Abstract
The currently circulating SARS-CoV-2 Omicron variant posed a big challenge for the ongoing pandemic prevention and control activities. The critical concern is whether the current vaccines and therapeutics are capable of fully controlling this variant. Omicron has several mutations mainly concentrated in the receptor-binding domain (RBD) which is the main target for neutralizing antibodies and vaccine-elicited sera, and it is reportedly evading immunity. However, the degree to which the Omicron evades immunity and its impact on the prevention and control activities requires recent and continuous scrutiny. Despite several reports are available, updated and recent discussions are important to tackle the ongoing pandemic especially due to the emerging SARS-CoV-2 variants. Therefore, new insights on designing effective preventive and control measures is utmost important. This review discusses the extent of immune evasion by the Omicron variant and forwards important directions which could have valuable contributions to design alternative strategies in fighting against SARS-Co-2 variants.
Introduction
After the first report of the SARS-CoV-2 Wuhan reference strain (Wuhan-Hu-1), several spike protein mutations have emerged. Surprisingly, there have been several mutations reported in the RBD of the spike protein leading to different strains with heightened transmissibility, virulence, and immune escape.Citation1 These spike protein mutations are giving rise to different variants. Based on the World Health Organization (WHO) classification, the variants are named as variants of concern (VOC), variants of interest (VOI), and variants under monitoring (VUM). VOC are variants with increased transmissibility or increased virulence or increased resistance to public health measures currently including Alpha, Beta, Gamma, Delta, and Omicron. Whereas VOI is variants with genetic changes predicted or proved to affect virus nature like virulence, transmissibility, immune escape, diagnostics, or therapeutic escape and cause multiple COVID-19 clusters anywhere currently including Lambda and Mu. SARS-CoV-2 VUM is one where the genetic changes are suspected to alter the virus characteristics but epidemiological evidence is unclear. Current VUM includes Pango lineages AZ.5, C.1.2, B.1.617.1, B.1.526, B.1.525, B.1.630, and B.1.640.Citation2
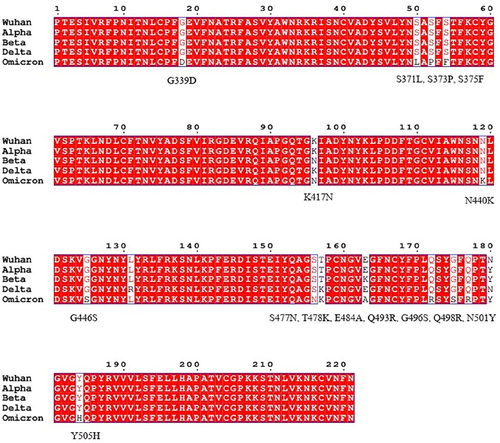
A new SARS-CoV-2 Omicron variant, currently circulating worldwide, was first identified in Botswana earlier in November and reported to WHO on November 24, 2021, from South AfricaCitation3–5 with potentially increased transmissibility, resistance to therapeutics and with heightened immune evasion.Citation6 The Omicron variant (B.1.1.529) is independently evolvedCitation7 and is highly mutated compared to the Wuhan reference strain, and other VOC (Alpha, Beta, Gamma, and Delta variants). The genome of SARS-CoV-2 Omicron variant harbors 18,261 mutations of which 97% of them are within the coding regionCitation8 resulting in subtypes.Citation9 Moreover, this variant is reported to include 36 mutations with 29 amino acid changes, six amino acid deletions, and a single amino acid insertionCitation10–12 in the spike protein. The Omicron variant (BA.1) has other subtypes; BA.1.1, BA.2 and BA.3. These subtypes share 11 amino acid substitutions in their RBD and BA.2 and BA.3 are predicted to have higher transmission potential.Citation13 The majority of the mutations are concentrated in the RBD (30 substitutions, 6 deletions, and 3 insertions)Citation14 which is the major target for neutralizing antibodies making the ongoing vaccination and immunotherapeutic efforts challenging. The Omicron RBD-ACE2 (angiotensin converting enzyme 2) complex shows multi-residue interactions due to the substituted amino acids (R493, S496, Y501, R498) which are not found in the ancestral RBD-ACE2 complexCitation15 and substitutions T478K, N501Y, Y505H, Q493R/K and Q498R are reported to enhance the binding energy to ACE2.Citation13,Citation16 This is suggestive of altered transmissibility, infectivity, and immune evasion of the Omicron variant. The Omicron RBD mutations compared to other VOC and the reference strain is described in .
Figure 1 SARS-CoV-2 Omicron RBD mutations compared to other variants. Multiple sequence alignment showing amino acid mutations on the RBD of Omicron compared to the Wuhan reference strain, alpha, beta and delta variants. SARS-CoV-2 spike protein sequences were obtained from the Global Initiative on Sharing All Influenza Data (GISAID) database (https://www.gisaid.org/) on December 22, 2021.Citation122 The 15 Omicron RBD amino acid mutations compared to the reference strain are indicated. The multiple sequence alignment was done by ESPript 3.x online server (https://espript.ibcp.fr/ESPript/cgi-bin/ESPript.cgi).Citation121
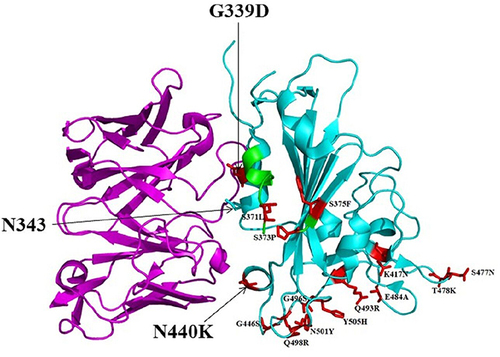
SARS-CoV-2 VOC demonstrated increased resistance to convalescent sera and monoclonal antibodies (mAbs)Citation17 and mutations located in the RBD are resistant to almost all neutralizing antibodies.Citation18–21 Additionally, emerging RBD mutations are reported to resist human leukocyte antigen (HLA-24)-restricted cellular immunity.Citation22,Citation23 Overall, the VOC exhibited comprehensive immune evasion requiring updated interventions and scrutiny.Citation24 Studies reported that the Omicron variant showed resistance to neutralization by convalescent sera, vaccine-induced antibody and mAbsCitation10,Citation12,Citation25–30 challenging the prevention and control activities. The interaction of Omicron RBD with a theoretically approved sarbecovirus mAB S309 Fab is illustrated in .
Figure 2 A cartoon representation of Omicron RBD (cyan) in complex with the therapeutically approved sarbecovirus mAb S309 Fab (magenta) (PDB entry: 7TN0). G339D and N440K mutations found near or within the S309 antigenic recognition site are indicated in red sticks. S309 recognizes N343 glycan indicating non-significant alteration of S309 binding to Omicron RBD. The Omicron RBD region comprising residues 366–375 is highlighted in green. This region is known to have deviated conformation compared to the Wuhan-Hu-1 RBD as it harbors S371L/S373P/S375F substitutions but without a significant effect on S309 neutralization.Citation98 The 15 Omicron RBD mutations compared to the Wuhan-Hu-1 reference strain are presented in red sticks.
