Abstract
Background
Nontuberculous mycobacteria (NTM) and their associated diseases remain neglected. Through minor modifications in our diagnostic algorithm, we observed an unexpected higher number of cultivable NTM isolates. Therefore, a retrospective study was performed thoroughly to investigate the effect of changed laboratory procedures on NTM isolation in a specialized tuberculosis hospital.
Methods
NTM isolation rates and composition of NTM species were compared for the two diagnostic algorithms: (1) by using traditional p-nitrobenzoic acid (PNB) selective medium as a preliminary test to identify NTM isolates among the positive cultures (procedure I) and (2) by using the MPT64 antigen detection method to distinguish between Mycobacterium tuberculosis complex (MTBC) isolates and possible NTM isolates after a positive MGIT960 liquid culture (procedure II).
Results
The NTM isolation rate in procedure II was significantly higher than the procedure I (18.08% vs 9.71%; P<0.001). A noticeable increase in the ratio of NTM isolates among the identified mycobacteria was observed over the studied years (ie, from 58.18% in 2019 to 72.93% in 2021), which indicated a more precise prescription of species identification test after prompt information was provided in procedure II. In addition, the consistency of the identified species using multiple specimens from the same patient did not present a significant difference between the procedures.
Conclusion
According to our study, NTM infection might be far more underestimated than it is. A diagnostic procedure combining MGIT960 culture and MPT64 antigen detection could timely and easily identify clues of NTM isolates and improve the diagnosis of NTM infections.
Introduction
Nontuberculous mycobacteria (NTM) are ubiquitous in environments,Citation1 and more than 200 NTM species or subspecies have been reported.Citation2 Although the majority of the NTM species are non-pathogenic, ~60 different NTM species have been reported to be isolated from the clinical specimen and demonstrate pathogenic potential.Citation3
In recent years, a better understanding of NTM and their related diseases, the availability of new molecular diagnostics, an increased life span of the global population, and increase in the number of immunocompromised conditions have all contributed to an increasing trend in NTM infection.Citation4–7 In many developed countries, the incidences of NTM diseases have surpassed tuberculosis (TB).Citation8–10 Like TB, NTM also most frequently affects the lungs. NTM infections could be readily misdiagnosed as TB since they present similar clinical symptoms and produce almost the same outcomes in pathological and radiological examination.Citation11–13 It has been reported that approximately 30% of the patients with suspected drug-resistant TB may be NTM infections,Citation14 which indicates that the actual NTM infection rates could be much higher than reported.
In China, before 2010, traditional Lowenstein–Jensen (L-J) solid medium culture was the dominant method for mycobacteria recovery. Meanwhile, the p-nitrobenzoic acid (PNB) containing differential medium was the only available method for identifying possible NTM isolates in a clinical laboratory. Generally, the Mycobacterium tuberculosis complex (MTBC) cannot grow on a medium containing PNB, while most NTM species can tolerate this reagent and develop robust colonies. Since 2010, BACTEC MGIT960 liquid culture has gained popularity across the country. Furthermore, in recent years, another preliminary NTM identification technique, ie, MPT64 antigen assay, has been applied in hospitals for verifying that the acid-fast bacilli (AFB) cultivated by MGIT960 culture belongs to the MTBC.
Beijing Chest Hospital is a TB-designated hospital. Before July 2020, both solid medium culture and MGIT960 liquid culture were performed. Drug susceptibility testing (DST) was performed for isolates recovered by both methods when the doctor prescribed this test. A PNB differential growth test was included in the DST panel (procedure I). In July 2020, the routine laboratory diagnostic algorithm switched to the MGIT960 culture method followed by MPT64 antigen detection for any culture-positive specimen (procedure II). For both procedures, multiple target genes are amplified and sequenced to characterize species and subspecies whenever the doctors prescribe the test. This study was intended to compare the NTM isolation rate and composition of the species in these two workflows and to explore the impact of changing laboratory algorithms on the diagnosis of pulmonary NTM diseases.
Materials and Methods
Ethics Statement
The protocols of this study were approved by the Ethics Committee of the Beijing Chest Hospital, Capital Medical University. The study was conducted in accordance with the Declaration of Helsinki. As patient records were anonymous prior to analysis, informed consent from the study patients was not obtained.
