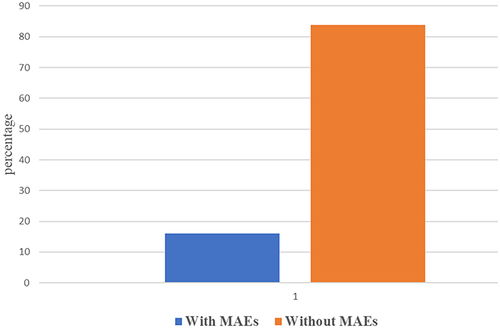Abstract
Background
Adverse events (AE) contribute to poor drug adherence and withdrawal, which contribute to a low treatment success rate. AE are commonly reported among multi-drug resistance tuberculosis (MDR-TB) patients in Ethiopia. However, predictors of AE among MDR-TB patients were limited in Ethiopia. Thus, the current study aimed to develop and validate a score to predict the risks of major AE among MDR-TB patients in Southern Ethiopia.
Methods
A retrospective follow-up study design was employed among MDR-TB patients from 2014–2019 in southern Ethiopia at selected hospitals. A least absolute shrinkage and selection operator algorithm was used to select the most potent predictors of the outcome. The adverse event risk score was built based on the multivariable logistic regression analysis. Discriminatory power and calibration were checked to evaluate the performance of the model. Bootstrapping method with 100 repetitions was used for internal model validation.
Results
History of baseline khat use, long-term drug regimen use, and having coexisting disorders (co-morbidity) were predictors of AEs. The score has a satisfactory discriminatory power (AUC = 0.77, 95% CI: 0.68, 0.82) and a modest calibration (Prob > chi2 = 0.2043). It was found to have the same c-statistics after validation by bootstrapping method of 100 repetitions with replacement.
Conclusion
A history of baseline khat use, co-morbidity, and long-term drug regimen use are helpful to predict individual risk of major adverse events in MDR-TB patients with a satisfactory degree of accuracy (AUC = 0.77).
Background
Globally, tuberculosis (TB) is the primary cause of mortality from infectious disease. It affects near to 4000 lives per day, 120,000 lives per month, and more than 1.2 million lives annually. The increasing burden of Multi-Drug Resistant Tuberculosis (MDR-TB) and the emerging of Extensively Drug Resistant Tuberculosis (XDR-TB) makes the global TB control challenging.Citation1 MDR-TB is defined as a patient who is resistant to at least isoniazid (INH) and rifampicin (R). In 2019, about 3.3% of new and 18% of previously treated cases had MDR-TB. The overall expected incidence of Rifampin Resistant (RR) TB was 465,000 per year. Of these, 78% of RR-TB cases had MDR-TB and 182,000 of them died.Citation1
More than 85% of MDR-TB is found in 30 high burden countries (HBCs). Africa is the second highest MDR-TB burden region which accounts for 25% of the global share, next to the WHO Southeast Asian region which accounts for 44% of the global share. The proportion of MDR-TB among previously treated cases ranges from 15–21.07%. In contrast, the incidence of MDR-TB was much lower among newly diagnosed TB cases, accounting for only 2%.Citation2,Citation3
Coronavirus disease (COVID-19) is feared to bring the global TB and MDR-TB crisis to the level it was in 2012 and 2015.Citation1,Citation4 As a result, it could contribute to the emergence of XDR-TB thus further worsening the problem. Furthermore, drugs for treatment of MDR-TB are given for a relatively long duration (i.e., up to 2 years) and a short-term regimen (i.e., up to 9 months) is usually associated with substantial toxicities and poor outcomes.Citation5,Citation6 An adverse drug event is defined as any unpleasant medical occurrence in a patient receiving any pharmacological product regardless of a necessarily causal relationship to the treatment received.Citation6 According to the literature, major AE among MDR-TB patients include drug-induced hepatitis, electrolyte imbalance, acute psychosis, acute kidney injury, peripheral neuropathy, and hypothyroidism.Citation5,Citation6 According to a systematic review study, about 17% of MDR-TB patients developed hypothyroidism.Citation7 Moreover, a study from South Africa showed that 19% of MDR-TB patients developed at least one form of major AE in their course of treatment.Citation8
The incidence of AE in Amhara region, Ethiopia was 5.79 per 100 person-months observations.Citation9 Major AEs are commonly caused by obesity,Citation10 anemia,Citation11 advanced age,Citation12,Citation13 tobacco use, poor dietary practice,Citation14,Citation15 and co-morbid conditions including HIV infection.Citation12,Citation16–19 Hence, this study aimed to develop a scoring algorithm for the prediction of major AEs among patients treated for MDR-TB, so that clinicians could take the necessary measures before the occurrence of an adverse event.
