Abstract
Objective
Nontuberculous mycobacteria (NTM) can cause pulmonary and extrapulmonary diseases. Tedizolid (TZD) is a new oxazolidinone with in vitro activity against NTM such as Mycobacterium avium complex (MAC), Mycobacterium fortuitum, and Mycobacterium abscessus complex. The aim of this study was to evaluate the TZD susceptibility profiles of clinical isolates of NTM.
Methods
The microdilution method was used to identify the minimum inhibitory concentration (MIC) of TZD and linezolid (LZD) for 133 clinical NTM isolates. Broth microdilution chequerboard assays were used to investigate the synergistic effects of TZD and three antibiotics on two reference isolates and eleven clinical isolates of NTM.
Results
The TZD MIC50 and MIC90 for M. abscessus complex were 2 and 4 μg/mL, 16 and >32 μg/mL for MAC, respectively. TZD exhibited lower MICs than that of LZD for most NTM, which were positively correlated. Due to the high MIC values of TZD against MAC, it is necessary to conduct drug sensitivity tests before TZD administration. TZD-clarithromycin combination had synergistic response on M. abscessus complex in 3 of the 8 isolates, which lasted only 3–5 days. TZD-cefoxitin had synergistic effect against all five M. fortuitum isolates.
Conclusion
Our study demonstrates that TZD had greater in vitro potency than LZD, and synergy studies suggested that TZD may be an important component of multi-drug treatment regimen.
Introduction
Nontuberculous mycobacteria (NTM) are opportunistic pathogens particularly in susceptible populations of all ages that infect pulmonary and extrapulmonary sites including the skin, soft tissues, lymph nodes, bones, and joints.Citation1 In 2010, it was reported that the prevalence of pulmonary NTM in the United States exceeds that of tuberculosis,Citation2 raising public awareness. The most common NTM pathogens are Mycobacterium avium complex (MAC), Mycobacterium kansasii, and Mycobacterium xenopi, among the slowly growing NTM (SGM), and Mycobacterium abscessus complex, among the rapidly growing NTM (RGM).Citation1 Many species of NTM are naturally multidrug resistant;Citation2 the infections caused by them are difficult to treat and usually require long-term combination therapy.
Linezolid (LZD), an oxazolidinone antibiotic, has been recommended as a first-line drug in the treatment of M. abscessus complex disease and as the second-line drug in the treatment of MAC pulmonary disease, due to its strong antibacterial activity.Citation3 However, its high recommended dosage of 600 mg once or twice per day incurs many adverse events such as cytopenia, and neuropathy restricts its long-term usage in clinical applications.Citation4 Tedizolid (TZD) has many of the same structural features as LZD, and inhibits protein synthesis by binding to the 23S rRNA of the 50S subunit, thereby blocking the formation of the 70S initiation complex.Citation5,Citation6 Tedizolid phosphate is a prodrug that is rapidly converted by endogenous phosphatases to TZD.Citation6 This structural difference confers higher aqueous solubility and bioavailability, and less steric hindrance than that of LZD.Citation7,Citation8 TZD has been approved by the US Food and Drug Administration (FDA) in 2014, and the European Medicine Agency in 2015 for the treatment of acute bacterial skin and soft tissue infections. TZD has presented high activity against Mycobacterium tuberculosis and exhibited greater potency than that of LZD.Citation9 In the hollow-fiber system model of intracellular MAC, TZD showed bactericidal activity, and is the backbone of short-course anti-MAC.Citation10 The recommended therapeutic dose of TZD has greater in vitro potency than that of LZD against inhibition of mitochondrial protein synthesis for a shorter duration, indicating its potent effect and fewer adverse reactions.Citation11 Furthermore, Yuste et al published the first clinical case of pulmonary NTM infection that shifted to prolonged treatment with TZD after hematological toxicity and gastrointestinal intolerance with LZD, and the patient tolerated the full course of TZD with no nausea or other side effects during this time.Citation12 TZD was recommended in the 2020 ATS/ERS/ESCMID/IDSA clinical practice guidelines for the treatment of NTM pulmonary disease when patients are intolerant or whose isolate is resistant to first-line drugs including clofazimine, moxifloxacin, and LZD.Citation1 However, further studies are required to elucidate the activity of TZD against NTM.