Data Collection
This study was conducted in the National Tuberculosis Clinical Laboratory at Beijing Chest Hospital in China. Two time periods, ie, before and after workflow change, were chosen for the comparison. Between January 2019 and May 2019, 6142 respiratory specimens, including 4664 sputum and 1478 bronchoalveolar lavage fluid (BALF) samples, were collected from the suspected pulmonary TB (PTB) patients, which were all processed for the mycobacteria culture by L-J slant and/or MGIT960. The PNB preliminary identification test (procedure I) was performed once this test was ordered. Between January 2021 and May 2021, 9815 specimens from the respiratory tract (including 7929 sputum and 1886 BALF samples) were collected from suspected PTB for culture by MGIT960, and MTP64 antigen was tested for every culture-positive vial (procedure II). The detailed data in 2020 was not analyzed because the COVID-19 pandemic radically decreased the patient number; therefore, we chose the two time periods with routine working loads to compare. However, the NTM isolation rate for 2020 was calculated to compare with other years.
PNB Differential Growth Testing
The PNB test is a part of a plate assay of a DST kit (MicroDSTTM, Zhuhai Yinke LIT., China). The DST assay was performed according to the kit’s instructions.Citation15 The strain which could grow vigorously in the medium containing 400 µg/mL PNB was reported as “NTM isolates”, otherwise as “MTBC isolates”.
MGIT960 and MPT64 Antigen Assay
The specimen was inoculated into MGIT vials and incubated at 37°C in the MGIT960 instrument (Becton, Dickinson and Company, United States). For vial that reported positive, AFB was observed by microscopic examination, and the culture supernatant was tested by an MPT64 antigen detection kit (Genesis Corporation, Hangzhou, China). The vial with AFB positive and MTP64 antigen-positive outcome would be reported as “MTBC-positive”, while with AFB positive but MTP64 antigen-negative outcome would be reported as “AFB-positive”.
Species/Subspecies Identification
Crude genomic DNA of each isolate was extracted and used as the template for subsequent PCR reactions. 16s rRNA coding gene, rpoB, and hsp65 were amplified using the following primers: 16S rRNA, 27f (AGAGTTTGATCCTGGCTCAG) and 907r (CCCCGTCAATTCATTTGAGTTT); rpoB, MF(CGACCACTTCGGCAACCG) and MR (AGCGGCTGCTGGGTGATCATC); hsp65, HSPF-P1 (ATCGCCAAGGAGATCGAGCT) and HSPF-P2 (AAGGTGCCGCGGATCTTGTT). The PCR protocols were performed as previously described,Citation16 and the products were sequenced by RuiBiotech (Beijing, China). The nucleotide sequences were compared with GenBank reference sequences (National Center for Biotechnology Information, NCBI).
Statistical Analysis
All the data were analyzed using SPSS v. 21.0. A Chi-square test was performed to compare the rates of categorical variables between groups (Fisher’s exact test when the expected number was less than five). The difference was considered statistically significant for P<0.05.
Results
NTM Isolation in Procedure I and II
In procedure I, a total of 1619 clinical strains were isolated from 1005 cases. Among these isolates, 1103 (68.13%) were prescribed DST, including 1000 PNB-negative (reported as “MTBC isolate”) and 103 PNB-positive (reported as “NTM isolate”) (). Eighty-seven PNB-positive isolates were prescribed species identification, and the remaining 16 isolates were identified by us for research purposes. Among them, 96 specimens were identified as NTM, whereas 7 specimens were identified as other infections. Therefore, the NTM isolation rate by isolate in this study period was 8.7% (96/1103).
Figure 1 Flowcharts of the two applied diagnostic procedures. (A) Procedure (I) L-J slant/MGIT960 with the PNB differential medium test; (B) Procedure II: MGIT960 with the MPT64 antigen test.
