Abstract
Background
Carbapenem-resistant Klebsiella pneumoniae (CRKP) infection is associated with high mortality and has become a major public problem threatening patients. This study aimed to explore risk factors for death in patients with Klebsiella pneumoniae (KP) and identify risk factors for CRKP infection.
Methods
The study retrospectively analyzed clinical characteristics and microbiological data from patients infected with KP from January 2019 to October 2021 to identify risk factors and mortality, using multivariate logistic regression analysis and Cox regression analysis.
Results
A total of 214 KP inpatients were enrolled in our study. The in-hospital mortality rate was significantly higher in patients infected with CRKP (13/68, 19.12%) than carbapenem-susceptible KP (CSKP) (2/146, 1.37%) and the difference was statistically significant (P= 0.03). Multivariate Cox regression analysis showed CRKP isolation (HR 12.26, 95% CI 2.43–61.68, P = 0.002), lower TP (HR 10.50, 95% CI 1.33–82.76, P = 0.03), antibiotic days of therapy >15 (HR 0.08, 95% CI 0.01–0.56, P= 0.01) and length of stay (LOS) (HR 0.03, 95% CI 0.002–0.61, P= 0.02) were independent risk factors for death from KP. Additionally, intensive care unit (ICU) stay (OR 21.69, 95% CI 4.50–118.76, P< 0.001) and previous carbapenem exposure (OR 5.26, 95% CI 1.38–21.19, P= 0.02) are independent risk factors for CRKP.
Conclusion
Our findings showed that patients infected with CRKP have a higher in-hospital mortality rate. Identifying the independent risk factors for CRKP infection may contribute to the management of CRKP and reduce the mortality of KP patients.
Introduction
Klebsiella pneumoniae (KP) is a major human pathogen associated with nosocomial infections, particularly in immune-compromised individuals.Citation1,Citation2 Carbapenems are widely recognized as the last line of therapy for multidrug-resistant KP,Citation2,Citation3 and therefore are extremely important in conventional medicine. However, the growing problem of Carbapenem-resistant Klebsiella pneumoniae (CRKP) poses a global threat, with the detection rate of CRKP in China rising sharply from 9.2% in 2010 to 27.1% in 2021 (http://www.chinets.com/Data/GermYear).Citation1–3 Moreover, CRKP has occurred by the acquisition of virulent or resistant plasmids, which are easily transferred between bacteria and cause nosocomial infections.Citation4,Citation5 The cost associated with CRKP is becoming alarming at present, making it necessary to push actions to tackle this problem.Citation6
Previous studies showed that intensive care unit (ICU) admission, exposure to carbapenems, surgery, mechanical ventilation, central venous catheterization, indwelling gastric tube and nasogastric intubation are independent risk factors for CRKP.Citation7,Citation8 In addition, multiple studies have concluded that CRKP could increase the mortality rate of KP to about 40–50%.Citation9,Citation10 Therefore, a comprehensive understanding of the clinical characteristics, risk factors, and outcomes of patients with CRKP infection will aid surveillance and diagnostic initiatives, and help clinicians adjust treatment strategies.Citation11
Despite the large amount of data generated by epidemiology, the detection rate of CRKP continues to increase year by year, and support for CRKP infection management remains inadequate. Meanwhile, risk factors for CRKP may vary in different clinical settings. Therefore, in this paper, we focus on getting insights into the risk factors and the mortality of patients with KP. Further, we also dissected the risk factors for patients with CRKP and Carbapenem-susceptible KP (CSKP). The results obtained in this study will help clinicians to identify the risk factors for the occurrence of CRKP infection, understand the prognosis of patients with KP, and provide new ideas for the prevention or most effective management of CRKP.
Methods
Data Collection and Study Design
This was a retrospective study conducted in Shunde Hospital of Southern Medical University (a 3000-bed tertiary hospital in Guangdong Province, China) from January 2019 to October 2021. All patients with CRKP detected during hospitalization were included in the study. Data collected from the hospital database include patients’ clinical and demographic characteristics, infection outcomes, ICU stay, invasive procedures, treatment options, and so on. For each bacterial isolate, antimicrobial sensitivity was analyzed from the laboratory database. Only the first episode of each patient was included in our study, and those with recurrent infections were excluded. In addition, incomplete cases or cases with cultivated CRKP prior to hospitalization were also excluded. To assess the risk factors and explored the prognosis among patients, the study was designed as follows. First, patients were divided into CSKP and CRKP groups, and the differences between the two groups were analyzed. Second, the risk factors for patients with CRKP infection were confirmed. Finally, KP patients were divided into survival and death groups according to their discharge outcomes to determine risk factors following KP infection.