Nonetheless, the time-kill assays of TZD did not show any bactericidal activity against M. abscessus complex.Citation13 Due to little clinical data, the in vitro effects of TZD on other NTMs are still vague. Additionally, TZD was reported to have a synergistic interaction with some antibiotics against NTM. The combination of TZD with clarithromycin showed an initial synergistic effect against M. abscessus complex, whereas indifferent interactions were noted when TZD was combined with tigecycline, ciprofloxacin, doxycycline, cefoxitin, and amikacin.Citation13–15 In another report, the combination of TZD with imipenem showed a moderate synergistic effect against M. abscessus ATCC 19977.Citation16 The combination of TZD and cefoxitin showed significant synergistic interaction against M. fortuitum.Citation15 TZD exhibited no notable synergistic interaction with other commonly used antimycobacterial drugs against MAC including clarithromycin, rifampicin, ethambutol, amikacin, and minocycline.Citation15 However, the strains included in these synergistic studies are limited.
Hence, in our study, we investigated the drug susceptibility status of TZD compared to that of LZD against a total of 133 clinical NTM strains in vitro, particularly those isolates have the high MIC values of LZD. Additionally, we evaluated the synergistic effect of TZD with other antibiotics that are effective against these clinical isolates.
Material and Methods
Isolates and Antibiotics
In total, 133 clinical isolates were collected by our laboratory from Huashan Hospital, Fudan University (Shanghai, China) between 2016 and 2021, including 64 respiratory isolates, 33 tissue samples (skin, puncture fluid, pus and wound), 5 blood system isolates, 3 urinary tract isolates, 1 central system isolate. Regrettably, the rest 27 isolates source records are missing. All NTM isolates collected between 2016 and 2021 except for MAC previously reported or Mycobacterium marinum were selected in this study. For M. marinum, we randomly selected 10 isolates of M. marinum collected in 2021. If a patient had multiple isolates, only the earliest isolate was included.
M. abscessus ATCC 19977, M. fortuitum ATCC 6841, Mycobacterium mucogenicum ATCC 49650, Mycobacterium smegmatis ATCC 700084, Mycobacterium peregrinum ATCC 700686, Mycobacterium intracellulare ATCC 13950, and M. kansasii ATCC 12478 were used as reference strains () performed in each round of drug susceptibility test that were purchased from the American Type Culture Collection (ATCC). All isolates were thawed and recovered in cation-adjusted Mueller-Hinton Broth (CAMHB) (Oxoid, Hampshire, UK) or 7H9 liquid medium (Difco, Becton Dickinson, Sparks, MD).
Table 1 The Drug Susceptibility Results of 7 Reference NTM Isolates
TZD, LZD and cefoxitin sodium salt were purchased from Aladdin (Shanghai, China). Imipenem and clarithromycin powders were purchased from Meilunebio (Shanghai, China).
All NTM isolates were identified at the species level using polymerase chain reaction (PCR) amplification and Sanger sequencing of 16S rRNA according to a previous study.Citation17 The subspecies of the 35 M. abscessus complex clinical isolates was identified by multi-locus sequencing of the rpoB, hsp65 and secA genes as reported.Citation26,Citation19
Drug Susceptibility Tests
The drug susceptibility tests of SGM were performed by using the broth microdilution method in 96-well plates according to the CLSI guidelines M24-A2.Citation18 We tested the TZD concentrations ranging from 0.125 to 32 mg/L and LZD from 0.5 to 128 mg/L in 2-fold serial dilutions in all 140 NTM isolates. The minimum inhibitory concentration (MIC) was defined as the concentration of antibiotic at which there was no visible growth. MIC50 and MIC90 values were, respectively, defined as drug concentrations that inhibited 50% and 90% of the isolates. All experiments were performed at least twice with the reference strains as the control. To obtain sufficient growth for the SGM isolates, we used 7H9 liquid medium supplemented with 0.05% Tween® 80 and 10% bovine serum albumin-dextrose-catalase (ADC) enrichment instead of CAMHB at 37°C for 14 days as the previous studies recommended,Citation25 and bacteria (McFarland standard of 1.0) were inoculated into 7H9 containing antibiotics. After 14–21 days of incubation at 37°C (30°C for M. marinum), the MIC was determined. The RGM bacteria (McFarland standard of 0.5) were inoculated in CAMHB containing antibiotics. For some RGM isolates, the growth is insufficient in the growth control well; therefore, MICs for RGM bacteria were determined after incubating at 37°C instead of 30°C for 3–5 days, depending on the growth of the positive control wells containing no antibiotic. As showed in a previous study to explore the relationship between LZD and TZD MIC,Citation15 we also ln(x+1) transformed the MIC values and performed a Spearman’s ρ two-tailed correlation test using a Gaussian approximation by the SPSS software version 20.