Comprehensive and concrete data on the degree to which the Omicron variant is affecting vaccination and immunotherapy is hardly explored. Therefore, new insights and scrutiny on designing effective preventive and control measures is utmost important. Owing to several mutations in its genome, the diagnosis and management of the Omicron variant is also another problem to be solved. A study in Finland suggested underdiagnoses of COVID-19 after the emergence of Omicron due to mild infections and diminished polymerase chain reaction (PCR) sensitivity.Citation31 Therefore, identifying the most reliable diagnosis is utmost helpful for better prevention and control of the variant. Variant specific PCR and nanopore sequencing aids a sensitive and rapid identification of SARS-CoV-2 variants.Citation32
As PCR is time consuming and resource demanding, rapid serological tests could be more helpful especially in resource limited settings. In this regard, Abbott BinaxNow antigen test demonstrated good detection ability of the Omicron.Citation33 However, designing rapid test kits for the fast diagnosis of patients with Omicron infection is amongst the recommended insights for better prevention and control outcomes. Although it requires a thorough validation,Citation34 application of nanofiber swabs is ideal to reduce false-negative results and detect a very low concentration of viral RNA.Citation35 Besides, application of specific antibody biomarkers, the CRISPR/Cas system, segment selective DNA/RNACitation36 and biosensor diagnostic kits targeting the spike proteinCitation37 could provide more effective diagnosis. Novel prevention measures are also quite important. Interestingly, the application of antibacterial and antifungal high performance nanosystems is suggested to effectively halt the aerosol transmission of SARS-CoV-2 variants through neutralizing or eradicating the virus.Citation38 Generally, analyzing and summarizing recent evidences is helpful for the management of COVID-19 due to the Omicron variant. This review discusses the extent of immune evasion by the Omicron variant and forwards important directions which could have valuable contributions to design alternative strategies in fighting against SARS-Co-2 variants.
Immune Evasion by the Omicron Variant
Aggregation of more than 15 mutations in the RBDCitation39 of the Omicron spike alters its transmissibility, infectivity, neutralization escape, vaccine evasion, and reduced efficiency of diagnosis. Although more studies are yet to answer, data suggested that the Omicron variant has heightened vaccine escape and mAb evasion than the Delta variant.Citation40–42 RBD-induced immune evasion is possible in Omicron owing to several factors; RBD showed immune escape in other VOC,Citation43 humoral immunity is a key for viral defenseCitation44 and RBD is the main target of neutralizing antibodies.Citation24,Citation45,Citation46 The immune evasion of the Omicron variant compared to other VOC is summarized in .
Table 1 Summary of Immune Evasion by the Omicron Variant Compared to Other Variants
Monoclonal Antibodies
Many mAbs have been approved for the emergency treatment of COVID-19. For example, sotrovimab reduced the risk of disease progression among patients with mild-to-moderate COVID-19.Citation47 Omicron is evading therapeutic mAbs,Citation48 however, more studies are required to have a complete understanding. Predicting immune escape mutations plays paramount importance to design effective therapeutics. An antibody escape calculator study demonstrated that mutations at sites 417, 484, and 490 are peaks of antibody escape while other sites have redundant and additive effects.Citation49 There is still limited data on immune evasion of Omicron against mAbs yet requiring further shreds of evidence to understand it better. Therefore focusing on these sites in the VOC could be important to design effective vaccines and antibodies.
Computationally, compared to the wild and Delta variant, the Omicron variant exhibited low binding affinity to human mAbs CR3022, B38, CB6, and P2B2F6.Citation28 Omicron also resisted casirivimab and imdevimab mAbs in a study.Citation12 Mutations T478K, Q493R, Q498R and E484A produced a significantly reduced RBD binding with mAbs Etesevimab, Bamlanivimab, and CT-p59 while conserved epitope targeting mAbs AZD1061 and Sotrovimab demonstrated slight reduction of binding energies to Omicron RBD.Citation50 Besides, in vitro neutralization was lost against Omicron among most receptor binding motif (RBM) targeting mAbs. Intriguingly, mAbs like S2K146 (ACE2-mimicking) and sarbecovirus, and mAbs with broad epitopes outside the RBM (sotrovimab, S2X259, and S2H97) exhibited unaltered potency against Omicron.Citation51
A broadly neutralizing antibody ZCB11 neutralized all authentic VOC including the Omicron. However, antibodies like ZCB3, ZCC10 and ZCD3 showed variable potency against other VOC while they lost their neutralization activity against the Omicron.Citation52 RBD mutations S371L, N440K, G446S and Q493R are reported to confer high antibody resistance resulting in abolished or impaired antibody neutralization. Here, most tested mAbs failed to neutralize the Omicron with at least ten-fold reduction of their neutralization potency.Citation53 Omicron evades therapeutic mAbs while substantial resistance was also observed against antibodies elicited by booster vaccine dose. mAbs bamlanivimab, etesevimab, casirivimab, imdevimab and regdanvimab exhibited 11–242 fold binding reduction (at 0.1 µgml−1) to Omicron infected cells compared to Delta while sotrovimab demonstrated similar neutralization against both variants.Citation54 The electrostatic potential of mAbs etesevimab, bamlanivimab, and CT-p59 was significantly dropped mostly due to Omicron substitutions T478K, Q493K, Q498R and E484A indicating that the RBD mutations are playing significant roles in immune evasion.Citation16
Mutations affecting viral stability are associated with viral fusion efficiency, transmission, adaptability and immunogenicity.Citation55 Omicron has improved stability in the environment which resulted in enhanced transmissibility. In addition, Omicron mutations perturb the conformation of epitopes recognized by most antibodiesCitation56 making it highly resistant to antibody-mediated neutralization.Citation57 To overcome this, therapeutic options through combining mAbs is recommended, but screening the efficacy some m|Abs is important before administration.Citation58
Convalescent Sera and Vaccination
Previous infection and vaccination have an invaluable role in fighting against SARS-CoV-2 infection. However, convalescent sera from COVID-19 patients and sera from BNT162b2, mRNA-1273, and Ad26.COV2.S vaccinated individuals showed reduced binding to the Omicron RBD than the wild Wuhan, Beta, and Delta RBDs.Citation10 Moreover, a comparatively much lesser neutralization activity of sera from convalescent patients and ChAdOx1, Spikevax, or BNT162b2 vaccinated individuals was observed for the Omicron variant. Interestingly, sera from infected and vaccinated or vaccinated and infected individuals exhibited strong neutralization of the Omicron variantCitation27,Citation59,Citation60 due to “super immunity”. However, most vaccinated individuals showed non-detectable neutralization to Omicron. On the contrary, antibodies from mRNA vaccine (BNT162b and mRNA-1273) boosted individuals demonstrated potent Omicron neutralization. But substantial Omicron escape was observed in recently vaccinated individuals.Citation61 Thus mRNA based booster doses are better in resisting immune evasion by emerging variants.Citation62
Sera from BNT162b2 or Coronavac recipients also showed surprisingly low neutralization to the Omicron variant. While BNT162b2 recipients showed detectable neutralizing antibodies (but with low Omicron neutralization ability compared to the ancestral, Beta, and Delta strains), Coronavac recipients did not produce any detectable antibodies against this variantCitation25 entailing low vaccine effectiveness. The geometric mean neutralization antibody titers (GMT) of BNT162b2 recipients for Omicron was 35.7–39.9-fold lower than the reference strain (229.4) and significantly lower than Beta and Delta variants. Although mRNA vaccines demonstrated better antibody production, it is key to answer the molecular basis of immune evasion to update vaccine efficacy.