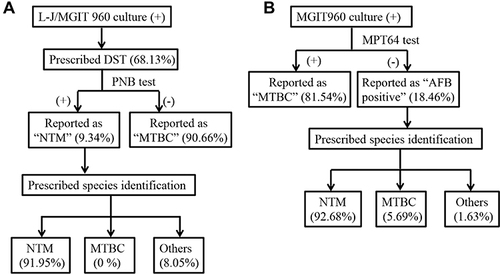
In procedure II, 1258 cases with 2161 specimens were positive for MGIT960 culture. Among these, 487 patients were tested with two or more specimens. MPT64 antigen detection was performed for all the culture-positive isolates. As shown in , among the 2161 culture-positive specimens, 1762 (81.54%) had MPT64 antigen-positive results (reported as “MTBC”), while the remaining 399 (18.46%) isolates were MPT64 antigen-negative (reported as “AFB-positive”). Among the 399 AFB-positive isolates, 246 isolates were prescribed species identification, and 228 specimens were identified as NTM. Hence, the NTM isolation rate by isolate in this study period was 17.11% [399*(228/246)/2161].
The total cases studied with the culture-positive results in each procedure are shown in . In procedure I, 690 of the 1005 culture-positive cases performed DST, and 72 of them were reported as “NTM isolate”. Then, 62 were prescribed species identification, whereas another ten species identification was performed by us. Among these, 67 were identified as NTM, and five were identified as other bacterial infections. The case’s actual isolation rate of NTM was 9.71% (67/690). Only two cases were identified to have discordant outcomes from different specimens in procedure I. Specifically, in one patient, the first isolate was identified as Nocardia vermiculate, and the second isolate was identified as MTBC. Another patient had three positive cultures, two of which were identified as M. parascrofulaceum and one as M. intracellulare.
Table 1 NTM Isolations in the Two Different Procedures
In procedure II, there were 1014 cases with MPT64 antigen-positive results (reported as “MTBC”), whereas 244 cases were MPT64 antigen-negative but AFB-positive (reported as “AFB-positive”). Among the 244 AFB-positive cases, 162 cases were further analyzed by sequencing analysis, which identified 151 cases as NTM, eight as MTBC, and three as bacteria other than mycobacteria. Cases identified as NTM infections accounted for 93.21% (151/162) of AFB-positive cases. Therefore, the corrected isolation rate of NTM by the case in procedure II was 18.08% [(244*93.21%)/1258]. The NTM isolation rate in procedure II was significantly higher than in procedure I (18.08% vs 9.71%; P<0.001). Twenty cases yielded both “MTBC-positive” and “AFB-positive” outcomes with different specimens, whereas for 10 of them, no further species identification was performed. Among the 10 cases (from the “AFB-positive” isolates) with known species outcomes, 6 cases were identified as NTM, whereas the other 4 were MTBC. Furthermore, there were another four cases with two or more AFB-positive culture results, but their species identification results were inconsistent.
The Species/Subspecies Composition of NTM Isolates in Procedure I and II
In procedure I, 72 PNB-positive cases with 103 isolates were further identified into species/subspecies levels. Among these, 67 cases with 96 isolates were identified as NTM species, and 5 cases with seven isolates were reported as other AFB bacterial infections. A total of 10 different species/subspecies were identified in 67 cases (). The four most prevalent clinical NTM species were M. intracellulare (40, 59.7%), M. abscessus (12, 17.91%), M. kansasii (6, 8.96%), M. avium (3, 4.48%), and other uncommon NTM species (6) ().
Figure 2 NTM species/subspecies identified in the two applied procedures. (A) Species compositions in Procedure I; (B) Species compositions in Procedure II.
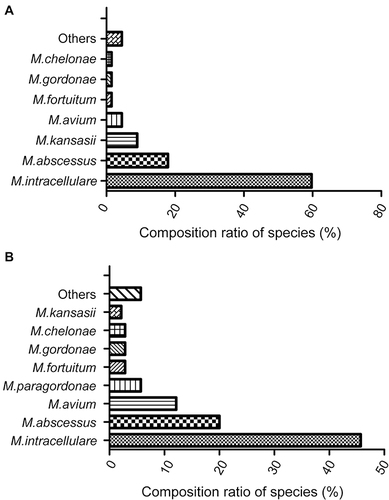
In procedure II, 140 NTM cases were analyzed into species/subspecies levels. From these, 16 clinical NTM species/subspecies were identified (). The five most prevalent species in terms of the number were M. intracellulare (64, 45.71%), M. abscessus (28, 20%), M. avium (17, 12.14%), M. paragordonae (8, 5.71%), M. fortuitum (4, 2.86%), M. gordonae (4, 2.86%), and M. chelonae (4, 2.86%), M. kansasii (3), and other uncommon NTM species (8). In both procedures, the top two most frequently isolated species were M. intracellulare and M. abscessus, but procedure I had a relatively higher percentage of M. intracellulare than procedure II (59.7% vs 45.71%; P=0.06).