The normality of MIC distribution of M. abscessus complex, M. fortuitum, MAC and M. kansasii was evaluated by Shapiro–Wilk test using SPSS. The comparison between M. abscessus complex subspecies was analyzed by Kruskal–Wallis test, with P ≤ 0.05 as statistically significant.
Synergy Studies and Genotype of erm(41)
Synergy testing was performed using the broth microdilution checkerboard method.Citation20 According to the MIC of TZD in our experiments, clinical isolates whose drug concentrations ranged around the MIC50 of its species used in the drug synergy test. TZD was evaluated in combination with clarithromycin and imipenem against 7 clinical isolates of M. abscessus complex and the reference strain ATCC 19977. The result of TZD–clarithromycin combination was read at both day 3 and day 14. The erm(41) genotype of all 7 clinical strains were sequenced as previous study.Citation27
TZD in combination with cefoxitin was evaluated against 4 clinical isolates of M. fortuitum and the reference strain ATCC 6841. The fractional inhibitory concentration index (FICI) was calculated as follows:
Interaction was classified as synergistic, indifferent, and antagonistic when the FICI value was ≤0.5, 0.5–4, and >4, respectively.
Results
MICs of LZD and TZD
The RGM species included M. abscessus complex (n = 35), M. fortuitum (n = 21), M. mucogenicum (n = 4), M. chelonae (n = 1), and M. ltetiense (n = 1). Among the 35 M. abscessus complex strains, there were 24 M. abscessus isolates, 11 M. massiliense isolates and no M. bolletii identified by multi-locus sequencing. The SGM species included MAC (n = 40), M. kansasii (n = 18), M. marinum (n = 10), M. virginiense (n = 1), and M. gordonae (n = 2).
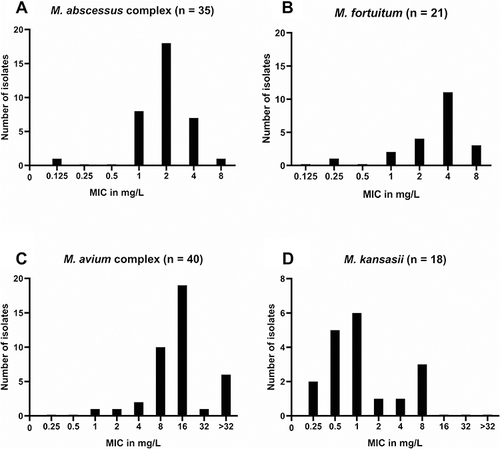
The MICs of TZD for clinical isolates of NTM are given in . The detailed MICs of each strain are given in Supplementary Table 1. The MIC50 and MIC90 of M. abscessus complex are 2 and 4 μg/mL for TZD, respectively. There was no significant difference between the 2 subspecies of the M. abscessus complex (P = 0.142 for TZD and 0.182 for LZD). The susceptibilities of the TZD MIC50 and MIC90 of M. fortuitum are 4 and 8 μg/mL, respectively, which are 4 times lower than those of LZD. The 40 MAC isolates had MIC50 and MIC90 values of 16 and >32 μg/mL for TZD, and 64 and 128 μg/mL for LZD, respectively. Analysis showed that MICs of TZD and LZD are strongly correlated for M. abscessus complex (Spearman’s ρ = 0.692, P < 0.001), M. fortuitum (Spearman’s ρ = 0.617, P < 0.05), and M. kansasi (Spearman’s ρ = 0.811, P < 0.001), and moderately correlated for MAC (Spearman’s ρ = 0.564, P < 0.001). The gaussian distribution was showed in the MIC distribution of TZD for M. abscessus complex (P = 0.265), M. fortuitum (P = 0.096) and M. kansasii (P = 0.32) in , except for MAC (P = 0.025).
Table 2 The Drug Susceptibility Results of 133 Clinical NTM Isolates
TZD Synergy Response with Other Antimicrobials
For the 7 clinical isolates of M. abscessus complex tested in TZD-clarithromycin combination, the erm(41) PCR product of the M. massiliense (n = 3) was shorter (~350 bp) than that of the M. abscessus (n = 4) (~700 bp), consistent with the previous study.Citation27 Among the 4 clinical isolates of M. abscessus which had longer erm(41), one isolate had a nonfunctional T28C mutation in it, while the other 3 isolates had a wildtype erm(41).
