Abstract
Background
Since oral direct-acting antiviral agents (DAAs) became available, the global hepatitis C treatment situation has undergone tremendous changes. However there are still many issues worthy of attention in treatment.
Methods
We selected 53 HCV-infected patients who were treated and followed up in the Peking University First Hospital from December 2017 to January 2021 to detect the RASs in HCV. Pearson correlation analysis was used to analyze HCV RNA and HCV cAg, the Fisher exact test and chi-square test was used to compare the effects of RASs on the rate of decline of HCV RNA and HCV core antigen (cAg) during DAA treatment.
Results
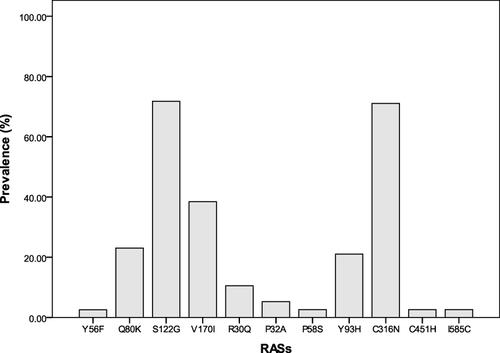
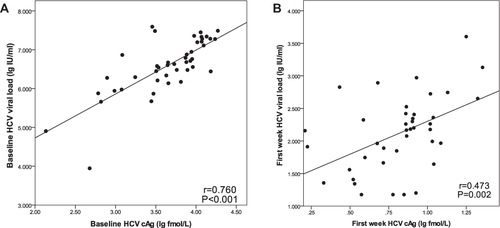
The RASs and its prevalence on the NS3 are mainly Y56F 2.56% (1/39), Q80K 23.08% (9/39), S122G 71.79% (28/39), and V170I 38.46% (15/39). On the NS5A were R30Q 10.53% (4/38), P32A 5.26% (2/38), P58S 2.63% (1/39), and Y93H 21.05% (8/38). On NS5B were C316N 71.05% (27/38), C451H 2.63% (1/38), and I585C 2.63% (1/38). There was no significant correlation between the RASs (Y93H, V179I, Q80K, S122G, C316N) and HCV genotype (p > 0.05). The baseline serum HCV RNA and HCV cAg had a significant medium-degree correlation (r = 0.601, p = 0.002). After 1 week of DAA treatment was weak correlation (r = 0.413, p = 0.032). Q80K, S122G, V170I, Y93H, and C316N had no effect on the clearance of HCV RNA and HCV cAg within the first week of DAA treatment (p>0.05).
Conclusion
The HCV genotype may have a limited impact on the presence of the five RASs (Y93H, V179I, Q80K, S122G, and C316N) as shown in this study. HCV RNA and HCV cAg have a correlation, especially at baseline is the highest; the appearance of some RASs has no effect on DAA treatment in most chronic hepatitis C patients.
Introduction
Direct-antiviral agents (DAAs) have greatly changed the treatment of chronic hepatitis C (CHC). HCV RNA detection has been widely used in the diagnosis and treatment of CHC. The HCV infection diagnosis relies on detectable HCV RNA, and whether HCV RNA can be detected after treatment is also considered a criterion of sustained virologic response (SVR), which implies clinical cure. HCV RNA testing requires expensive reagents, highly technical requirements, long testing time, and high testing costs.Citation1,Citation2 On the other hand, although HCV RNA detection has been popularized in most areas around the world, the definition of SVR depends on a highly sensitive HCV RNA detection method with a lower limit of detection (LLOD) of 15 IU/mL. At present, non-highly sensitivity HCV RNA detection methods are used in many regions. Low-sensitivity HCV RNA detection methods may result in misdiagnosis because of their inability to detect low viral load HCV in the serum. Therefore, it is still of great significance to find a simple test with high diagnostic accuracy to help eliminate HCV, especially in low- and middle-income countries.Citation3 HCV core antigen (cAg) is a viral protein released into the blood during HCV assembly. In the past few years, an HCV-cAg detection method has been established and gradually improved.Citation4–6 Many studies have shown that there is a good correlation between HCV cAg and HCV RNA higher than 3000 IU/mL, but when the viral load is low, the correlation and consistency between the two are unclear.Citation7,Citation8 HCV cAg is promising to replace the HCV RNA measurement because it is easy to detect and is inexpensive. On the other hand, HCV is an RNA virus, which is susceptible to mutation. Many studies have confirmed that the resistance-associated substitutions (RASs) produced by HCV replication can make HCV become resistance to DAA drugs and can even lead to treatment failure.Citation9 This study mainly explored the prevalence of RASs at NS3, NS5A, and NS5B in patients with chronic hepatitis C (CHC), the correlation between HCV RNA and HCV cAg, and the effect of RASs on the clearance of HCV RNA and HCV cAg.