NTM Identified in BALF
A total of three NTM cases were identified from BALF in procedure I, including M. intracellulare (1), M. abscessus (1), and M. kansasii (1). A total of 17 NTM cases were identified from BALF in procedure II, including M. intracellulare (7), M. avium (6), M. abscessus (3), and M. kansasii (1). The NTM species identified in BALF were less than sputum (3 vs 10 and 4 vs 16 in the procedure I and II, respectively). Meanwhile, the NTM species identified in BALF were mainly the top four or five species isolated in each process.
The Consistency of NTM Identification in Each Procedure
In procedure I, 68 cases underwent NTM identification, among which 19 cases were confirmed as NTM by sputum at least twice, whereas only one case showed inconsistent identification outcomes between tests. Thus, the consistency of NTM identification in the procedure I was 94.74% (18/19). In contrast, in procedure II, 144 patients were identified with NTM infection, among which 39 cases had consistent identification results, whereas 4 cases had shown inconsistent outcomes on multiple tests. Therefore, the consistency of NTM identification was 90.7% (39/43) with procedure II. However, no significant difference between the procedures was observed in terms of their consistency (P>0.05).
NTM Identification Ratio During the Three Studied Years
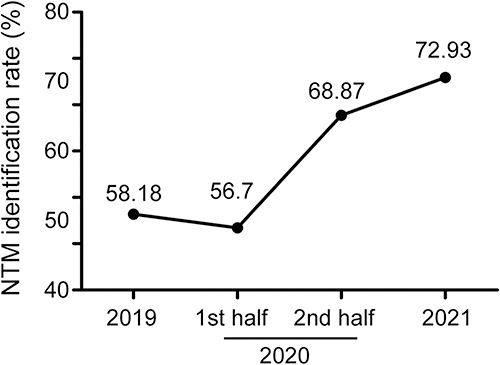
To understand the association of changes in diagnostic algorithm with NTM isolation, a statistical analysis was performed using the results of all isolates prescribed to undergo species identification between 2019 and 2021 in our hospital. As shown in , the proportion of NTM isolates among the identified mycobacteria increased over the studied time, ie, from 58.18% (498/856) in 2019 to 72.93% (617/846) in 2021. Notably, in 2020, the ratio of NTM increased radically in the second half of the year compared with the first half [68.87% (261/379) vs 56.7% (110/194)] among the isolates prescribed the species identification test.
Discussion
The prevalence of NTM is continuously increasing globally, which requires and attracts more and more attention.Citation17,Citation18 In addition to compromised immune response, the primary reason for increased case findings is the improved technology used for species identification and increased clinical attention to NTM.Citation4–6,Citation13,Citation17 Previous studies have reported that NTM isolation rates were highly variable in different regions of China, with high prevalence in southern China and low prevalence in the north.Citation19,Citation20 Results from a meta-analysis have shown that the overall isolation rate of NTM among Mycobacterial isolates in China was approximately 11.57%,Citation21 while in the region of Beijing, which is located in north China, was around 5%. Wang et al analyzed the prevalence of NTM during 2008–2011 using the clinical data from our hospital and found that only 2.6% of all mycobacterial isolates belonged to NTM.Citation22 We also routinely analyzed the NTM isolation rate in our hospital each year after 2014, such as procedure I, which remained at a similar isolation rate of 5–10% for many years (unpublished data). However, after changing the diagnostic algorithms in July 2020, an unexpectedly high isolation rate (more than 15%) was obtained, so we paid attention to the issues reflected in this study. Therefore, we retrospectively analyzed the NTM identification data in our hospital before and after the change of testing procedures to shed light on the realistic NTM infection and raise awareness of the related disease that remained ignored in the past. Our results showed that the NTM isolation rate in the new procedure, which combined MGIT960 culture with MPT64 antigen detection, became almost double than the old procedure, ie, PNB differential medium which was conventionally used as the major screening method for NTM isolation (18.08% vs 9.71%; P<0.001). Furthermore, the newly applied procedure identified more NTM species than the old procedure (16 vs 10).