Methods
Study Design and Setting
A retrospective follow-up study design was employed among MDR-TB patients between September 2014 and September 2019. In Southern Ethiopia, treatment for MDR-TB is provided in eight public hospitals, namely Arbaminch Referral Hospital, Yirgalem General Hospital, Queen Elleni Memorial Referral Hospital, Butajira, Mizan Tepi, Jinka, Dilla, and Sawla Hospitals. The detailed information about the study setting has been published elsewhere.Citation20
Population and Sample
All MDR-TB patients who started treatment in Southern Ethiopia between September 2014 and September 2019 were the source population. From the eight hospitals found in Southern Ethiopia, four hospitals (Yirgalem, Queen Elleni Memorial Hospital, Butajira, and Dilla) were selected purposively since these hospitals initiated MDR-TB treatment early and had a high patient burden. Then all patients who fulfilled the inclusion criteria were included in the study.
Variables of the Study
The outcome variable, major adverse drug event, was defined as a patient who developed at least one of the following side effects: nephrotoxicity, hepatotoxicity, hypokalaemia, hypothyroidism, and hematologic abnormalities. The details about the variables of the study have been published elsewhere.Citation20
Data Collection Tools, Procedures, and Quality Control
Data were collected using a data extraction checklist from patient medical charts and registration books. All the methods were performed under the relevant guidelines and regulations. To keep the data quality, a two-day training was given to data collectors and supervisors. Moreover, pre-test was done in Yirgalem General Hospital and Wachamo University Queen Elleni Mohamed Memorial Referral Hospital on 5% of the sample. Finally, completeness and consistency were checked daily, and double entry was made on 5% of the sample.
Data Processing and Analysis
The data were entered using Epidata version 3.1 and exported to Stata version 14 and R version 4.0.4 for analysis. Counts and percentages were used to summarize the descriptive findings. Mean with standard deviation (SD) and median with interquartile range (IQR) was used to summarize continuous variables in case of a normal and skewed data, respectively.
The least Absolute Shrinkage and Selection Operator (LASSO) algorithm was used to choose the most important predictor variables. We preferred using penalized regression method to minimize overfitting, hence developing an unbiased and most parsimonious AEs risk prediction score.Citation21 The model with optimum shrinkage factor and minimum cross-validation mean deviance was selected by LASSO regression.
The multivariable logistic regression analysis was done using potential predictors identified by LASSO regression. The individual risk score using quantifiable tool was built on multivariable logistic analysis. The score performance evaluation was done by assessing the discriminatory power and calibration. The discriminatory power of the score was quantified by calculating the c-statistics or area under curve (AUC) of the receiver operating characteristics curve (ROC curve). The prediction score was assessed qualitatively using Swets’ criteria, in which values range from 0.5–0.6 (bad), 0.6–0.7 (poor), 0.7–0.8 (satisfactory), 0.8–0.9 (good), and 0.9–1.0 (excellent).Citation22 The calibration power of the score was presented graphically using a calibration plot. A bootstrap resampling with 100 repetitions of the original set was performed for internal model validation.
Results
Socio-Demographic and Behavioral Characteristics of MDR-TB Patients
Between September 2014 and September 2019, 381 patients were initiated with MDR-TB treatment. Of these, 329 (86.35%) of participants fulfilled the inclusion criteria. The remaining 52 (13.65%) were excluded due to unknown outcome status. A total of 52 (15.8%) study participants had a history of baseline khat use and 42 (12.8%) of participants had a history of alcohol use. Only 10 (3.04%) of patients had smoking history ().
Table 1 Socio-Demographic and Behavioral Characteristics of MDR-TB Patients in Southern Ethiopia, 2014–2019 (N = 329)
Clinical Characteristics of MDR-TB Patients
A total of 319 (96.96%) MDR-TB patients had pulmonary tuberculosis. More than three-fourths (80.2%) of MDR-TB patients were treated using a long-term drug regimen. In this study, 72.64% and 70.5% MDR-TB patients were positive for baseline sputum smear and culture, respectively. Nearly three-fourths (76.9%) of MDR-TB patients had previous history of TB treatment. About 12% of MDR-TB patients had co-morbidity. Most of the patients, 246 (74.8%) had lung cavitation and infiltration. About three-fourths (75.07%) of study participants were in ambulatory care ().