Diagnostic and Drug Susceptibility Test
Vitek2 (Biomerieux, France) was used to confirm the species identification and drug susceptibility tests. Clinical specimens were mainly sputum samples, and other samples included mid-stream urine, whole blood, pleural and abdominal fluid, etc. Carbapenem resistance is defined as MIC ≥2 μg/mL of ertapenem or MIC ≥4 μg/mL of meropenem or imipenem, according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI).Citation12 According to the standards of the Centers for Disease Control (CDC), CRKP is diagnosed when non-susceptibility is found to at least one carbapenem class antibiotic (including imipenem and meropenem).Citation13
Statistical Analysis
Continuous variables with normal distribution were evaluated using Either Student’s t-test or Mann–Whitney U-Test, resulting in median (interquartile range [IQR]). Categorical variables were compared by the Chi-squared test and expressed as counts or counts/total (percentages). Identification of risk factors for in-hospital mortality by univariate and multivariate Cox regression analysis. Kaplan–Meier (KM) survival curves were generated with Log-rank test analysis. Logistic regression analyses were performed to confirm independent risk factors for CRKP, the results were listed as odds ratios (95% confidence interval). All analyses were performed with R version 3.6.3 (The R Foundation, Vienna, Austria). P < 0.05 was considered statistically significant, and all analyses were two-tailed.
Results
Clinical and Demographic Characteristic of Patients with KP
A total of 214 KP inpatients were enrolled in our study. Most of the positive specimens were obtained from sputum samples (53.74%), followed by mid-stream urine (21.50%), whole blood (14.49%), pleural and abdominal fluid (2.34%), etc. According to the resistance of isolates to carbapenems, the clinical and demographic characteristics of patients with KP isolates were shown in . Compared to patients with CSKP, those who suffered from CRKP had diabetes mellitus, lung infection, lower TP, had stayed in ICU, or had undergone invasive procedures, including surgery. Furthermore, patients with CRKP tend to be hospitalized for longer than 30 days. In addition, those who received antibiotics treatments before KP isolation were more likely to develop CRKP than those with CSKP.
Table 1 Clinical and Demographic Characteristics of patients with KP
Risk Factors for in-Hospital Mortality in Patients with KP Infection
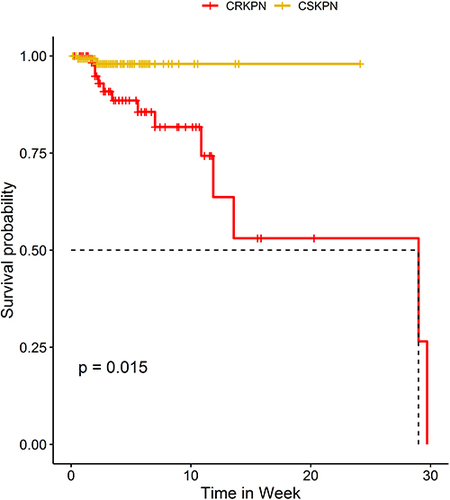
The univariate analyses to identify potential risk factors for in-hospital mortality of KP include CRKP isolation (HR 5.59, 95% CI 1.21–21.91, P =0.03), lower TP (HR 13.79, 95% CI 1.78–106.86, P= 0. 01), ICU stay (HR 13.95, 95% CI 1.77–110.23, P =0.01), antibiotic days of therapy>15 (HR 0.10, 95% CI 0.02–0.47, P =0.004), drug combination therapy (HR 4.05, 95% CI 1.07–15.42, P =0.04), LOS >30day (HR 0.03, 95% CI 0.002–0.42, P =0.04). Multivariate analysis of these 214 patients showed that variable associated with in-hospital mortality in patients with KP were: CRKP isolation (HR 12.26, 95% CI 2.43–61.68, P= 0.002), lower TP (HR 10.50, 95% CI 1.33–82.76, P= 0.03), antibiotic days of therapy>15 (HR 0.08, 95% CI 0.01–0.56, P= 0.01), LOS>30day (HR 0.03, 95% CI 0.002–0.61, P = 0.02) (). The in-hospital mortality rate of patients with KP was 7.01% (15/214). Nevertheless, it was significantly higher for patients with CRKP (13/68, 19.12%) than patients with CSKP (2/146, 1.37%). Survival curve analysis also confirmed that infection with CRKP has a higher risk of mortality (P =0.02) ().