Materials and Methods
Patients
Patients with HCV infection were selected from the Department of Infectious Diseases, Peking University First Hospital, from December 2017 to January 2021 for treatment and follow-up. Inclusion criteria were as follows: 1) Age > 18 years old; and 2) DAA-naive. Exclusion criteria were as follows: 1) with HCC or malignant tumors; 2) Women with hemoglobin ˂8g/dl; men with hemoglobin ˂9g/dl3) undergoing immunosuppressive therapy; and 4) pregnant or unable to take appropriate contraceptive measures. According to the patient’s HCV genotype, liver function, renal function status, complications and concomitant medications before treatment, we referred to the guidelines to choose a DAA drug regimen.Citation10 For genotype 1b patients, we chose asunaprevir/daclatasvir (ASV/DCV) for 24 weeks, obipari combined with dasabuvir (paritaprevir/ombitasvir/ritonavir/dasabuvir (OBV/PTV/R+DSV) for 12 weeks, or elbasvir/grazoprevir tablets (EBR/GZR)); for patients with genotype type 2a we selected sofosbuvir combined with velpatasvir (SOF/VEL) for 12 weeks or glecaprevir/pibrentasvir (GLE/PIB) for 8 weeks. Our study protocol was approved by the Ethics Committee of Peking University First Hospital and was adhere ethical guidelines in the Declaration of Helsinki. Written informed consent was obtained from all patients.
Sequencing
We extracted RNA from serum samples and performed reverse transcription. After obtaining the cDNA, nested polymerase chain reactions (PCR) amplification was performed to amplify the target regions of NS3/4A, NS5A, and NS5B, and all PCR products were subjected to Sanger sequencing. Using AJ238799.1 as the reference sequence, the sequence information was compared and analyzed with Vector NTI Suite 9.0 software and submitted to the HCV resistance database for analysis and comparison (http://hcv.bioinf.mpi-inf.mpg.de/index.php). RNA extraction used QIAamp Viral RNA Mini Kit (Qiagen, Germany) reverse transcription PCR, and nested PCR used RevertAid First Strand cDNA Synthesis Kit (ThermoFisher Scientific, USA) and Premix Taq enzyme (Takara, Japan). Sequencing was performed on an ABI Prism 3730xl Genetic Analyzer (Applied Biosystems, CA).
Detection of Hepatitis C Virus
Quantitative detection of HCV RNA and HCV cAg was performed at baseline, the first week of treatment, the fourth week of treatment, and the end of treatment. HCV RNA was quantified using COBAS TaqMan HCV Test kit (Roche, Switzerland), sample processing was performed by COBAS AmpliPrep instrument (Roche, Switzerland), and automatic amplification and detection was performed by COBAS TaqMan analyzer (Roche, Switzerland). The HCV cAg test used the Hepatitis C Virus Antigen Assay Kit (Abbott, USA) and was quantitatively detected by the I2000 Abbott Automatic Chemiluminescence Immunoassay Analyzer (Abbott, USA).
Statistical Analysis
Microsoft Excel was used for data collection and management. The data are as mean ± standard deviation (x ± s) or described in the form of counts. Pearson correlation analysis was used to analyze HCV RNA and HCV cAg between the baseline and the first week of DAA treatment. The difference between the two samples used the t-test, Fisher’s exact test, or Chi-Square Test Data of continuous numerical variables that did not conform to the normal distribution are represented by the median interquartile range, and the rank sum test was used for comparison between groups. SPSS 21.0 was used for data analysis, and p < 0.05 was considered to be statistically significant.