Table 2 Clinical Characteristics of MDR TB Patients in Southern Ethiopia, 2014–2019 (N = 329)
Prevalence of Major Adverse Events
In this study, 54 (16.41%) of the study subjects developed a major adverse event. The results are summarized in .
Feature Selection and Cross-Validation Function Plot
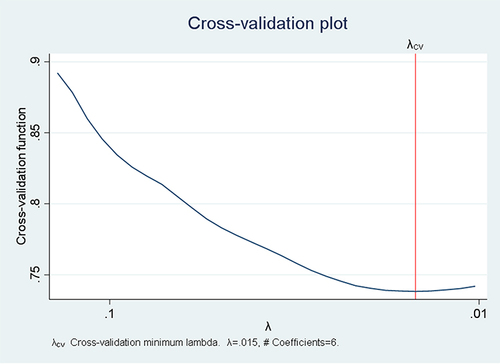
Twenty-nine models were generated using the lasso estimator and the 10-fold cross-validation selection method. The 25th model with an optimum penalty factor (lambda) of 0.015 and minimum cross-validation mean deviance was selected. Among the 19 co-variants entered in LASSO regression, six potential features were selected (). The cross-validation plot showed that the cross-validation function plot is minimum at a lambda value of 0.015 ().
Table 3 Optimum Shrinkage Factor (Lambda) and Potential Predictors Identified by Lasso Regression by 10-Fold Cross-Validation Selection Method
Development of an Individualized Prediction Score
The multivariable logistic regression model was fitted using the identified potential predictors. Of all, drug regimen, khat use and co-morbidity were found to be significant predictors of AEs ().
Table 4 Predictors of Major Adverse Events Identified by Lasso Regression (N = 329)
Risk Score of the Final Model and Its Performance
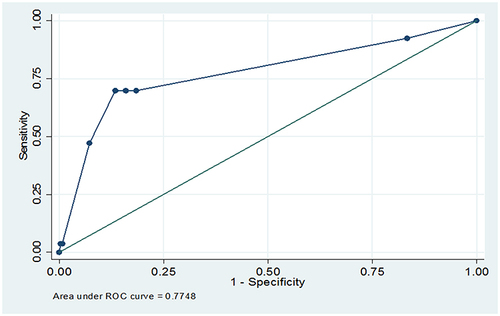
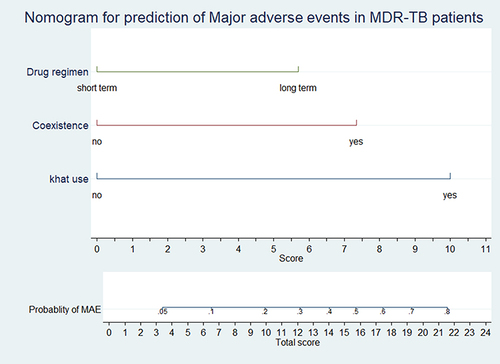
The score for the presence of co-morbidity, long-term regimen, and khat use were 7.5, 5.5, and 10, respectively. The total score = 7.5+5.5+10 = 23. According to the risk score, the probability of AEs for the total score of 23 would be more than 80% (). The discriminatory power was found to be (AUC = 0.77, 95% CI: 0.68, 0.82) (). The calibration test of the fitted model was (Prob > chi2 = 0.2043) ().
Figure 3 Risk score for the individualized prediction of AEs among MDR TB patients in Southern Ethiopia, 2014–2019 (N=329).
Validation of the Developed Risk Prediction Model
The score was trained and internally validated in the original dataset without splitting the data as the sample size was not adequate to split it into training and validation dataset. A bootstrap resampling method with 100 repetitions was employed for internal validation. The model was found to have c-statistics of 0.77 after validation, a similar discriminatory power with the original score.
Discussion
History of baseline khat use, long-term drug regimen use, and having co-morbidity were predictors of AEs. The score has a discriminatory power of (AUC = 0.77, 95% CI: 0.68, 0.82) and a calibration test of (Prob > chi2 = 0.2043).