Table 2 Univariate and Multivariate COX Regression Analysis of Risk Factors for in-Hospital Mortality Among Patients with KP
Risk Factors for the Development of CRKP Infection in Patients
Univariate analysis showed that, compared with patients with CSKP, patients with CRKP were more likely to have old age, lower TP, diabetes mellitus, lung infection, ICU stay, or to have undergone invasive procedures, including surgery. As a supplement, patients with CPKP also had LOS>30 and they were more likely to have been exposed to extensive antimicrobial therapy before infecting KP (). Further, the multivariate logistic regression analysis in summarizes independent risk factors for developing CRKP versus CSKP: ICU stay (OR 21.69, 95% CI:4.50–118.76, P < 0.001), and previous carbapenem exposure (OR 5.26, 95% CI:1.38–21.19, P = 0.02).
Table 3 Univariate and Multivariate Logistic Regression Analysis of Risk Factors for Patients with CRKP
Discussion
The spread of CRKP has become a major global health event and a new crisis for humanity.Citation14 Developing new antibiotics and strictly controlling their use is the most effective means of curbing the spread of CRKP, but it takes time and considerable effort. Whole-genome sequencing of drug-resistant strains will likely uncover novel drug-resistant biomarkers, but extensive systematic studies are also required for further confirmation.Citation15 Therefore, we conducted this retrospective study to identify risk factors for CRKP infection and in-hospital mortality with the aim of guiding future interventions and reducing the spread of CRKP.
In this paper, those who suffered from CRKP have longer hospital and ICU stay, and frequent use of invasive procedures, compared to patients with CSKP. Carbapenems are often considered a last resort to treat infections with multi-resistant strains. A study has confirmed that the use of carbapenem antibiotics was closely related to the production of carbapenemase by KP.Citation16 Our results demonstrated that previous carbapenem exposure was an independent risk factor for CRKP. Consistently, several studies of hospitalized patients in specific populations have revealed similar risk factors identified in our study.Citation17,Citation18 Furthermore, we also found that ICU stay is a critical risk factor for CRKP infection development, which is in line with several previous publications.Citation19–21 It must be admitted that KP usually colonizes the respiratory tract or intestine due to frequent use of invasive procedures (eg endotracheal intubation) and can lead to mucosal barrier damage. However, it is unlikely that patients in ICU will be able to avoid prolonged exposure to invasive equipment or antibiotics, especially carbapenems, due to their weakened autoimmune, critical illness, and vulnerability to infection.Citation22 Interestingly, some studies identified that KP colonization is a significant risk factor for infection in ICU, and acquisition of CRKP during ICU hospitalization may be due to colonization of existing CRKP in the gastrointestinal tract, particularly after widespread use of antibiotics.Citation23,Citation24 Therefore, it is critical to implement colonization screening and intervention strategies in ICU Settings, especially to enhance equipment care, to further prevent colonization and transmission of CRKP.
The paper also identified that CRKP isolation, low TP, LOS>30, and antibiotic days of therapy>15 were independent risk factors for in-hospital mortality of KP. The in-hospital mortality for CRKP of 19.11% was higher than that of the CSKP of 1.37%, and the difference was statistically significant (P =0.03), which is consistent with the results of Liu et al.Citation18 In immune-compromised patients, the mortality associated with CRKP infection was higher than that observed in our study, particularly when considering patients undergoing transplantation (55.8%), or patients with hematologic malignancies and solid tumors (56–73%).Citation25–28 In contrast, the mortality of CRKP in this study was significantly lower than in other literature, probably because many Chinese chose to be discharged home at the end of their life. These differences may be due to differences in inclusion criteria and study design chosen by different investigators. In addition, patient conditions, co-morbidities, as well as geographic variations, should be taken into account. Our study also analyzed risk factors for KP in-hospital mortality. The results suggest that CRKP isolation is associated with high in-hospital mortality, which is similar to those reported in previous publications.Citation29,Citation30
Admittedly, there are several limitations to our study. First, our study was a single-center retrospective analysis with a small sample size or selection bias. Second, The risk factors we identified are limited, and their applicability to other settings needs to be further validated in prospective studies. Third, further research is needed to conduct drug resistance gene detection in the CRKP group to explore the relationship between CRKP drug resistance types and clinical characteristics. Additionally, in future iterations of the project, we hope to improve the identification of MDR and XDR infections.
Conclusion
In conclusion, patients with CRKP showed poor outcomes and high mortality. CRKP isolation, low total protein, long hospital stay, and antibiotic days of therapy>15 were independent risk factors for mortality of patients with KP. Furthermore, ICU stay and previous carbapenem exposure are associated with an increased risk of CRKP. Awareness of these risk factors will help guide future interventions and hopefully reduce the transmission of CRKP, thereby reducing the mortality of KP and the risk of developing CRKP. Our findings also can be applied to the advancement of knowledge in the broader area of antimicrobial resistance and infection control.