Results
Characteristic of Patients
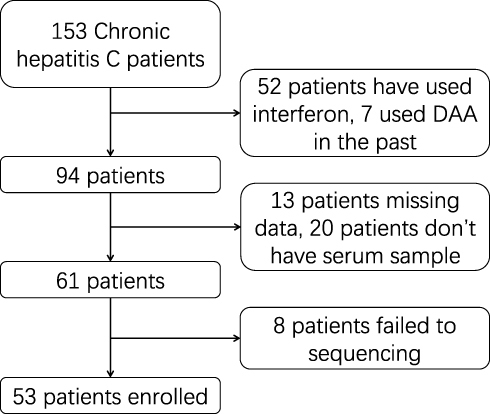
There were 53 patients who met the requirements among 153 CHC patients. The clinical characteristics of the 53 patients are shown in . Flow chart of enrolled patients shows in . The average age was 57.1 (46.25–67.75); 20 patients were males and 33 were females. There were 44 patients with HCV genotype 1b and 9 patients with type 2a. The average HCV RNA was 6.509 ± 0.824 lg IU/mL, ALT was 53.07 (19.45–67.50 IU/mL, and AST was 46.28 (20.00–65.10)) IU/mL.
Table 1 Patients Characteristics at Baseline
Prevalence of RASs in Patients
The 39, 38, and 38 gene sequences of the NS3, NS5A, and NS5B regions were successfully obtained. Results are shown in . The RASs on the NS3 gene segment were mainly Y56F, Q80K, S122G, and V170I. The prevalence of Y56F was 2.56% (1/39), Q80K was 23.08% (9/39), S122G was 71.79% (28/39), and V170I was 38.46% (15/39). The RASs on the NS5A gene fragment were mainly R30Q, P32A, P58S, and Y93H. The prevalence of R30Q was 10.53% (4/38), P32A was 5.26% (2/38), and P58S was 2.63% (1/39), and Y93H was 21.05% (8/38); RASs on NS5B gene fragments were mainly C316N, C451H, and I585C; the prevalence of C316N was 71.05% (27/38), C451H was 2.63% (1 /38), and I585C was 2.63% (1/38).
Analysis of RASs Based on HCV Genotype
The study analyzed the RASs measured in the experiments according to genotype and the presence or absence of certain RASs. Results are shown in the . The results showed that there was no significant correlation between the distribution rates of the five most frequently occurring RASs and genotypes (1b or 2a) in the study (p > 0.05).
Table 2 Fisher Exact Test Between RASs and Genotype
Correlation Between HCV RNA and HCV cAg
The baseline serum HCV RNA and HCV cAg had a significant medium-degree correlation (r = 0.601, p = 0.002). After 1 week of DAA treatment, although the two were correlated, the correlation was lower (r = 0.413, p = 0.032). The presence or absence of Q80K, S122G, V170I, Y93H, and C316N had no effect on the clearance of HCV RNA and HCV cAg within the first week of DAA treatment (p values are all greater than 0.05) ().
Analysis of RASs
The patients were divided into groups according to the presence or absence of different RAS, and we analyzed whether there was a difference in the clearance of HCV RNA and HCV cAg in the first week when the patient had or did not have a certain RAS. The results are shown in . When patients with Q80K, S122G, V170I, Y93H, and C316N were compared with patients without these RASs, there was no difference in the degree of HCV RNA or HCV cAg decline in the first week (p > 0.05).
Table 3 The Effect of RASs on the Clearance of HCV RNA or HCV cAg in the First Week
Further analysis was performed using the 10^5 lg IU/mL and 10^6 lg IU/mL cut-off values to define low vs high baseline viral load. We performed a chi-square test on the variables and calculated the odds ratio (OR) value. The results are shown in the . The results showed that the presence or absence of Q80K, S122G, C170I, Y93H, and C316N did not significantly affect the level of baseline viral load (p > 0.05).
Table 4 Fisher Exact Test Between RASs and Baseline HCV RNA
Discussion
HCV infection is covert and progresses slowly, so there are no obvious symptoms in the early stage. In many infected people, HCV infection is not diagnosed in a timely manner. At present, patients are first screened for anti-HCV, and when anti-HCV is found to be positive, then HCV RNA is further tested.Citation11 If HCV RNA is positive, an HCV infection is confirmed. However, both of these procedures currently have certain limitations: Anti-HCV usually appears and can be detected 70 days or more after infection, so it cannot be used for early diagnosis of HCV infection.Citation12 Anti-HCV exists in the body for a long time, and even after HCV has been cleared from the body, anti-HCV can still be detected. Therefore, anti-HCV positivity cannot confirm whether the patient has a current infection or a previous infection. In addition, anti-HCV testing also has a false positive rate. HCV RNA testing is the “gold standard” for HCV infection diagnosis, but HCV RNA testing requires sophisticated testing equipment and entails high testing costs. At the same time, lower-sensitivity HCV RNA testing may lead to missed diagnosis, so finding an alternative detection method still has research value.