Prediction scores that are being developed are found to be the cornerstones of modern medicine and clinical care. However, these scores are usually context-specific for handling the most important factors in that particular setting. Then, the already developed scores should be externally validated to be used in other settings. We derived and internally validated a more parsimonious and easier to use risk score for assessing potential major AEs in the treatment of MDR-TB patients.
The risk prediction score developed for major adverse events has a satisfactory level of accuracy with AUC of 0.77. The discriminatory performance is higher than a prediction score developed in Mexico (c-statistics = 0.68) for prediction of TB treatment failure, death, and drug resistance,Citation23 for poor MDR-TB treatment outcome in China (AUROC = 0.69),Citation24 the risk score developed to predict dropouts in MDR-TB patients in Brazil (c-statistics = 0.65),Citation25 prediction of hospital readmission in general medical patients (AUC = 0.61),Citation26 and a risk score to predict the risk of advanced nasopharyngeal carcinoma (c-index = 0.748).Citation27
However, the prediction model developed in this study was lower than the risk scores developed to predict MDR-TB treatment failure (c-index = 0.8),Citation25 risk of atrial fibrillation (AUC = 0.78),Citation28 pediatrics mortality (c-index = 0.88),Citation29 and adverse outcomes in patients with pulmonary embolism (AUC = 0.85).Citation30 These prediction models were done using a prospective study design where there is a possibility of identifying all key prognostic indicators, a possible reason for the higher discriminatory performance of the aforementioned scores than the current prediction score. However, developing patient-specific risk prediction scores using retrospective data is still important in resource-limited settings, as is the case in Ethiopia.
The current study identified that khat chewing, co-existing disorder (co-morbidity), and MDR-TB drug regimen were predictors of AEs. A risk prediction score developed using these identified key predictors can be used to strictly follow high-risk patients. This is the first risk prediction model developed on major adverse events among MDR-TB patients in Ethiopia. However, most of the identified predictors such as co-existence and drug regimen have been identified by other studies.Citation9,Citation11,Citation12,Citation19 Co-morbidities such as HIV can increase the risk of major adverse events in anti-MDR-TB treatment for two reasons. The first reason is the possible drug interaction between the anti-MDR-TB treatment drugs and other medications given for co-morbidities such as antiretroviral medications. The second reason is that co-morbidities such as HIV can further compromise the immunity of the affected individuals. According to a recent study, patients on a long-term anti-MDR-TB regimen had higher odds of major adverse events. The involvement of first-line drugs in a short-term regimen is less toxic than the second-line anti-MDR-TB drugs. Furthermore, hepatotoxicity and other poor outcomes could be more likely as patients will be on second-line medicines for an extended period.
The other key predictor of major adverse events was baseline khat use. Khat chewing is a common habit in the southern part of Ethiopia relative to other parts of the country. Khat users are invariably smokers and alcohol drinkers.
The risk score was developed using easily identifiable prognostic determinants which can be ascertained early at patient enrolment, and can be used by clinicians as a simple clinical tool in MDR-TB management and care. However, the study has some limitations. The first limitation was related to the retrospective nature of the data, in which potential predictors of major AEs might have been missed.
In our set-up, the injectable short-term regimen was initiated for the treatment of MDR-TB since 2017. Then because of life-threatening irreversible side-effects such as ototoxicity, the regimen was entirely converted into oral anti-MDR-TB drugs. The short-term regimen consisting of bedaquiline, levofloxacin, pyrazinamide, a high dose of isoniazid (INH), clofazimine, prothionamide, andethambutol has been initiated since the first month of 2021. These could alter the actual burden of AEs among MDR-TB patients. Furthermore, the type of drug that causes the major adverse event was not reported since patients used similar medications without considering the findings of the DST. Besides, the score was not externally validated using an independent external dataset. Therefore, further studies using a prospectively collected dataset are advisable.
Conclusion
The risk score developed will be used to estimate the individual patient’s risk of a major adverse event among patients on MDR-TB treatment. History of khat use, presence of comorbidity, and long-term drug regimen can predict major AEs among MDR-TB patients.