BNT162b2 vaccine-elicited sera also showed a severely reduced neutralization of the Omicron variant than the Delta one. Sera collected after six months of two doses of BNT162b2 vaccinated individuals exhibited a significantly reduced NT50 (microneutralization titers resulting in 50% virus neutralization) against the Omicron compared to the Delta variant. Similarly, the Omicron NT50 of sera from different doses of BNT162b2 vaccinated individuals (collected after two weeks and three months of vaccination) was severely reduced compared to the Delta one. A 10 times reduction of Omicron NT50 was also observed in sera collected from ChAdOx1 and BNT162b2 vaccinated individuals after six months.Citation12
Further, sera from cohorts of ChAdOx1 and BNT162b2 (two doses) vaccinated individuals showed substantial neutralization reduction against the Omicron compared to Beta and Delta variants.Citation14 Most convalescent patients and individuals vaccinated with a single dose of Ad26.COV2.S and Sputnik V or BBIBP-CorV showed no Omicron neutralization activity. On the contrary, mRNA-1273, BNT162b2, and AZD1222 vaccinated individuals retained neutralization against the Omicron, but with a significant fold reduction of pairwise neutralizing antibody titers (ID50) compared to the Wuhan-Hu-1 strain.Citation51 Besides, a decrease in the mean neutralization activity of sera from convalescent patients against Omicron was observed compared to the D614G reference strain. Similarly, Omicron resisted convalescent neutralization evidenced by a reduced ID50 valueCitation63 indicating enhanced immune escape by the Omicron. Similarly, GMTs against Omicron were below the lower detection limit in sera collected 14 days post two doses of BBIBP-CorV vaccinated individuals.Citation64 Although, vaccinated individuals exhibit a cross reactivity to all VOC, it is with a waning immunity after six months.Citation65,Citation66 Therefore, predicting the causes of and solutions to waning of immunity is also crucial.
Compared to the Wuhan-Hu-1 strain, plasma from two doses of mRNA vaccinated individuals showed a 30 to 180-fold reduced potency against the Omicron while the median deficit of neutralization activity in the convalescents was 30 to 60-fold. Compared to the D614G variant, Omicron exhibited a 22-fold escape from vaccine-elicited neutralization. Interestingly, those infected or received booster mRNA vaccine doses showed a 38 to 154-fold increase in neutralizing the Omicron.Citation59 Neutralization was also effective in infected and BNT162b2 vaccinated individuals from South Africa but extensive neutralization reduction was observed in vaccinated but not-infected individuals. Data predicted that previously infected and vaccinated individuals confer 73% prevention from symptomatic Omicron infection which is quite higher than a 35% protection in non-infected individuals.Citation67
Sera from mRNA-1273 vaccinated individuals showed comparatively a heightened neutralization reduction to Omicron variant compared to the D614G and Beta variants.Citation62 A significant Omicron neutralization reduction was also observed in sera from mRNA double vaccinated, boosted, convalescent and double vaccinated, and convalescent and boosted individuals where convalescent individuals had no measurable antibody titers for the Omicron. Convalescents with complete or booster doses retained better neutralization of the Omicron but still with a reduced neutralization potency.Citation68 Collectively, Omicron showed significant evasion of humoral immunity.Citation69
Cellular Immunity
Whether the Omicron variant has a capacity of evading cellular immunity is yet to be fully studied. So far, L452R (in B.1.427/429) and Y453F (in B.1.298) are reported to escape from the HLA-24-Citation22 and HLA-A24-restricted cellular immunity (CD8+ T cells),Citation70 and mutations in MHC-I-restricted epitopes including those in the spike protein evade in vitro CD8+ T cell responses.Citation71 L452R and Y453F substitutions and the presence of mutations in the T cell epitopes are not reported in the Omicron requiring future scrutiny. But the absence of the two substitutions (L452R and Y453F) does not guarantee unaltered cellular response to the Omicron.
A study reported a very small proportion of convalescents harbored a single amino acid substitution in the HLA-restricted epitopes of the Omicron spike suggesting the inability of Omicron to fully escape from CD8+ T cells.Citation72 According to a recent study on vaccinated individuals, compared to the Wuhan strain, T cell recognition of the Omicron spike protein was reduced by 47%Citation73 indicating possibility of cellular immune evasion. SARS-CoV-2 has been evolving continuously but without significant evasion of cellular immunity. This suggests that T cell responses from the previous infection and/or vaccination retain their potential of controlling the Omicron and other VOC.Citation72,Citation74 Current vaccines elicit highly cross reactive CD8+ T cell responses that robustly protect severe disease by the Omicron.Citation75 In addition, natural infection and mRNA vaccine elicited CD4+ T cell response is conserved across all VOCCitation76 indicating the potential of cellular immunity protecting from severe COVID-19.Citation77–81 However, a reduction of CD8+ T cell reactivityCitation82 and less consistency of CD8+ T cells than CD4+ T cellsCitation83 was observed suggesting evasion of HLA Omicron recognition.
Discussion and Perspectives
The Omicron variant is somehow weird mutant as compared to the previous VOC. Due to multiple mutations across its genome, its origin is producing ambiguity where some studies claimed evidence of mouse origin.Citation84 Here the authors suggested that the spike protein mutations of the Omicron variant are known to promote adaptation and affinity to mouse cells. These suggestions tell us the need to urgently design new vaccines, drugs, therapeutics, and prevention and control strategies to cope with the new wave of the pandemic. Thus, one health approach should be strengthened to control future zoonotic origins of the virus.