This study’s analysis is conducted based on real-world data in a clinical laboratory. It is not very meaningful to compare the turnaround time of the two procedures directly because whether and when the strain identification test is carried out depends on whether and when the patient comes to see a doctor after receiving the positive culture report. Therefore, we roughly calculated the total time needed for the strain identification with the actual inspection time of each procedure. In procedure I, for most of the slow-growing mycobacteria, it took about 3–4 weeks to acquire positive culture results by solid culture or 1–2 weeks by liquid culture. The DST (including the PNB growth test) took nearly another 2 weeks using the plate assay method. The species identification test was generally performed within a week after the prescription from the doctor was obtained. Hence, from specimen collection to species identification, nearly 2 months were needed. For rapid-growing species, the total time could be 2–3 weeks less. In contrast, the currently used procedure can report MTBC-positive or AFB-positive about 1–2 weeks after specimen collection. After that, species identification can be carried out in another week. Hence, the total time needed was ~2 weeks and ~3 weeks for rapid or slow-growing species. Notably, the new procedure significantly shortens the turnaround time and reduces the aimless prescription of the NTM identification test. In the old procedure, doctors often prescribed a species identification simultaneously with a mycobacterial culture, which was not mainly based on a suspected NTM infection, but rather because of the extended time required by the procedure and non-compliance of the patient afterward. Therefore, it was reasonable to observe a lower NTM ratio in the isolates prescribed species identification test in procedure I than in procedure II (58.18% in 2019 and 72.93% in 2021). The plausible reason for this increase was that with a quicker clue of NTM in procedure II, the specific species identification test would be ordered more precisely by the doctors. Timely identification of NTM species is essential to initiate appropriate treatment.Citation23,Citation24
It was not surprising to have four strains with “AFB-positive” outcomes that were further clarified as MTBC after species identification. As described in kinds of literature, the main reasons that cause these misinterpreted outcomes of the MPT64 antigen test include low titer of antigen at an early stage when the vial yielded a culture-positive signal, plus amutation in the MPT64 antigen encoding gene.Citation25,Citation26 Another limitation of the MPT64 antigen test is its inability to characterize the TB-NTM co-existence. If the antigen was detected, MTBC is suggested, but there is no other clue to preclude the co-existence of NTM.
The NTM species composition in the two procedures demonstrated some differences. Although the two most prevalent species in both procedures were M. intracellulare and M. abscessus, procedure I had a noticeable higher percentage of M. intracellulare than procedure II (59.7% vs 45.71%), but the difference was not significant. Additionally, while procedure I had a higher chance of identifying M. kansassii than procedure II, M. avium had an opposite tendency between the two procedures. The reasons which caused these differences remain unknown. The PNB growth test, as well as the MPT64 antigen test, identified some non-mycobacterial AFB-positive species, such as Gordonia bronchialis and Tsukamurella tyrosinosolvens. Both of these bacteria are opportunistic pathogens, and the diseases caused by them need to be differentiated from TB in clinical practice. Therefore, it is not always trustable to judge AFB-positive isolates without MPT64 antigen as NTM. Thus, subsequent species identification would be helpful to avoid this low possibility of misdiagnosis.
According to the criteria of the Official American Thoracic Society/Infectious Disease Society of America for NTM diagnosis,Citation23,Citation24 NTM can only be microbiologically diagnosed when either positive culture results from at least two separate sputum specimens or one BALF are obtained. Therefore, we compared the consistency of species identification outcomes between the two procedures and observed similar and high consistency rates (94.74% vs 90.7%; P>0.05). To some extent, these outcomes suggest that the clinical significance of the two procedures are comparable, which gives credit to procedure II in case finding. However, both procedures yielded some infrequently isolated NTM species, which are believed to have low clinical significance. Doctors should always be alert to the possibility of misleading information even when the species identification outcomes fit the recommended diagnosis criteria. In contrast to sputum, the outcomes of BALF specimens seemed to have better clinical significance, as the species identified were mainly the reported pathogenic species.