The synergistic response of TZD-clarithromycin combination was observed in 3 clinical isolates of the M. abscessus and this synergistic response of TZD-clarithromycin lasted 3–5 days, but rapidly showed inducible clarithromycin resistance. As for the other 4 isolates susceptible to clarithromycin, we did not observe the synergistic response on day 3 (Average FICI = 0.84) or day 14 (Average FICI = 1.016). The FIC and erm(41) genotype of each M. abscessus complex isolate is shown in .
Table 3 FICIs of Tedizolid in Combination with Clarithromycin Against M. abscessus complex (n = 8)
The combination of TZD and TZD-imipenem showed indifferent interactions (FICI = 0.82) () against 4 isolates of M. abscessus complex, and the other 4 clinical isolates were highly resistant to imipenem.
Table 4 FICIs of Tedizolid in Combination with Clarithromycin and Imipenem for M. abscessus complex, and Tedizolid in Combination with Cefoxitin for M. fortuitum
For M. fortuitum, TZD-cefoxitin exhibited a synergistic effect against all five isolates (average FICI = 0.36).
Discussion
The drug susceptibility of NTM clinical strains is important for evaluating the effect of TZD. In this study, the MIC50 and MIC90 of TZD for M. abscessus complex were 2 and 4 μg/mL, respectively. This result is consistent with previous studies in which the TZD MIC50 and MIC90 were 1–4 and 2–8 μg/mL, respectively.Citation13,Citation15,Citation21–23
The TZD MIC50 and MIC90 for MAC were relatively high at 16 and 32 μg/mL, respectively (). In a previous study, both the TZD MIC50 and MIC90 for MAC were 8 μg/mL.Citation21 Similarly, another study showed the TZD MIC50 and MIC90 for M. avium were 2 and 16 μg/mL, respectively, and the MIC ranged from 4 to 8 μg/mL for M. intracellulare.Citation15 However, a previous study from Beijing, China, showed high-level MIC values to TZD, in which the TZD MIC50 and MIC90 for M. avium and M. intracellulare were 32 and >32 μg/mL, respectively.Citation23 Therefore, the relative higher MIC values for MAC isolates in our study could have national and geographical reasons such as the high use of antibiotics in China. Furthermore, all studies including ours showed the high MIC values of TZD against MAC; thus, it is necessary to carry out drug sensitivity tests before TZD administration. Meanwhile, TZD MIC distribution in our study was unimodal for M. abscessus complex and M. fortuitum, nearly bimodal for M. kansasii and MAC, which was consistent with the previous study.Citation15 The breakpoint for TZD was set as 1mg/L in a previous study in 2017, in which the exposure–response experiment in the hollow-fiber system model of intracellular MAC was performed at the TZD dose of 200mg/day.Citation10 However, we did not use the recommended breakpoint, because 1mg/L is exactly on the first peak of MIC distribution for M. kansasii, MAC, and M. abscessus complex, that may divide the wild type population and subsequently leads to once as susceptible and once as resistant in the resistance replicate test, which usually not identical and lay within a relatively small range. As there is insufficient basis for setting breakpoint based on current data, the setting of breakpoint still requires more clinical data and further study.
A previous study showed that TZD’s penetration into lung epithelial lining fluid and alveolar macrophages is greater than that of LZD.Citation24 Additionally, TZD had stronger potency against NTM, which may be an effective substitute for LZD in the treatment of NTM-related infectious diseases. Furthermore, we found the MICs of TZD and LZD were positively correlated for M. abscessus complex, M. fortuitum, M. kansasi, and MAC; this observation is partially consistent with those in a study by Ruth et alCitation15 wherein TZD and LZD MICs were correlated for MAC but not for M. abscessus complex. This may be because >10% M. abscessus complex had an MIC >32 μg/mL in LZD and a limited number of isolates in their study; therefore, there was no significant difference (Spearman’s ρ = 0.2304, P = 0.1192). Due to the correlation between TZD and LZD, we do not recommend directly replacing LZD with TZD in the treatment of drug-resistant strains without first verifying the susceptibility of TZD.