Abbreviations
BMI, Body Mass Index; CI, Confidence Interval; CXR, Chest X-ray; CV, Cross validation; DR, Drug-Resistance; DST, Drug Susceptibility Test; LASSO, Least Absolute Shrinkage and Selection Operator; MDR, Multidrug-Resistant; AEs, Major Adverse Events; SRR, Rifampicin Resistance; SNNPR, Southern Nation, Nationalities, and People’s Region; TIC, Treatment Initiation Centers; TB, Tuberculosis; WHO, World Health Organization; XDR, Extensively Drug-Resistant.
Data Sharing Statement
The data will be available on a reasonable request from the corresponding author.
Ethical Approval and Consent to Participate
Ethical grant was obtained from the University of Gondar institutional review board (IRB) and permission was secured from SNNPR health departments. Informed consent was not taken directly from the study subjects since the data were collected retrospectively. Confidentiality of information was kept. The study was conducted in accordance with the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
We authors declare that there is no conflict of interest.
Acknowledgments
We would like to forward our gratitude to the University of Gondar for providing small financial support for the data collection. Moreover, we also extend our thanks to the data collectors and supervisors.
Additional information
Funding
References
- Wang W, Wang J, Zhao Q, et al. Contribution of rural-to-urban migration in the prevalence of drug resistant tuberculosis in China. Eur J Clin Microbiol Infect Dis. 2011;30(4):581–586. doi:10.1007/s10096-010-1125-6
- Eshetie S, Gizachew M, Dagnew M, et al. Multidrug resistant tuberculosis in Ethiopian settings and its association with previous history of anti-tuberculosis treatment: a systematic review and meta-analysis. BMC Infect Dis. 2017;17(1):1–12. doi:10.1186/s12879-017-2323-y
- Girum T, Muktar E, Lentiro K, Wondiye H, Shewangizaw M. Epidemiology of multidrug-resistant tuberculosis (MDR-TB) in Ethiopia: a systematic review and meta-analysis of the prevalence, determinants and treatment outcome. Trop Dis Travel Med V. 2018;4(1):1–12. doi:10.1186/s40794-018-0065-5
- Stop TB partner ship. Availabe from: http://www.stoptb.org/countries/tbdata.asp. Accessed May 02, 2021.
- Falzon D, Schünemann HJ, Harausz E, et al. World Health Organization treatment guidelines for drug-resistant tuberculosis, 2016 update. Eur Respir J. 2017;49(3):1602308. doi:10.1183/13993003.02308-2016
- World Health Organization. A practical handbook on the pharmacovigilance of medicines used in the treatment of tuberculosis: enhancing the safety of the TB patient. 2012.
- Tola HH, Holakouie-Naieni K, Lejisa T, et al. Is hypothyroidism rare in multidrug resistance tuberculosis patients on treatment? A systematic review and meta-analysis. PLoS One. 2019;14(6):e0218487. doi:10.1371/journal.pone.0218487
- Schnippel K, Berhanu RH, Black A, et al. Severe adverse events during second-line tuberculosis treatment in the context of high HIV co-infection in South Africa: a retrospective cohort study. BMC Infect Dis. 2016;16(1):1–10. doi:10.1186/s12879-016-1933-0
- Merid MW, Gezie LD, Kassa GM, Muluneh AG, Akalu TY, Yenit MK. Incidence and predictors of major adverse drug events among drug-resistant tuberculosis patients on second-line anti-tuberculosis treatment in Amhara regional state public hospitals; Ethiopia: a retrospective cohort study. BMC Infect Dis. 2019;19(1):1–12. doi:10.1186/s12879-019-3919-1
- Carroll M, Lee M, Cai Y, et al. Frequency of adverse reactions to first-and second-line anti-tuberculosis chemotherapy in a Korean cohort. Int J Tuberc Lung Dis. 2012;16(7):961–966. doi:10.5588/ijtld.11.0574
- Lorent N, Sebatunzi O, Mukeshimana G, Van den Ende J, Clerinx J. Incidence and risk factors of serious adverse events during antituberculous treatment in Rwanda: a prospective cohort study. PLoS One. 2011;6(5):e19566. doi:10.1371/journal.pone.0019566