HCV RNA is a sign of virus replication, and HCV cAg is a viral protein released into the blood during the virus assembly process.Citation3 In this study, quantitative detection of HCV RNA and HCV cAg was performed on patients at baseline and during the first week of treatment, and it was found that HCV RNA and HCV cAg were correlated during treatment. The correlation between the HCV RNA and HCV cAg was strong at baseline, but it was weakened when the value of virus was low in the first week of treatment. The reason may be that DAA drugs directly block the virus replication process, affecting HCV RNA more rapidly and directly, and the virus protein clearance occurs after clearing HCV RNA, so that the HCV cAg reaction is slightly delayed. Quantitative HCV cAg testing in the fourth week of treatment can still detect very low levels of HCV cAg in some patients, which also indicates that the clearance of core antigen may be delayed compared with HCV RNA. Compared with HCV RNA, HCV cAg testing has some advantages, such as simple operation and lower cost. When highly sensitive HCV RNA detection is limited, HCV cAg testing may help establish the HCV infection diagnosis.
Timely and accurate diagnosis is of great significance to reduce the chronicity of HCV disease and control its spread. Previous studies have shown that among several major factors that can lead to the failure of DAA treatment, RASs is extremely important. In this study, NS3, NS5A, and NS5B were sequenced in CHC patients. Y56F, Q80K, S122G, and V170I were found in the NS3 fragment; R30Q, P32A, P58S, and Y93H were found in the NS5A fragment; and C316N, C451H, and I585C were found in the NS5B fragment. We found that the prevalence of most RASs was roughly the same as that reported previously, but the prevalence of Y93H was higher than previously reported (20.51% vs 8.27%).Citation13 The reason may be that the included patients were mainly from North China, and the distribution of RASs shows regional differences, according to previous studies. In previous studies, the incidence of RASs was shown to vary among different HCV genotypes. For example, the reported incidence of Y93H in genotype 1b was 4.86%, while in 2a it was 0.91%. In this study, there was no significant correlation between the RASs and HCV genotypes. The reason may be that the sample size of this study is small, and the statistical results may be biased and do not reflect a more accurate condition. On the other hand, this result may also reflect that the relationship between RASs and genotype may not be as large as originally thought.Citation13 In addition,Citation13,Citation14 although some patients had related RASs, all these patients achieved SVR after DAA treatment. All types of RAS have no effect on the decline of the virus within the first week of DAA treatment. There may be several reasons for this result. First of all, the current DAA regimens for hepatitis C antiviral therapy consist of combination drugs targeting two or more HCV gene loci, and DAA drugs targeting only one gene loci are almost no longer used for single treatment, thus making the treatment more effective. In previous studies, the susceptibility of HCV strains was tested in vitro.Citation15,Citation16 Second, age, comorbidities, immune status, and other factors also affect the clearance of HCV in the body and the treatment in CHC. The results of this study indicate that, even if RASs are present in the HCV genes, their effect on virus clearance is still small. DAAs therapy can still quickly clear the virus and ultimately attain SVR.
Because of the low natural prevalence of some RASs, not all RASs were analyzed for statistical differences in this study. We analyzed whether RASs affect baseline HCV RNA, and the results showed that RASs in the study did not affect baseline HCV RNA. Generally, RASs can reduce the adaptability of HCV and reduce its replication ability. Therefore, the strains containing RASs are non-dominant strains with a lower frequency. HCV has a high mutation rate. In HCV infection, a variety of virus strains with different mutation sites can often be detected. Multiple virus strains made no difference in the total amount of HCV RNA in patients in this study. However, it is worth noting that under the action of DAAs, these non-dominant virus strains containing RASs may be transformed into dominant strains, which will reduce the antiviral activity of DAA and lead to treatment failure.
Conclusions
Y56F, Q80K, S122G, and V170I were found in the NS3 fragment. R30Q, P32A, P58S, and Y93H were found in the NS5A fragment, and C316N, C451H, and I585C were found in the NS5B fragment. The prevalence of Y93H was higher than previously reported. The HCV genotype may have a limited impact on the presence of the five RASs (Y93H, V179I, Q80K, S122G, C316N) in this study. HCV RNA is related to HCV cAg. The correlation between the two was strong at baseline, and when the virus load was low after 1 week of treatment, the correlation between the two was weak. Q80K, S122G, V170I, Y93H, and C316N detected in this study had no effect on virus clearance after 1 week of DAA treatment and baseline virus load.