Synergy tests showed that TZD exhibited significant interactions with cefoxitin against M. fortuitum, which was consistent with the results of a previous study.Citation15 Although the combination of TZD with imipenem showed a moderate synergistic effect against M. abscessus ATCC 19977,Citation16 this synergy was not observed in our clinical strains. In addition to the broth microdilution checkerboard method, TZD-amikacin combination showed modest, concentration-dependent synergy against MAC relative to the Bliss independence model by time–kill kinetics assays, whereas TZD-ethambutol combination was synergistic for M. avium at lower concentrations.Citation15 TZD showed concentration-dependent bactericidal activity against M. avium and bacteriostatic activity against M. abscessus in time-kill experiments, and the Bliss independent model focused on the superposition of doses. In the hollow-fiber system model of intracellular MAC, Deshpande et al identified a novel four-drug combination (ceftazidime/avibactam, rifabutin, TZD, and moxifloxacin) exhibiting kill rates better than that of standard therapy.Citation16 Similarly, the results of synergy tests in the current study suggested that TZD may be an important component of multi-drug treatment regimen, although further research on exposure dosages is required.
However, our study has several limitations. First, probably since most of our NTM isolates were from patients who had been on antimicrobial therapy, a large amount of RGM isolates were not growing well in 30°C although we tried to perform the drug susceptibility tests in 30°C. The MICs for all RGM bacteria in our study uniformly grew and were determined after incubating at 37°C for 3–5 days, depending on the growth of the positive control wells containing no antibiotic. We recorded the MICs timely and included the standard strains as quality controls in each batch. Although we changed the incubation temperature to 37°C, the TZD and LZD MIC of our quality control strains were comparable to the previous studies,Citation15,Citation22 therefore, the results still have reference value. Second, the isolates were collected from a geographically limited group of patients who visited Shanghai. Additionally, a limited number of NTM isolates were collected. Hence, the drug susceptibility and synergic combination results should be verified in more clinical strains.
In conclusion, our results showed that TZD has lower MICs than those of LZD when tested against NTM-related diseases, which indicated that TZD had greater in vitro potency than LZD. Furthermore, the results of MICs of TZD and LZD were correlated in vitro, based on the synergistic interactions with cefoxitin and clarithromycin against M. fortuitum and only initially 3/7 of the clinical isolates of M. abscessus complex, respectively.
Ethics Approval and Informed Consent
The research for the current study has been approved by the Institutional Review Board (IRB) of Huashan Hospital, Fudan University (Number: 2022-291). Our study is a retrospective study without direct contact with the patient, and did not involve personal privacy or commercial interests, so the informed consent was waived. This study is in accordance with the Helsinki Declaration and the information of all patients included in the study remained confidential.
Disclosure
The authors declare no conflicts of interest in this work.
Additional information
Funding
References
- Daley CL, Iaccarino JM, Lange C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline: executive summary. Clin Infect Dis. 2020;71(4):E1–E36. doi:10.1093/cid/ciaa241
- Adjemian J, Olivier KN, Seitz AE, Holland SM, Prevots DR. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am J Respir Crit Care Med. 2012;185(8):881–886. doi:10.1164/rccm.201111-2016OC
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416. doi:10.1164/rccm.200604-571ST
- Winthrop KL, Ku JH, Marras TK, et al. The tolerability of linezolid in the treatment of nontuberculous mycobacterial disease. Eur Respir J. 2015;45(4):1177–1179. doi:10.1183/09031936.00169114
- Kisgen JJ, Mansour H, Unger NR, Childs LM. Tedizolid: a new oxazolidinone antimicrobial. Am J Health Syst Pharm. 2014;71(8):621–633. doi:10.2146/ajhp130482
- Burdette SD, Trotman R, Saravolatz LD. Tedizolid: the first once-daily oxazolidinone class antibiotic. Clin Infect Dis. 2015;61(8):1315–1321. doi:10.1093/cid/civ501