The Omicron disease cases are reported to be mildCitation85 with a lower risk of hospital admission,Citation86 however, unvaccinated individuals and risk groups may experience severe disease and death.Citation87 Thus, serious attention and public health measures need to be in place as a highly transmissible Omicron variant could overwhelm the health care systems. It is noted that this variant is at least 2–3 times more infectious than even the Delta one. Besides, there are still questions to be fully addressed such as diseases severity, the degree of cellular immune evasion, and efficacy of the current vaccines in controlling infection with the Omicron. Challenges are still there especially the presence of a large number of non-vaccinated and immunocompromised individuals, immature immune response in pediatric population and limited resource in developing countries can lead to the emergence of new variants.Citation5,Citation40,Citation88
In addition to the RBD, mutations outside the RBD could also strongly affect immunizations and treatment as the D614G has been proved to stabilize the spike trimerCitation89 resulting in enhanced RBD-ACE2 interactionCitation90 and transmissibility.Citation91 Most SARS-CoV-2 mutations occur in and around the RBD and the protease cleavage siteCitation1 where continuous mutation surveillance is important for targeted drug and vaccine design. L452R is absent in Omicron; however, induced L452R substitution increased omicron fusogenicity, infectivity and host glycolysisCitation92 entailing conscious consideration of possible mutations. Therefore, exclusively relying on the ancestral spike protein sequences for designing vaccines and/or immunotherapy is not recommended.Citation93 Studies reported reduced binding of Omicron RBD to ACE2.Citation10 For example, the K417N mutation is known to reduce the affinity of Omicron RBD to ACE2, but new salt bridges and hydrogen bonds in addition to the N501Y substitution (which increases the affinity) are assumed to compensate the ACE2 affinity reducing mutations.Citation29,Citation94–96 On the contrary, other studies reported enhanced affinity of Omicron to ACE2.Citation15,Citation50,Citation51,Citation97,Citation98 This indicates incompleteness of the available evidences about the host-pathogen interaction of the Omicron variant. Thus, it is recommended that treatments blocking the RBD-ACE2 interaction will no longer be the primary choices due to the continuous genetic variability;Citation97 however, non-ACE2 blocking mAbs demonstrated better neutralization against the Omicron.Citation99
Considering nanoscale particles is a promising approach in fighting infectious diseases. In this regard, nanobodies of neutralizing antibodies have been developed that reach distant targets with subsequent restriction of SARS-CoV-2 propagation. Likewise nano-enabled mRNA vaccines have comparatively the most effective protection ability through eliciting durable humoral and cellular immunity in addition to overcoming viral immune evasion.Citation100,Citation101 A combination of neutralizing antibodies (nAbs) with non-overlapping epitopes could also be effective against the VOC.Citation102 Besides, neutralizing Abs/vaccines accompanied with drugs inhibiting signaling pathways that orchestrate SARS-CoV-2 immunopathology could also improve treatment outcomes.Citation103 Further, a combination of cocktail mAbs with broad epitopes are reported to retain Omicron neutralization.Citation48,Citation53,Citation104,Citation105 But, broad epitope recognizing mAbs like sotrovimab are not recommended for hospitalized and intubated COVID-19 patients. Thus further efforts are necessary to address these individuals. However, considering conserved epitopes, targeting a broad array of epitopes beyond the RBM and applying additional booster doses should still be in practice.
Mutations on the Omicron RBD (K417N, E484a and Q493R) are reported to abolish mAb binding through electrostatic contact rearrangements and steric hindrance.Citation98 Thus, designing structurally suitable vaccines and/or antibodies targeting a broad array of conserved epitopes in the spike are important. Neutralizing mAbs demonstrated in vivo efficacy for the treatment and prevention of SARS-CoV-2.Citation106–109 In this regard, studies reported that broadly neutralizing mAbs overcome Omicron antigenic shift.Citation51 Moreover, even though the Omicron showed extensive immune evasion, its escape from mRNA-based vaccines is incomplete.Citation67
Promisingly, broadly neutralizing mAbs, sera from booster doses of mRNA-based vaccines, vaccinated and infected or infected and vaccinated individuals retained better neutralization efficacy against the Omicron variant making the prevention and control activities hopeful. Of note, third vaccination doses or three exposures to infection elicit superior neutralization that can cope with the emerging VOC.Citation51,Citation110,Citation111 With no doubt, booster mRNA vaccine doses are quite better than two doses.Citation112,Citation113 But mRNA vaccines induced SARS-CoV-2 neutralizing antibodies wane overtimeCitation114 unless boosted by breakthrough infection. Therefore, increasing mRNA vaccination coverage with additional booster doses is vital; though, whether the protection is consistent or transient is undetermined.Citation57 Moreover, elderly people are assumed to be protected from Omicron infection with a fourth antigenic exposure after a booster dose of mRNA vaccines.Citation115 Studies also recommended pre-exposure mAb prophylaxis especially in those unable to mount immune response.Citation54
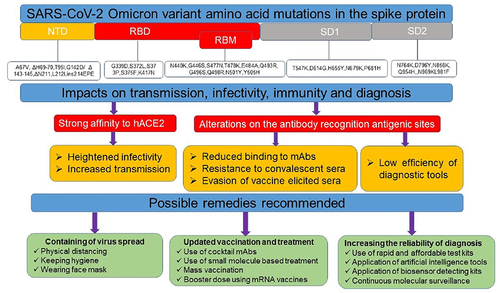
Alternatively, application of small molecule drugs is also a promising approach to treat viral infections. Accordingly, a study reported that the Omicron is highly sensitive to molnupiravir, nirmatrelvir and the combination.Citation116 But, the probability of reinfection with Omicron is reportedly high and thus, besides the use of potent vaccination and therapeutic approaches, considering state-of-The art diagnosing modalities, fostering the recommended prevention measures, mass testing, appropriate patient tracking, and applying interdisciplinary approaches is highly recommended for fast and reliable diagnosis, prevention, treatment and control of the current Omicron variant as well as the likely emerging new SARS-CoV-2 variants.Citation34,Citation117 The emergence of new variant is inevitable Therefore, developing effective diagnostic tools, vaccines and therapeutics, prevention and control measures are the only ways out of the pandemicCitation118,Citation119 ().
Figure 3 Schematic summary of the impacts of Omicron mutations on diagnosis, treatment and immune evasion. Several mutations especially on the spike protein of SARS-CoV-2 Omicron variant significantly affected the ongoing prevention and control strategies and thus multidisciplinary approaches are highly valuable. Amino acid substitutions were taken from Shah et al.Citation16
Concluding Remarks
Collectively, evidences showed that the Omicron variant substantially evades both vaccine elicited sera and therapeutic antibodies while data on cellular immunity are still limited. So far, studies used different study populations, different types of experimental virus, collect sera in different vaccination intervals, use different types of vaccines, and employed different experimental setups which make it hard to produce concrete conclusions. Cellular immunity is an important immunity wing to control viral infections with broad epitope recognition. Thus, more controlled studies are required to decipher whether the Omicron evades cellular immunity. Overall, additional longitudinal studies are recommended to bring about collective pieces of evidence for a better understanding of the impact of the Omicron variant on immune evasion, infectivity, diagnostics, and transmissibility.
The spread of the Omicron variant is still devastating. Although several approaches of treatment and vaccination modalities are employed, controlling the Omicron variant is yet medically unmet. Utilizing more affordable and reliable diagnosis, treatment and vaccination strategies is still a big assignment to the scientific world. Vaccination equity, employing the most effective vaccine and drug delivery approaches, boosting the awareness of the society towards vaccination and prevention, and continuous molecular surveillance to track emerging variants are highly recommended. Moreover, developing smart artificial intelligence systems and re-evaluating the available diagnostic, treatment and vaccine delivery approaches could overcome the current disease prevention and control roadblocks.Citation38 As prevention is considered better than cure, strengthening behavioral measures, continuous application of the recommended prevention and control measures, and practicing proper sanitation and hygiene are still crucial ways to effectively manage the COVID-19 pandemic.Citation34,Citation120
Abbreviations
ACE2, angiotensin converting enzyme; RBD, receptor binding domain; SARS-CoV-2, severe acute respiratory syndrome virus 2; nAbs, neutralizing antibodies; mAbs, monoclonal antibodies; VOC, variants of concern; VUM, variants under monitoring; VOI, variants of interest; WHO, World Health Organization; COVID-19, coronavirus disease 19.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest in relation to this work.