Although the methods used in the present study are not new, and the MPT64 antigen test has been performed in our laboratory for many years, the comparison of these two diagnostic algorithms has never been reported. At the beginning of using this MPT64 antigen test, it was performed when the clinicians ordered. Hence, we had not found any evident consequence of using this technique. Then, our routine diagnostic algorithm was changed due to some administrative requirement reason, and surprisingly, an obviously increasing in NTN isolation was observed. We realized that not because of the MPT64 antigen test itself, but a necessity MPT64 antigen test after a positive culture by MGIT960 acquired account for this increase. As a specialty lab on TB diagnosis, this finding really surprised us. We also believed that the present study’s findings would provide a valuable reference to adapt or refine the lab technologies for other diagnostic laboratories, especially in some developed countries or resource-limited areas. Our study has some limitations. Since the diagnosis of NTM pulmonary disease is difficult and time-consuming, many participants might not have a precise diagnosis during their visits. Therefore, we did not investigate the final diagnosis of the participants. Additionally, this is a real-world study based on laboratory data; thus, we can only analyze the data of patients who were prescribed the tests. Bias might exist.
Conclusion
NTM infection might be far more underestimated than it is. A diagnostic procedure combining MGIT960 culture and MPT64 antigen detection could timely and easily identify the NTM isolates and improve the diagnosis of NTM infections. Based on the actual data, the present study will offer a rapid, economic, and applicable diagnostic algorithm to identify NTM from TB in clinical practice.
Abbreviations
NTM, nontuberculous mycobacteria; MTBC, Mycobacterium tuberculosis complex; PNB, p-nitrobenzoic acid; TB, tuberculosis; L-J, Lowenstein–Jensen; AFB, acid-fast bacilli; DST, drug susceptibility testing; BALF, bronchoalveolar lavage fluid; PTB, pulmonary TB.
Role of Sponsors
The sponsor had no role in the design of the study, the collection, and analysis of the data, or the preparation of the manuscript.
Disclosure
The authors declare no conflict of interest.
Additional information
Funding
References
- Johansen MD, Herrmann JL, Kremer L. Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat Rev Microbiol. 2020;18(7):392–407. doi:10.1038/s41579-020-0331-1
- Tortoli E, Fedrizzi T, Meehan CJ, et al. The new phylogeny of the genus Mycobacterium: the old and the news. Infect Genet Evol. 2017;56:19–25. doi:10.1016/j.meegid.2017.10.013
- van der Werf MJ, Kodmon C, Katalinic-Jankovic V, et al. Inventory study of non-tuberculous mycobacteria in the European Union. BMC Infect Dis. 2014:14. doi:10.1186/1471-2334-14-14
- Casanova JL, Abel L. Genetic dissection of immunity to mycobacteria: the human model. Annu Rev Immunol. 2002;20:581–620. doi:10.1146/annurev.immunol.20.081501.125851
- Daley CL. Nontuberculous mycobacterial disease in transplant recipients: early diagnosis and treatment. Curr Opin Organ Transplant. 2009;14(6):619–624. doi:10.1097/MOT.0b013e3283327cd6
- Henkle E, Winthrop KL. Nontuberculous mycobacteria infections in immunosuppressed hosts. Clin Chest Med. 2015;36(1):91–99. doi:10.1016/j.ccm.2014.11.002
- Wu UI, Holland SM. Host susceptibility to non-tuberculous mycobacterial infections. Lancet Infect Dis. 2015;15(8):968–980. doi:10.1016/S1473-3099(15)00089-4