Data Sharing Statement
Availability of dataset for review could be requested. All data reserved by first author, Hongyu Chen.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Julicher P, Galli C. Identifying cost-effective screening algorithms for active hepatitis C virus infections in a high prevalence setting. J Med Econ. 2018;21(1):1–10. doi:10.1080/13696998.2017.1369983
- Nguyen LT, Gray E, O’Leary A, Carr M, De Gascun CF; Irish Hepatitis CORN. The role of hepatitis c virus core antigen testing in the era of direct acting antiviral therapies: what we can learn from the protease inhibitors. PLoS One. 2016;11(10):e0163900. doi:10.1371/journal.pone.0163900
- Feng B, Yang RF, Jiang HJ, et al. Correlation analysis of hepatitis C virus core antigen and low viral loads: can core antigen replace nucleic acid test? Clin Exp Med. 2020;20(1):131–141. doi:10.1007/s10238-019-00588-1
- Takahashi K, Okamoto H, Kishimoto S, et al. Demonstration of a hepatitis C virus-specific antigen predicted from the putative core gene in the circulation of infected hosts. J Gen Virol. 1992;73(3):667–672. doi:10.1099/0022-1317-73-3-667
- Freiman JM, Tran TM, Schumacher SG, et al. Hepatitis C core antigen testing for diagnosis of hepatitis c virus infection: a systematic review and meta-analysis. Ann Intern Med. 2016;165(5):345–355. doi:10.7326/M16-0065
- Chang C, Hung CH, Wang JH, Lu SN. Hepatitis C core antigen highly correlated to HCV RNA. Kaohsiung J Med Sci. 2018;34(12):684–688. doi:10.1016/j.kjms.2018.08.002
- Cetiner S, Cetin Duran A, Kibar F, Yaman A. Performance comparison of new generation HCV core antigen test versus HCV RNA test in management of hepatitis C virus infection. Transfus Apher Sci. 2017;56(3):362–366. doi:10.1016/j.transci.2017.02.005
- Lamoury FMJ, Hajarizadeh B, Soker A, et al. Evaluation of a hepatitis C virus core antigen assay in plasma and dried blood spot samples. J Mol Diagn. 2018;20(5):621–627. doi:10.1016/j.jmoldx.2018.05.010
- Bartels DJ, Sullivan JC, Zhang EZ, et al. Hepatitis C virus variants with decreased sensitivity to direct-acting antivirals (DAAs) were rarely observed in DAA-naive patients prior to treatment. J Virol. 2013;87(3):1544–1553. doi:10.1128/JVI.02294-12
- World Health Organization. Guidelines for the care and treatment of persons diagnosed with chronic hepatitis C virus infection. Geneva: World Health Organization; 2018. Available from: https://www.who.int/hepatitis/publications/hepatitis-c-guidelines-2018/en/. Accessed June 13, 2022.
- Ghany MG, Morgan TR, Panel A-IHCG. Hepatitis C guidance 2019 update: American association for the study of liver diseases-infectious diseases society of America recommendations for testing, managing, and treating hepatitis C virus infection. Hepatology. 2020;71(2):686–721. doi:10.1002/hep.31060
- De Paschale M, Manco MT, Arpino O, et al. Threshold value of LIAISON XL anti-HCV screening assay predicting positive immunoblotting results. J Med Virol. 2017;89(10):1817–1822. doi:10.1002/jmv.24831
- Liu Z, Mao X, Wu J, et al. World-wide prevalence of substitutions in HCV genome associated with resistance to direct-acting antiviral agents. Clin Gastroenterol Hepatol. 2019;19(9):1906–1914.
- Sarrazin C, Dvory-Sobol H, Svarovskaia ES, et al. Prevalence of resistance-associated substitutions in HCV NS5A, NS5B, or NS3 and outcomes of treatment with ledipasvir and sofosbuvir. Gastroenterology. 2016;151(3):501–512. doi:10.1053/j.gastro.2016.06.002
- Chen Q, Perales C, Soria ME, et al. Deep-sequencing reveals broad subtype-specific HCV resistance mutations associated with treatment failure. Antiviral Res. 2020;174:104694. doi:10.1016/j.antiviral.2019.104694
- Han B, Martin R, Xu S, et al. Sofosbuvir susceptibility of genotype 1 to 6 HCV from DAA-naive subjects. Antiviral Res. 2019;170:104574. doi:10.1016/j.antiviral.2019.104574