Acknowledgments
This study is supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDB29030104), the National Natural Science Fund (Grant No.: 31870731 and 31971129), the Fundamental Research Funds for the Central Universities, and the 100 Talents Program of the Chinese Academy of Sciences. HMM is supported by the University of Science and Technology of China scholarship program. KK is supported by CSC postdoctoral fellowship.
References
- Guruprasad L. Human SARS CoV-2 spike protein mutations. Proteins. 2021;89(5):569–576. doi:10.1002/prot.26042
- WHO. Tracking SARS-CoV-2 variants; 2021. Available from: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/. Accessed July 19, 2022.
- O’Toole Á, Scher E, Underwood A, et al. Assignment of epidemiological lineages in an emerging pandemic using the pangolin tool. Virus Evol. 2021;7(2):veab064. doi:10.1093/ve/veab064
- Classification of Omicron (B. 1.1. 529): SARS-CoV-2 variant of concern; 2021. Available from: https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern. Accessed July 19, 2022.
- Gao SJ, Guo H, Luo G. Omicron variant (B.1.1.529) of SARS-CoV-2, a global urgent public health alert! J Med Virol. 2022;94:1255–1256. doi:10.1002/jmv.27491
- COVID C, Team R. SARS-CoV-2 B. 1.1. 529 (Omicron) variant—United States, December 1–8, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(50):1731–1734. doi:10.15585/mmwr.mm7050e1
- Jung C, Kmiec D, Koepke L, et al. Omicron: what makes the latest SARS-CoV-2 variant of concern so concerning? J Virol. 2022;96(6):e02077–21. doi:10.1128/jvi.02077-21
- Bansal K, Kumar S. Mutational cascade of SARS-CoV-2 leading to evolution and emergence of omicron variant. Virus Res. 2022;315:198765. doi:10.1016/j.virusres.2022.198765
- Majumdar S, Sarkar R. Mutational and phylogenetic analyses of the two lineages of the Omicron variant. J Med Virol. 2022;94(5):1777–1779. doi:10.1002/jmv.27558
- Schubert M, Bertoglio F, Steinke S, et al. Human serum from SARS-CoV-2 vaccinated and COVID-19 patients shows reduced binding to the RBD of SARS-CoV-2 Omicron variant. BMC Med. 2022;20:102. doi:10.1186/s12916-022-02312-5
- Kumar S, Thambiraja TS, Karuppanan K, Subramaniam G. Omicron and delta variant of SARS‐CoV‐2: a comparative computational study of spike protein. J Med Virol. 2022;94:1641–1649. doi:10.1002/jmv.27526
- Wilhelm A, Widera M, Grikscheit K, et al. Reduced neutralization of SARS-CoV-2 Omicron variant by vaccine sera and monoclonal antibodies. medRxiv Preprint. 2021. doi:10.1101/2021120721267432
- Kumar S, Karuppanan K, Subramaniam G. Omicron (BA.1) and sub-variants (BA.1, BA.2 and BA.3) of SARS-CoV-2 spike infectivity and pathogenicity: a comparative sequence and structural-based computational assessment. bioRxiv. 2022. doi:10.1002/jmv.27927
- Dejnirattisai W, Huo J, Zhou D, et al. Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell. 2022;185(3):467–84.e15. doi:10.1016/j.cell.2021.12.046
- Lubin JH, Markosian C, Balamurugan D, et al. Structural models of SARS-CoV-2 Omicron variant in complex with ACE2 receptor or antibodies suggest altered binding interfaces. bioRxiv. 2021. doi:10.1101/2021.12.12.472313
- Shah M, Woo HG. Omicron: a heavily mutated SARS-CoV-2 variant exhibits stronger binding to ACE2 and potently escapes approved COVID-19 therapeutic antibodies. Front Immunol. 2022;12. doi:10.3389/fimmu.2021.830527
- Hu J, Peng P, Wang K, et al. Emerging SARS-CoV-2 variants reduce neutralization sensitivity to convalescent sera and monoclonal antibodies. Cell Mol Immunol. 2021;18(4):1061–1063. doi:10.1038/s41423-021-00648-1
- Cao Y, Su B, Guo X, et al. Potent neutralizing antibodies against SARS-CoV-2 identified by high-throughput single-cell sequencing of convalescent patients’ B cells. Cell. 2020;182(1):73–84. e16. doi:10.1016/j.cell.2020.05.025
- Ju B, Zhang Q, Ge J, et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020;584(7819):115–119. doi:10.1038/s41586-020-2380-z
- Lv Z, Deng Y-Q, Ye Q, et al. Structural basis for neutralization of SARS-CoV-2 and SARS-CoV by a potent therapeutic antibody. Science. 2020;369(6510):1505–1509. doi:10.1126/science.abc5881
- Shi R, Shan C, Duan X, et al. A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature. 2020;584(7819):120–124. doi:10.1038/s41586-020-2381-y
- Motozono C, Toyoda M, Zahradnik J, et al. An emerging SARS-CoV-2 mutant evading cellular immunity and increasing viral infectivity. bioRxiv. 2021. doi:10.1101/20210402438288
- Motozono C, Toyoda M, Zahradnik J, et al. An emerging SARS-CoV-2 mutant evading cellular immunity and increasing viral infectivity. Cell Host Microbe. 2021;29(7):1124–36.e11. doi:10.1016/j.chom.2021.06.006
- Mengist HM, Kombe Kombe AJ, Mekonnen D, Abebaw A, Getachew M, Jin T. Mutations of SARS-CoV-2 spike protein: implications on immune evasion and vaccine-induced immunity. Semin Immunol. 2021;55:101533. doi:10.1016/j.smim.2021.101533
- Lu L, Mok BW-Y, Chen L, et al. Neutralization of SARS-CoV-2 Omicron variant by sera from BNT162b2 or Coronavac vaccine recipients. Clin Infect Dis. 2021. doi:10.1093/cid/ciab1041
- Chen J, Wang R, Gilby NB, Wei G-W. Omicron (B.1.1.529): infectivity, vaccine breakthrough, and antibody resistance. J Chem Inf Model. 2022;62(2):412–422. doi:10.1021/acs.jcim.1c01451
- Rössler A, Riepler L, Bante D, Laer D, Kimpel J. SARS-CoV-2 B.1.1.529 variant (Omicron) evades neutralization by sera from vaccinated and convalescent individuals. medRxiv Preprint. 2021. doi:10.1101/2021120821267491
- Ranjan P, Neha DC, Devar KA, Das P. The influence of new SARS-CoV-2 variant Omicron (B.1.1.529) on vaccine efficacy, its correlation to Delta variants: a computational approach. bioRxiv Preprint. 2021. doi:10.1101/20211206471215
- Mannar D, Saville JW, Zhu X, et al. SARS-CoV-2 Omicron variant: antibody evasion and cryo-EM structure of spike protein–ACE2 complex. Science. 2022;375(6582):760–764. doi:10.1126/science.abn7760
- Tian D, Sun Y, Xu H, Ye Q. The emergence and epidemic characteristics of the highly mutated SARS-CoV-2 Omicron variant. J Med Virol. 2022;94(6):2376–2383. doi:10.1002/jmv.27643
- Ahava M, Jarva H, Jääskeläinen A, Lappalainen M, Vapalahti O, Kurkela S. Rapid increase in SARS-CoV-2 seroprevalence during the emergence of Omicron variant, Finland. medRxiv. 2022;41(6):997–999.