Abbreviations
ICU, intensive care unit; KP, Klebsiella pneumoniae; CRKP, Carbapenem-resistant Klebsiella pneumoniae, CSKP, carbapenem-susceptible Klebsiella pneumoniae; LOS, length of stay.
Data Sharing Statement
For reasons of medical research ethics and confidentiality, trial data are not publicly available. However, in addition to personal private information, other research data may also be obtained from corresponding authors upon reasonable request by the Ethics Committee.
Ethics Approval and Consent to Participate
The Study involving human participants received ethical approval from the Institutional Review Board (IRB) of Shunde Hospital, Southern Medical University. The IRB approval number is 2020071. For all patients, the purpose of the study was clearly explained, and written informed consent was obtained prior to admission, stating that medical records (medical records, laboratory tests, etc.) would be kept completely on the hospital’s medical record system during hospitalization. Researchers, ethics committees, and drug regulators will be allowed access to medical records. This study was conducted in accordance with the Declaration of Helsinki.
Disclosure
The authors have no conflict of interest in this work.
Acknowledgment
The authors would like to thank those who participated in this study.
References
- Lee BY, Bartsch SM, Wong KF, et al. The potential trajectory of carbapenem-resistant Enterobacteriaceae, an emerging threat to health-care facilities, and the impact of the centers for disease control and prevention toolkit. Am J Epidemiol. 2016;183(5):471–479. doi:10.1093/aje/kwv299
- Brink AJ. Epidemiology of carbapenem-resistant gram-negative infections globally. Curr Opin Infect Dis. 2019;32(6):609–616. doi:10.1097/qco.0000000000000608
- Munoz-Price LS, Poirel L, Bonomo RA, et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis. 2013;13(9):785–796. doi:10.1016/s1473-3099(13)70190-7
- Bialek-Davenet S, Criscuolo A, Ailloud F, et al. Genomic definition of hypervirulent and multidrug-resistant Klebsiella pneumoniae clonal groups. Emerg Infect Dis. 2014;20(11):1812–1820. doi:10.3201/eid2011.140206
- Feng Y, Lu Y, Yao Z, Zong Z. Carbapenem-resistant hypervirulent Klebsiella pneumoniae of sequence type 36. Antimicrob Agents Chemother. 2018;62(7). doi:10.1128/aac.02644-17
- Huang W, Qiao F, Zhang Y, et al. In-hospital medical costs of infections caused by carbapenem-resistant Klebsiella pneumoniae. Clin Infect Dis. 2018;67(suppl_2):S225–s230. doi:10.1093/cid/ciy642
- He G, Huang J, Huang S, et al. Risk factors affecting clinical outcome in patients with carbapenem-resistant k. Pneumoniae: a retrospective study. Med Sci Monit. 2020;26:e925693. doi:10.12659/msm.925693
- Qian Y, Bi Y, Liu S, Li X, Dong S, Ju M. Predictors of mortality in patients with carbapenem-resistant Klebsiella pneumoniae infection: a meta-analysis and a systematic review. Ann Palliat Med. 2021;10(7):7340–7350. doi:10.21037/apm-21-338
- Bratu S, Landman D, Haag R, et al. Rapid spread of carbapenem-resistant Klebsiella pneumoniae in New York city: a new threat to our antibiotic armamentarium. Arch Intern Med. 2005;165(12):1430–1435. doi:10.1001/archinte.165.12.1430
- Gasink LB, Edelstein PH, Lautenbach E, Synnestvedt M, Fishman NO. Risk factors and clinical impact of Klebsiella pneumoniae carbapenemase-producing k. Pneumoniae. Infect Control Hosp Epidemiol. 2009;30(12):1180–1185. doi:10.1086/648451
- Xu L, Sun X, Ma X. Systematic review and meta-analysis of mortality of patients infected with carbapenem-resistant Klebsiella pneumoniae. Ann Clin Microbiol Antimicrob. 2017;16(1):18. doi:10.1186/s12941-017-0191-3
- Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing: twenty-four informational supplement. CLSI document m100-s24; 2014.