- Rybak JM, Marx K, Martin CA. Early experience with tedizolid: clinical efficacy, pharmacodynamics, and resistance. Pharmacotherapy. 2014;34(11):1198–1208. doi:10.1002/phar.1491
- Locke JB, Finn J, Hilgers M, et al. Structure-activity relationships of diverse oxazolidinones for linezolid-resistant Staphylococcus aureus strains possessing the CFR methyltransferase gene or ribosomal mutations. Antimicrob Agents Chemother. 2010;54(12):5337–5343. doi:10.1128/AAC.00663-10
- Ruiz P, Causse M, Vaquero M, Casal M. In vitro activity of tedizolid against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2019;63(4). doi:10.1128/AAC.01939-18
- Deshpande D, Srivastava S, Pasipanodya JG, Lee PS, Gumbo T. Tedizolid is highly bactericidal in the treatment of pulmonary Mycobacterium avium complex disease. J Antimicrob Chemother. 2017;72(suppl_2):i30–i35. doi:10.1093/jac/dkx305
- Flanagan S, McKee EE, Das D, et al. Nonclinical and pharmacokinetic assessments to evaluate the potential of tedizolid and linezolid to affect mitochondrial function. Antimicrob Agents Chemother. 2015;59(1):178–185. doi:10.1128/AAC.03684-14
- Yuste JR, Berto J, Del PJ, Leiva J. Prolonged use of tedizolid in a pulmonary non-tuberculous mycobacterial infection after linezolid-induced toxicity. J Antimicrob Chemother. 2017;72(2):625–628. doi:10.1093/jac/dkw484
- Compain F, Soroka D, Heym B, et al. In vitro activity of tedizolid against the Mycobacterium abscessus complex. Diagn Microbiol Infect Dis. 2018;90(3):186–189. doi:10.1016/j.diagmicrobio.2017.11.001
- Tang YW, Cheng B, Yeoh SF, Lin R, Teo J. Tedizolid activity against clinical Mycobacterium abscessus complex isolates-an in vitro characterization study. Front Microbiol. 2018;9:2095. doi:10.3389/fmicb.2018.02095
- Ruth MM, Koeken V, Pennings LJ, et al. Is there a role for tedizolid in the treatment of non-tuberculous mycobacterial disease? J Antimicrob Chemother. 2020;75(3):609–617. doi:10.1093/jac/dkz511
- Le Run E, Arthur M, Mainardi JL. In vitro and intracellular activity of imipenem combined with tedizolid, rifabutin, and avibactam against Mycobacterium abscessus. Antimicrob Agents Chemother. 2019;63(4). doi:10.1128/AAC.01915-18
- Tortoli E. Impact of genotypic studies on mycobacterial taxonomy: the new mycobacteria of the 1990s. Clin Microbiol Rev. 2003;16(2):319–354. doi:10.1128/CMR.16.2.319-354.2003
- Woods GL, Brown-Elliott BA, Conville PS, et al. Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes. Wayne (PA): Clinical and Laboratory Standards Institute; 2018.
- Zelazny AM, Root JM, Shea YR, et al. Cohort study of molecular identification and typing of Mycobacterium abscessus, Mycobacterium massiliense, and Mycobacterium bolletii. J Clin Microbiol. 2009;47(7):1985-1995. doi:10.1128/JCM.01688-08
- Odds FC. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother. 2003;52(1):1. doi:10.1093/jac/dkg301
- Vera-Cabrera L, Brown-Elliott BA, Wallace RJ, et al. In vitro activities of the novel oxazolidinones DA-7867 and DA-7157 against rapidly and slowly growing mycobacteria. Antimicrob Agents Chemother. 2006;50(12):4027–4029. doi:10.1128/AAC.00763-06
- Brown-Elliott BA, Wallace RJ, Land GA. In vitro susceptibility testing of tedizolid against Nontuberculous mycobacteria. J Clin Microbiol. 2017;55(6):1747–1754. doi:10.1128/JCM.00274-17
- Wen S, Gao X, Zhao W, et al. Comparison of the in vitro activity of linezolid, tedizolid, sutezolid, and delpazolid against rapidly growing mycobacteria isolated in Beijing, China. Int J Infect Dis. 2021;109:253–260. doi:10.1016/j.ijid.2021.06.055
- Housman ST, Pope JS, Russomanno J, et al. Pulmonary disposition of tedizolid following administration of once-daily oral 200-milligram tedizolid phosphate in healthy adult volunteers. Antimicrob Agents Chemother. 2012;56(5):2627–2634. doi:10.1128/AAC.05354-11
- Jaffre J, Aubry A, Maitre T, et al. Rational choice of antibiotics and media for Mycobacterium avium complex drug susceptibility testing. Front Microbiol. 2020;11:81. doi:10.3389/fmicb.2020.00081
- Macheras E, Roux AL, Bastian S, et al. Multilocus sequence analysis and rpoB sequencing of Mycobacterium abscessus (sensu lato) strains. J Clin Microbiol. 2011;49(2):491–499. doi:10.1128/JCM.01274-10
- Shallom SJ, Gardina PJ, Myers TG, et al. New rapid scheme for distinguishing the subspecies of the Mycobacterium abscessus group and identifying Mycobacterium massiliense isolates with inducible clarithromycin resistance. J Clin Microbiol. 2013;51(9):2943–2949. doi:10.1128/JCM.01132-13