- Dächert C, Muenchhoff M, Graf A. Rapid and sensitive identification of omicron by variant-specific PCR and nanopore sequencing: paradigm for diagnostics of emerging SARS-CoV-2 variants. Med Microbiol Immunol. 2022;211(1):71–77. doi:10.1007/s00430-022-00728-7
- Regan J, Flynn JP, Choudhary MC, et al. Detection of the Omicron variant virus with the Abbott BinaxNow SARS-CoV-2 rapid antigen assay. Open Forum Infect Dis. 2022;9(3). doi:10.1093/ofid/ofac022
- Mostafavi E, Dubey AK, Teodori L, Ramakrishna S, Kaushik A. SARS-CoV-2 Omicron variant: a next phase of the COVID-19 pandemic and a call to arms for system sciences and precision medicine. MedComm. 2022;3(1):e119–e. doi:10.1002/mco2.119
- Rabiee N, Rabiee M, Sojdeh S, et al. Porphyrin molecules decorated on metal-organic frameworks for multi-functional biomedical applications. Biomolecules. 2021;11(11):1714. doi:10.3390/biom11111714
- Phan QA, Truong LB, Medina-Cruz D, Dincer C, Mostafavi E. CRISPR/Cas-powered nanobiosensors for diagnostics. Biosens Bioelectron. 2022;197:113732. doi:10.1016/j.bios.2021.113732
- Sharma PK, Kim E-S, Mishra S. Ultrasensitive and reusable graphene oxide-modified double-interdigitated capacitive (DIDC) sensing chip for detecting SARS-CoV-2. ACS Sensors. 2021;6(9):3468–3476. doi:10.1021/acssensors.1c01437
- Tiwari S, Juneja S, Ghosal A, et al. Antibacterial and antiviral high-performance nanosystems to mitigate new SARS-CoV-2 variants of concern. Curr Opin Biomed Eng. 2022;21:100363. doi:10.1016/j.cobme.2021.100363
- ECDPC. Implications of the spread of the SARS-CoV-2 B.1.1.529 variant of concern (Omicron) for the EU/EEA - first update. 2 December 2021. Stockholm: ECDC; 2021. Available from: https://www.ecdc.europa.eu/en/publications-data/covid-19-threat-assessment-spread-omicron-first-update. Accessed July 19, 2022.
- Chen J, Wang R, Gilby NB, Wei G-W. Omicron (B.1.1.529): infectivity, vaccine breakthrough, and antibody resistance. ArXiv. 2021;62:arXiv:2112.01318v1.
- Li M, Lou F, Fan H. SARS-CoV-2 variant Omicron: currently the most complete “escapee” from neutralization by antibodies and vaccines. Signal Transduct Target Ther. 2022;7(1):28. doi:10.1038/s41392-022-00880-9
- Hu J, Peng P, Cao X, et al. Increased immune escape of the new SARS-CoV-2 variant of concern Omicron. Cell Mol Immunol. 2022;19(2):293–295. doi:10.1038/s41423-021-00836-z
- Harvey WT, Jackson B, Jackson B, et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19(7):409–424. doi:10.1038/s41579-021-00573-0
- Ng KW, Wrobel AG, Gamblin SJ, Kassiotis G, Kassiotis G. Heterologous humoral immunity to human and zoonotic coronaviruses: aiming for the achilles heel. Semin Immunol. 2021;55:101507. doi:10.1016/j.smim.2021.101507
- Bošnjak BSS, Willenzon S, Willenzon S, et al. Low serum neutralizing anti-SARS-CoV-2 S antibody levels in mildly affected COVID-19 convalescent patients revealed by two different detection methods. Cell Mol Immunol. 2021;18(4):936–944. doi:10.1038/s41423-020-00573-9
- Zahradník JMS, Shemesh M, Shemesh M, et al. SARS-CoV-2 variant prediction and antiviral drug design are enabled by RBD in vitro evolution. Nat Microbiol. 2021;6(9):1188–1198. doi:10.1038/s41564-021-00954-4
- Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385(21):1941–1950. doi:10.1056/NEJMoa2107934
- VanBlargan LA, Errico JM, Halfmann PJ, et al. An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies. Nat Med. 2022;1–6. doi:10.1038/s41591-021-01678-y
- Greaney AJ, Starr TN, Bloom JD. An antibody-escape calculator for mutations to the SARS-CoV-2 receptor-binding domain. bioRxiv Preprint. 2021. doi:10.1101/20211204471236
- Woo MS, Woo HG. Omicron: a heavily mutated SARS-CoV-2 variant exhibits stronger binding to ACE2 and potently escape approved COVID-19 therapeutic antibodies. Front Immunol. 2022;12:830527.
- Cameroni E, Bowen JE, Rosen LE, et al. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. Nature. 2022;602:664–670. doi:10.1038/s41586-021-04386-2
- Zhou B, Zhou R, Chan JF-W, et al. An elite broadly neutralizing antibody protects SARS-CoV-2 Omicron variant challenge. bioRxiv Preprint. 2022. doi:10.1101/20220105475037
- Liu L, Iketani S, Guo Y, et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature. 2022;602(7898):676–681. doi:10.1038/s41586-021-04388-0
- Planas D, Saunders N, Maes P, et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2022;602(7898):671–675. doi:10.1038/s41586-021-04389-z
- Laha S, Chakraborty J, Das S, Manna SK, Biswas S, Chatterjee R. Characterizations of SARS-CoV-2 mutational profile, spike protein stability and viral transmission. Infect Genet Evol. 2020;85:104445. doi:10.1016/j.meegid.2020.104445
- Cui Z, Liu P, Wang N, et al. Structural and functional characterizations of infectivity and immune evasion of SARS-CoV-2 Omicron. Cell. 2022;185(5):860–71.e13. doi:10.1016/j.cell.2022.01.019
- Hoffmann M, Krüger N, Schulz S, et al. The Omicron variant is highly resistant against antibody-mediated neutralization: implications for control of the COVID-19 pandemic. Cell. 2022;185(3):447–56.e11. doi:10.1016/j.cell.2021.12.032
- Takashita E, Kinoshita N, Yamayoshi S, et al. Efficacy of antibodies and antiviral drugs against Covid-19 Omicron variant. N Engl J Med. 2022;386(10):995–998. doi:10.1056/NEJMc2119407
- Schmidt F, Muecksch F, Weisblum Y, et al. Plasma neutralization properties of the SARS-CoV-2 Omicron variant. medRxiv Preprint. 2021. doi:10.1101/2021121221267646
- Seidel A, Jahrsdörfer B, Körper S, et al. SARS-CoV-2 vaccination of convalescents boosts neutralization capacity against SARS-CoV-2 Delta and Omicron that can be predicted by anti-S antibody concentrations in serological assays. medRxiv Preprint. 2022. doi:10.1101/2022011722269201
- Garcia-Beltran WF, St. Denis KJ, Hoelzemer A, et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell. 2021;185(3):457–66.e4. doi:10.1016/j.cell.2021.12.033
- Doria-Rose NA, Shen X, Schmidt SD, et al. Booster of mRNA-1273 vaccine reduces SARS-CoV-2 Omicron escape from neutralizing antibodies. medRxiv Preprint. 2021. doi:10.1101/2021.12.15.21267805
- Zhang L, Li Q, Liang Z, et al. The significant immune escape of pseudotyped SARS-CoV-2 variant Omicron. Emerg Microbes Infect. 2022;11(1):1–5. doi:10.1080/22221751.2021.2017757
- Ai J, Zhang H, Zhang Y, et al. Omicron variant showed lower neutralizing sensitivity than other SARS-CoV-2 variants to immune sera elicited by vaccines after boost. Emerg Microbes Infect. 2021;11:477–481.