- Chung-Delgado K, Revilla-Montag A, Guillen-Bravo S, et al. Factors associated with anti-tuberculosis medication adverse effects: a case-control study in Lima, Peru. PLoS One. 2011;6(11):e27610. doi:10.1371/journal.pone.0027610
- Abera W, Cheneke W, Abebe G. Incidence of antituberculosis-drug-induced hepatotoxicity and associated risk factors among tuberculosis patients in Dawro Zone, South Ethiopia: a cohort study. Int J Mycobacteriol. 2016;5(1):14–20. doi:10.1016/j.ijmyco.2015.10.002
- Chiang Y-C, Lin Y-M, Lee J-A, Lee C-N, Chen H-Y. Tobacco consumption is a reversible risk factor associated with reduced successful treatment outcomes of anti-tuberculosis therapy. Int J Infect Dis. 2012;16(2):e130–e5. doi:10.1016/j.ijid.2011.10.007
- Gupta KB, Gupta R, Atreja A, Verma M, Vishvkarma S. Tuberculosis and nutrition. Lung India. 2009;26(1):9. doi:10.4103/0970-2113.45198
- Mouton JP, Njuguna C, Kramer N, et al. Adverse drug reactions causing admission to medical wards: a cross-sectional survey at 4 hospitals in South Africa. Medicine. 2016;95(19):e3437. doi:10.1097/MD.0000000000003437
- Ahmad N, Javaid A, Sulaiman SAS, Afridi AK, Khan AH, Khan AH. Occurrence, management, and risk factors for adverse drug reactions in multidrug resistant tuberculosis patients. Am J Ther. 2018;25(5):e533–e540. doi:10.1097/MJT.0000000000000421
- Furin JJ, Mitnick CD, Shin SS, et al. Occurrence of serious adverse effects in patients receiving community-based therapy for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2001;5(7):648–655.
- Resende LSO, Santos-Neto ETD. Risk factors associated with adverse reactions to antituberculosis drugs. J Bras Pneumol. 2015;41(1):77–89. doi:10.1590/S1806-37132015000100010
- Bogale L, Tsegaye T, Abdulkadir M, Akalu TY. Unfavorable treatment outcome and its predictors among patients with multidrug-resistance tuberculosis in Southern Ethiopia in 2014 to 2019: a multi-center retrospective follow-up study. Infect Drug Resist. 2021;14:1343–1355. doi:10.2147/IDR.S300814
- Pavlou M, Ambler G, Seaman SR, et al. How to develop a more accurate risk prediction model when there are few events. BMJ. 2015;351.doi: 10.1136/bmj.h3868
- Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988 Jun 3;240(4857):1285-93.
- Abdelbary BE, Garcia-Viveros M, Ramirez-Oropesa H, Rahbar MH, Restrepo BI. Predicting treatment failure, death and drug resistance using a computed risk score among newly diagnosed TB patients in Tamaulipas, Mexico. Epidemiol Infect. 2017;145(14):3020–3034. doi:10.1017/S0950268817001911
- Alene KA, Viney K, Gray DJ, McBryde ES, Xu Z, Clements ACA. Development of a risk score for prediction of poor treatment outcomes among patients with multidrug-resistant tuberculosis. PLoS One. 2020;15(1):e0227100. doi:10.1371/journal.pone.0227100
- Arroyo LH, Ramos ACV, Yamamura M. Predictive model of unfavorable outcomes for multidrug-resistant tuberculosis. Revista de Saude Publica. 2019;53:77. doi:10.11606/s1518-8787.2019053001151
- Hasan O, Meltzer DO, Shaykevich SA, et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2010;25(3):211–219. doi:10.1007/s11606-009-1196-1
- Tang X-R, Li Y-Q, Liang S-B, et al. Development and validation of a gene expression-based signature to predict distant metastasis in locoregionally advanced nasopharyngeal carcinoma: a retrospective, multicentre, cohort study. Lancet Oncol. 2018;19(3):382–393. doi:10.1016/S1470-2045(18)30080-9
- Schnabel RB, Sullivan LM, Levy D, et al. Development of a risk score for atrial fibrillation (Framingham heart study): a community-based cohort study. Lancet. 2009;373(9665):739–745. doi:10.1016/S0140-6736(09)60443-8
- Straney L, Clements A, Parslow RC, et al. Paediatric index of mortality 3: an updated model for predicting mortality in pediatric intensive care. Pediatr Crit Care Med. 2013;14(7):673–681. doi:10.1097/PCC.0b013e31829760cf
- Wicki J, Perrier A, Perneger TV, Bounameaux H, Junod AF. Predicting adverse outcome in patients with acute pulmonary embolism: a risk score. Thromb Haemost. 2000;84(10):548–552. doi:10.1055/s-0037-1614065