- Baldwin SL, Larsen SE, Ordway D, Cassell G, Coler RN. The complexities and challenges of preventing and treating nontuberculous mycobacterial diseases. PLoS Neglect Trop Dis. 2019;13(2):e0007083. doi:10.1371/journal.pntd.0007083
- Adjemian J, Olivier KN, Seitz AE, Holland SM, Prevots DR. Prevalence of nontuberculous mycobacterial lung disease in US medicare beneficiaries. Am J Resp Crit Care. 2012;185(8):881–886. doi:10.1164/rccm.201111-2016OC
- Gordin FM, Masur H. Current Approaches to Tuberculosis in the United States. J Am Med Assoc. 2012;308(3):283–289. doi:10.1001/jama.2012.7505
- Tortoli E, Rogasi PG, Fantoni E, Beltrami C, De Francisci A, Mariottini A. Infection due to a novel mycobacterium, mimicking multidrug-resistant Mycobacterium tuberculosis. Clin Microbiol Infect. 2010;16(8):1130–1134. doi:10.1111/j.1469-0691.2009.03063.x
- Raju RM, Raju SM, Zhao YL, Rubin EJ. Leveraging advances in tuberculosis diagnosis and treatment to address nontuberculous mycobacterial disease. Emerg Infect Dis. 2016;22(3):365–369. doi:10.3201/eid2203.151643
- Piersimoni C, Scarparo C. Pulmonary infections associated with non-tuberculous mycobacteria in immunocompetent patients. Lancet Infect Dis. 2008;8(5):323–334. doi:10.1016/S1473-3099(08)70100-2
- Shahraki AH, Heidarieh P, Bostanabad SZ, et al. “Multidrug-resistant tuberculosis” may be nontuberculous mycobacteria. Eur J Intern Med. 2015;26(4):279–284. doi:10.1016/j.ejim.2015.03.001
- Huo FM, Ma YF, Liu RM, et al. Interpretation of discordant rifampicin susceptibility test results obtained using GeneXpert vs phenotypic drug susceptibility testing. Open Forum Infect Dis. 2020;7(8):ofaa279.
- Liu G, Chen ST, Yu X, et al. Bacteriological and virulence study of a Mycobacterium chimaera isolate from a patient in China. Antonie Van Leeuwenhoek. 2015;107(4):901–909. doi:10.1007/s10482-015-0382-x
- Forbes BA, Hall GS, Miller MB, et al. Practice guidelines for clinical microbiology laboratories: mycobacteria. Clin Microbiol Rev. 2018;31(2). doi:10.1128/CMR.00038-17.
- McGrath EE, Anderson PB. Increased prevalence of non-tuberculous mycobacteria infection. Lancet. 2007;370(9581):28. doi:10.1016/S0140-6736(07)61044-7
- Yu X, Liu PNA, Liu G, et al. The prevalence of non-tuberculous mycobacterial infections in mainland China: systematic review and meta-analysis. J Infect. 2016;73(6):558–567. doi:10.1016/j.jinf.2016.08.020
- Pang Y, Tan YJ, Chen J, et al. Diversity of nontuberculous mycobacteria in eastern and southern China: a cross-sectional study. Eur Respir J. 2017;49(3):1601429. doi:10.1183/13993003.01429-2016
- Zhou L, Xu D, Liu HC, Wan KL, Wang RB, Yang ZC. Trends in the prevalence and antibiotic resistance of non-tuberculous mycobacteria in Mainland China, 2000–2019: systematic review and meta-analysis. Front Public Health. 2020;8. doi:10.3389/fpubh.2020.00295
- Wang XB, Li H, Jiang GL, et al. Prevalence and drug resistance of nontuberculous mycobacteria, Northern China, 2008–2011. Emerg Infect Dis. 2014;20(7):1252–1253. doi:10.3201/eid2007.131801
- Daley CL, Iaccarino JM, Lange C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur Respir J. 2020;56(1):2000535. doi:10.1183/13993003.00535-2020
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Resp Crit Care. 2007;175(4):367–416. doi:10.1164/rccm.200604-571ST
- Chikamatsu K, Aono A, Yamada H, et al. Comparative evaluation of three immunochromatographic identification tests for culture confirmation of Mycobacterium tuberculosis complex. BMC Infect Dis. 2014;14:1–7.
- Yu MC, Chen HY, Wu MH, et al. Evaluation of the rapid MGIT TBc identification test for culture confirmation of Mycobacterium tuberculosis complex strain detection. J Clin Microbiol. 2011;49(3):802–807. doi:10.1128/JCM.02243-10