- Faustini S, Shields A, Banham G, et al. Cross reactivity of spike glycoprotein induced antibody against Delta and Omicron variants before and after third SARS-CoV-2 vaccine dose in healthy and immunocompromised individuals. J Infect. 2022;S0163–S4453(22):00002.
- Burns M, Bartsch Y, Boribong B, et al. Durability and cross-reactivity of SARS-CoV-2 mRNA vaccine in adolescent children. medRxiv Preprint. 2022. doi:10.1101/2022.01.05.22268617
- Cele S, Jackson L, Khoury DS, et al. SARS-CoV-2 Omicron has extensive but incomplete escape of Pfizer BNT162b2 elicited neutralization and requires ACE2 for infection. medRxiv Preprint. 2021. doi:10.1101/2021120821267417
- Carreño JM, Alshammary H, Tcheou J, et al. Activity of convalescent and vaccine serum against SARS-CoV-2 Omicron. Nature. 2022;602:682–688. doi:10.1038/s41586-022-04399-5
- Cao Y, Wang J, Jian F, et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature. 2022;602:657–663. doi:10.1038/s41586-021-04385-3
- Motozono C, Toyoda M, Zahradnik J, et al. SARS-CoV-2 spike L452R variant evades cellular immunity and increases infectivity. Cell Host Microbe. 2021;29(7):1124–36. e11.
- Agerer B, Koblischke M, Gudipati V, et al. SARS-CoV-2 mutations in MHC-I–restricted epitopes evade CD8+ T cell responses. Sci Immunol. 2021;6(57):eabg6461. doi:10.1126/sciimmunol.abg6461
- Redd AD, Nardin A, Kared H, et al. Minimal cross-over between mutations associated with Omicron variant of SARS-CoV-2 and CD8+ T cell epitopes identified in COVID-19 convalescent individuals. bioRxiv Preprint. 2021. doi:10.1101/20211206471446
- De Marco L, D’Orso S, Pirronello M, et al. Preserved T cell reactivity to the SARS-CoV-2 Omicron variant indicates continued protection in vaccinated individuals. bioRxiv Preprint. 2021. doi:10.1101/2021.12.30.474453:2021.12.30.474453
- Redd AD, Nardin A, Kared H, et al. CD8+ T cell responses in COVID-19 convalescent individuals target conserved epitopes from multiple prominent SARS-CoV-2 circulating variants. Open Forum Infect Dis. 2021;8(7):ofab143. doi:10.1093/ofid/ofab143
- Liu J, Chandrashekar A, Sellers D, et al. Vaccines elicit highly conserved cellular immunity to SARS-CoV-2 Omicron. Nature. 2022. doi:10.1038/s41586-022-04465-y
- Mazzoni A, Vanni A, Spinicci M, et al. SARS-CoV-2 spike-specific CD4+ T cell response is conserved against variants of concern, including omicron. Front Immunol. 2022;13:801431. doi:10.3389/fimmu.2022.801431
- Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529):eabf4063. doi:10.1126/science.abf4063
- Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184(4):861–880. doi:10.1016/j.cell.2021.01.007
- Goel RR, Painter MM, Apostolidis SA, et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science. 2021;374(6572):abm0829. doi:10.1126/science.abm0829
- McMahan K, Yu J, Mercado NB, et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021;590(7847):630–634. doi:10.1038/s41586-020-03041-6
- Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181(7):1489–501. e15. doi:10.1016/j.cell.2020.05.015
- Naranbhai V, Nathan A, Kaseke C, et al. T cell reactivity to the SARS-CoV-2 Omicron variant is preserved in most but not all individuals. Cell. 2022;185(6):1041–1051. doi:10.1016/j.cell.2022.01.029
- GeurtsvanKessel CH, Geers D, Schmitz KS, et al. Divergent SARS CoV-2 Omicron-reactive T- and B cell responses in COVID-19 vaccine recipients. Sci Immunol. 2022;7:eabo2202. doi:10.1126/sciimmunol.abo2202
- Wei C, Shan K-J, Wang W, Zhang S, Huan Q, Qian W. Evidence for a mouse origin of the SARS-CoV-2 Omicron variant. J Genet Genom. 2021;48(12):1111–1121. doi:10.1016/j.jgg.2021.12.003
- Diseases NIfC. Frequently asked questions for the B.1.1.529 mutated SARS-CoV-2 lineage in South Africa. Johannesburg, South Africa: National Institute for Communicable Diseases; 2021. Available from: https://www.nicd.ac.za/. Accessed December 3, 2021.