- Kollef MH, Micek ST. Strategies to prevent antimicrobial resistance in the intensive care unit. Crit Care Med. 2005;33(8):1845–1853. doi:10.1097/01.ccm.0000171849.04952.79
- Jacobs DM, Safir MC, Huang D, Minhaj F, Parker A, Rao GG. Triple combination antibiotic therapy for carbapenemase-producing Klebsiella pneumoniae: a systematic review. Ann Clin Microbiol Antimicrob. 2017;16(1):76. doi:10.1186/s12941-017-0249-2
- Igbinosa O, Dogho P, Osadiaye N. Carbapenem-resistant Enterobacteriaceae: a retrospective review of treatment and outcomes in a long-term acute care hospital. Am J Infect Control. 2020;48(1):7–12. doi:10.1016/j.ajic.2019.07.006
- del Mar Tomas M, Cartelle M, Pertega S, et al. Hospital outbreak caused by a carbapenem-resistant strain of Acinetobacter baumannii: patient prognosis and risk-factors for colonisation and infection. Clin Microbiol Infect. 2005;11(7):540–546. doi:10.1111/j.1469-0691.2005.01184.x
- Pan H, Lou Y, Zeng L, et al. Infections caused by carbapenemase-producing Klebsiella pneumoniae: microbiological characteristics and risk factors. Microb Drug Resist. 2019;25(2):287–296. doi:10.1089/mdr.2018.0339
- Liu J, Wang H, Huang Z, et al. Risk factors and outcomes for carbapenem-resistant Klebsiella pneumoniae bacteremia in onco-hematological patients. J Infect Dev Ctries. 2019;13(5):357–364. doi:10.3855/jidc.11189
- Schwaber MJ, Klarfeld-Lidji S, Navon-Venezia S, Schwartz D, Leavitt A, Carmeli Y. Predictors of carbapenem-resistant Klebsiella pneumoniae acquisition among hospitalized adults and effect of acquisition on mortality. Antimicrob Agents Chemother. 2008;52(3):1028–1033. doi:10.1128/aac.01020-07
- Tian L, Tan R, Chen Y, et al. Epidemiology of Klebsiella pneumoniae bloodstream infections in a teaching hospital: factors related to the carbapenem resistance and patient mortality. Antimicrob Resist Infect Control. 2016;5:48. doi:10.1186/s13756-016-0145-0
- Giannella M, Trecarichi EM, De Rosa FG, et al. Risk factors for carbapenem-resistant Klebsiella pneumoniae bloodstream infection among rectal carriers: a prospective observational multicentre study. Clin Microbiol Infect. 2014;20(12):1357–1362. doi:10.1111/1469-0691.12747
- Gómez Rueda V, Zuleta Tobón JJ. Risk factors for infection with carbapenem-resistant Klebsiella pneumoniae: a case-case-control study. Colomb Med. 2014;45(2):54–60. doi:10.25100/cm.v45i2.1417
- Martin RM, Cao J, Brisse S, et al. Molecular epidemiology of colonizing and infecting isolates of Klebsiella pneumoniae. MSphere. 2016;1(5). doi:10.1128/mSphere.00261-16
- Gorrie CL, Mirceta M, Wick RR, et al. Gastrointestinal carriage is a major reservoir of Klebsiella pneumoniae infection in intensive care patients. Clin Infect Dis. 2017;65(2):208–215. doi:10.1093/cid/cix270
- Xiao T, Zhu Y, Zhang S, et al. A retrospective analysis of risk factors and outcomes of carbapenem-resistant Klebsiella pneumoniae bacteremia in nontransplant patients. J Infect Dis. 2020;221(Suppl 2):S174–s183. doi:10.1093/infdis/jiz559
- Satlin MJ, Calfee DP, Chen L, et al. Emergence of carbapenem-resistant Enterobacteriaceae as causes of bloodstream infections in patients with hematologic malignancies. Leuk Lymphoma. 2013;54(4):799–806. doi:10.3109/10428194.2012.723210
- Freire MP, Pierrotti LC, Filho HH, et al. Infection with Klebsiella pneumoniae carbapenemase (kpc)-producing Klebsiella pneumoniae in cancer patients. Eur J Clin Microbiol Infect Dis. 2015;34(2):277–286. doi:10.1007/s10096-014-2233-5
- Ramos-Castañeda JA, Ruano-Ravina A, Barbosa-Lorenzo R, et al. Mortality due to kpc carbapenemase-producing Klebsiella pneumoniae infections: systematic review and meta-analysis: mortality due to kpc Klebsiella pneumoniae infections. J Infect. 2018;76(5):438–448. doi:10.1016/j.jinf.2018.02.007
- Dai G, Xu Y, Kong H, Xie W, Wang H. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection and associated clinical outcomes. Am J Transl Res. 2021;13(6):7276–7281.
- Zhang F, Zhong J, Ding H, et al. Analysis of risk factors for carbapenem-resistant Klebsiella pneumoniae infection and its effect on the outcome of early infection after kidney transplantation. Front Cell Infect Microbiol. 2021;11:726282. doi:10.3389/fcimb.2021.726282