key issues and challenges. Infect Dis Poverty. 2021;10(1):17. doi:10.1186/s40249-021-00809-433750460
- Xionling C, Qingfang W, Liming Z, et al. The epidemiological characteristics and treatment outcome of multi-drug resistant tuberculosis among migrating individuals in Shenzhen. Chin Prev Med. 2018;19(9):647–650.
- Li R, Ruan Y, Sun Q, et al. Effect of a comprehensive programme to provide universal access to care for sputum-smear-positive multidrug-resistant tuberculosis in China: a before-and-after study. Lancet Glob Health. 2015;3(4):e217–e228. doi:10.1016/S2214-109X(15)70021-525794675
- Pradipta IS, Forsman LD, Bruchfeld J, Hak E, Alffenaar JW. Risk factors of multidrug-resistant tuberculosis: a global systematic review and meta-analysis. J Infect. 2018;77(6):469–478. doi:10.1016/j.jinf.2018.10.00430339803
- Faustini AHA, Perucci CA. Risk factors for multidrug resistant tuberculosis in Europe: a systematic review. Thorax. 2006;61(2):158–163. doi:10.1136/thx.2005.04596316254056
- Eshetie S, Gizachew M, Dagnew M, et al. Multidrug resistant tuberculosis in Ethiopian settings and its association with previous history of anti-tuberculosis treatment: a systematic review and meta-analysis. BMC Infect Dis. 2017;17(1):219. doi:10.1186/s12879-017-2323-y28320336
- Ambaye GY, Tsegaye GW. Factors associated with multi-drug resistant tuberculosis among TB patients in selected treatment centers of Amhara Region: a case-control study. Ethiop J Health Sci. 2021;31(1):25–34.34158749
- World Health Organization. Xpert MTB/RIF Implementation Manual: Technical and Operational ‘How-To’; Practical Considerations. Geneva: World Health Organization; 2014.
- Liu Z, Dong H, Wu B, et al. Is rifampin resistance a reliable predictive marker of multidrug-resistant tuberculosis in China: a meta-analysis of findings. J Infect. 2019;79(4):349–356. doi:10.1016/j.jinf.2019.08.00431400354
- Smith SE, Kurbatova EV, Cavanaugh JS, Cegielski JP. Global isoniazid resistance patterns in rifampin-resistant and rifampin-susceptible tuberculosis. Int J Tuberc Lung Dis. 2012;16(2):203–205. doi:10.5588/ijtld.11.044522136739
- McQuaid CF, Vassall A, Cohen T, Fiekert K, White RG. The impact of COVID-19 on TB: a review of the data. Int J Tuberc Lung Dis. 2021;25(6):436–446. doi:10.5588/ijtld.21.014834049605