- Hussey H, Davies M-A, Heekes A, et al. Assessing the clinical severity of the Omicron variant in the Western Cape Province, South Africa, using the diagnostic PCR proxy marker of RdRp target delay to distinguish between Omicron and Delta infections – a survival analysis. Int J Infect Dis. 2022;118:150–154. doi:10.1016/j.ijid.2022.02.051
- Cele S, Jackson L, Khoury DS, et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature. 2022;602(7898):654–656. doi:10.1038/s41586-021-04387-1
- Chen -L-L, Chua GT, Lu L, et al. Omicron variant susceptibility to neutralizing antibodies induced in children by natural SARS-CoV-2 infection or COVID-19 vaccine. Emerg Microbes Infect. 2022;11(1):543–547. doi:10.1080/22221751.2022.2035195
- Zhang J, Cai Y, Xiao T, et al. Structural impact on SARS-CoV-2 spike protein by D614G substitution. Science. 2021;372(6541):525–530. doi:10.1126/science.abf2303
- Yurkovetskiy L, Wang X, Pascal KE, et al. Structural and functional analysis of the D614G SARS-CoV-2 spike protein variant. Cell. 2020;183(3):739–51.e8. doi:10.1016/j.cell.2020.09.032
- Hou YJ, Chiba S, Halfmann P. SARS-CoV-2 D614G variant exhibits efficient replication ex vivo and transmission in vivo. Science. 2020;370(6523):1464–1468. doi:10.1126/science.abe8499
- Zhang Y, Zhang T, Fang Y, Liu J, Ye Q, Ding L. SARS-CoV-2 spike L452R mutation increases Omicron variant fusogenicity and infectivity as well as host glycolysis. Signal Transduct Target Ther. 2022;7(1):76. doi:10.1038/s41392-022-00941-z
- Wang B, Goh YS, Prince T, et al. Resistance of SARS-CoV-2 variants to neutralization by convalescent plasma from early COVID-19 outbreak in Singapore. NPJ Vaccines. 2021;6(1):125. doi:10.1038/s41541-021-00389-2
- Zhu X, Mannar D, Srivastava SS, et al. Cryo-electron microscopy structures of the N501Y SARS-CoV-2 spike protein in complex with ACE2 and 2 potent neutralizing antibodies. PLoS Biol. 2021;19(4):e3001237. doi:10.1371/journal.pbio.3001237
- Mannar D, Saville JW, Zhu X, et al. Structural analysis of receptor binding domain mutations in SARS-CoV-2 variants of concern that modulate ACE2 and antibody binding. Cell Rep. 2021;37(12):110156. doi:10.1016/j.celrep.2021.110156
- Liu H, Zhang Q, Wei P, et al. The basis of a more contagious 501Y. V1 variant of SARS-COV-2. Cell Res. 2021;31(6):720–722. doi:10.1038/s41422-021-00496-8
- Saxena SK, Kumar S, Ansari S, et al. Characterization of the novel SARS‐CoV‐2 Omicron (B. 1.1. 529) variant of concern and its global perspective. J Med Virol. 2022;94(4):1738–1744. doi:10.1002/jmv.27524
- McCallum M, Czudnochowski N, Rosen LE, et al. Structural basis of SARS-CoV-2 Omicron immune evasion and receptor engagement. Science. 2022;375(6583):864–868. doi:10.1126/science.abn8652
- Duan X, Shi R, Liu P, et al. A non-ACE2-blocking neutralizing antibody against Omicron-included SARS-CoV-2 variants. Signal Transduct Target Ther. 2022;7:23. doi:10.1038/s41392-022-00879-2
- Khurana A, Allawadhi P, Khurana I, et al. Role of nanotechnology behind the success of mRNA vaccines for COVID-19. Nano Today. 2021;38:101142. doi:10.1016/j.nantod.2021.101142
- Nel AE, Miller JF. Nano-enabled COVID-19 vaccines: meeting the challenges of durable antibody plus cellular immunity and immune escape. ACS Nano. 2021;15(4):5793–5818. doi:10.1021/acsnano.1c01845
- Wang F, Li L, Dou Y, et al. Etesevimab in combination with JS026 neutralizing SARS-CoV-2 and its variants. Emerg Microbes Infect. 2022;11(1):548–551. doi:10.1080/22221751.2022.2032374
- Silva CM, Wanderley CWS, Veras FP, et al. Gasdermin-D activation by SARS-CoV-2 trigger NET and mediate COVID-19 immunopathology. medRxiv Preprint. 2022. doi:10.1101/2022012422269768
- Zhou H, Tada T, Dcosta BM, Landau NR. Neutralization of SARS-CoV-2 Omicron BA.2 by therapeutic monoclonal antibodies. bioRxiv Preprint. 2022. doi:10.1101/20220215480166
- Tada T, Zhou H, Dcosta BM, et al. Increased resistance of SARS-CoV-2 Omicron variant to neutralization by vaccine-elicited and therapeutic antibodies. bioRxiv Preprint. 2021. doi:10.1101/20211228474369
- Hansen J, Baum A, Pascal KE, et al. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science. 2020;369(6506):1010–1014. doi:10.1126/science.abd0827
- Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384(3):238–251. doi:10.1056/NEJMoa2035002
- Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325(7):632–644. doi:10.1001/jama.2021.0202
- Zost SJ, Gilchuk P, Case JB, et al. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature. 2020;584(7821):443–449. doi:10.1038/s41586-020-2548-6
- Lusvarghi S, Pollett SD, Neerukonda SN, et al. SARS-CoV-2 Omicron neutralization by therapeutic antibodies, convalescent sera, and post-mRNA vaccine booster. bioRxiv Preprint. 2021. doi:10.1101/20211222473880
- Wratil PR, Stern M, Priller A, et al. Three exposures to the spike protein of SARS-CoV-2 by either infection or vaccination elicit superior neutralizing immunity to all variants of concern. Nat Med. 2022;28(3):496–503. doi:10.1038/s41591-022-01715-4
- Muik A, Lui BG, Wallisch A-K, et al. Neutralization of SARS-CoV-2 Omicron by BNT162b2 mRNA vaccine–elicited human sera. Science. 2022;375(6581):678–680. doi:10.1126/science.abn7591
- Pérez-Then E, Lucas C, Monteiro VS, et al. Neutralizing antibodies against the SARS-CoV-2 Delta and Omicron variants following heterologous CoronaVac plus BNT162b2 booster vaccination. Nat Med. 2022;1. doi:10.1038/s41591-022-01705-6
- Evans JP, Zeng C, Carlin C, et al. Neutralizing antibody responses elicited by SARS-CoV-2 mRNA vaccination wane over time and are boosted by breakthrough infection. Sci Transl Med. 2022;14:1–13.
- Bruel T, Pinaud L, Tondeur L, et al. SARS-CoV-2 Omicron neutralization and risk of infection among elderly after a booster dose of Pfizer vaccine. medRxiv. 2022. doi:10.1101/2022.03.30.22273175
- Li P, Wang Y, Lavrijsen M, et al. SARS-CoV-2 Omicron variant is highly sensitive to molnupiravir, nirmatrelvir, and the combination. Cell Res. 2022;32(3):322–324. doi:10.1038/s41422-022-00618-w
- Brakenhoff TB, Franks B, Goodale BM, et al. A prospective, randomized, single-blinded, crossover trial to investigate the effect of a wearable device in addition to a daily symptom diary for the remote early detection of SARS-CoV-2 infections (COVID-RED): a structured summary of a study protocol for a randomized controlled trial. Trials. 2021;22(1):412. doi:10.1186/s13063-021-05241-5
- Ao D, Lan T, He X, et al. SARS-CoV-2 Omicron variant: immune escape and vaccine development. MedComm. 2022;3(1):e126. doi:10.1002/mco2.126
- Hirabara SM, Serdan TDA, Gorjao R, et al. SARS-COV-2 variants: differences and potential of immune evasion. Front Cell Infect Microbiol. 2022;11. doi:10.3389/fcimb.2021.781429
- Okpeku M. Possibility of COVID-19 eradication with evolution of a new omicron variant. Infect Dis Poverty. 2022;11(1):30. doi:10.1186/s40249-022-00951-7
- Robert X, Gouet P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014;42(W1):W320–W4. doi:10.1093/nar/gku316
- Global Initiative on Sharing All Influenza Data (GISAID) database; 2021. Available from: https://www.gisaid.org/. Accessed December 22, 2021.
