Abstract
Purpose
The Chinese government has authorized the emergency use of an inactivated vaccine for COVID-19 in children and adolescents aged 3 to 17 years. This study aimed to investigate parents’ attitudes towards vaccinating their children against COVID-19 and influencing factors.
Patients and Methods
Through an online questionnaire survey, we collected self-reported children’s demographic characteristics, physical conditions and parents’ attitudes towards COVID-19 vaccination for children. The parents in the unwilling group received online consultation about the benefits and risks of COVID-19 vaccine and were asked to complete the questionnaire again.
Results
A total of 868 participants were recruited from July 2021 to August 2021 in Nanjing, China. Overall, 76.0% of parents were willing to accept vaccination for children. Parents’ willingness increased with children’s age (P=0.018) and height (P=0.034), but decreased if the children fell sick within previous one month (P=0.030). Most of the unwilling parents gave a higher score to the risk of vaccination (53.76 VS 40.18). Unsafety (63.8%) and unfamiliarity (24.0%) were their major concerns. After consultation with a health worker, 24% of the unwilling parents turned willing.
Conclusion
Children’s age and recent physical condition are related to parents’ attitudes towards vaccination for children against COVID-19. The major concerns of parents are unsafety and unfamiliarity. Parents view health workers as a reliable source of vaccine information. A successful consultation with health workers to understand the benefits and risks of vaccination can increase parents’ willingness. This study provides insight into parents’ attitudes towards vaccination for children against COVID-19 in China and related influencing factors. Our findings could be referenced in establishing policies for vaccinating children against COVID-19 in China.
Introduction
The coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is still ravaging the world. In the United States, about 1.5 million children aged 11–17 years contracted COVID-19 and more than 300 people younger than 18 years died in 2020.Citation1 Although COVID-19 in childhood has a benign course, severe multisystem inflammatory syndrome still develops in a certain portion of children.Citation2 Besides, to curb SARS-CoV-2 transmission, the herd immunity threshold should be approximately 55% to 84%.Citation3,Citation4 Therefore, extending vaccination to children and adolescents is essential to establish a stable immune barrier against COVID-19.Citation5
In China, adults (aged ≥18 years) are vaccinated with home-made inactivated vaccines that can induce efficient immunogenicity but rare side effects in Phase 1, 2 and 3 trials.Citation6–8 This vaccination has effectively reduced laboratory-confirmed COVID-19 cases, and the odds of hospitalization, ICU care, and death in a real-world setting.Citation9,Citation10 Further, a double-blind phase 1/2 clinical trial showed that the inactivated CoronaVac vaccine was safe and well tolerated, and could induce humoral responses in children and adolescents aged 3–17 years in China. To the best of our knowledge, it is the first to report the immunogenicity and safety of COVID-19 candidate vaccine in children as young as 3 years.Citation11 On August 3, 2021, the Chinese government approved that an inactivated SARS-CoV-2 vaccine (CoronaVac) can be used in emergency in individuals aged 3 to 17 years. Since then, the teenagers aged 12–17 years have been vaccinated in an increasing number of areas.
However, vaccine hesitancy remains popular in the parents of to-be-vaccinated children. Parents’ attitudes are a major factor affecting the vaccination rate among children. This study intends to investigate the attitudes of Chinese parents towards vaccinating their children against COVID-19 and influencing factors.
Materials and Methods
Study Design and Participants
Our study recruited parents whose children aged ≤18 years and had visited Children’s Hospital of Nanjing Medical University. Each of the parents agreed to participate in this investigation. An identical structured questionnaire interview was conducted online through social media WeChat and the public account of Children’s Hospital of Nanjing Medical University based on WeChat between late July and late August 2021, using WeChat ID as a unique identifier. An online platform “Questionnaire Star” was used to collect children’s demographic characteristics, physical conditions, and parents’ attitudes about COVID-19 vaccination based on the Checklist for Reporting Results of Internet E-Surveys (CHERRIES). The questionnaire is presented as Supplementary Material.
These questions were asked: “Since an inactivated vaccine has been approved for emergency use in children aged 3–17 years, would you give it to your child?”, “What score between 0 (no risk) and 100 (highest risk) would you use to evaluate the risk in vaccinating your child against COVID-19?”. If unwilling to vaccinate their children against COVID-19, the parents were asked of the reasons. In addition, the unwilling parents were informed of the benefits and risks, as well as the significance of vaccination through a telephone consultation by a health worker, and then asked to complete the questionnaire again voluntarily. Besides, children’s age, gender, height, weight, preterm birth, cesarean delivery, recent physical condition, and risk of COVID-19 epidemic in local areas, as well as parents’ age, education level and annual household income were collected from the online questionnaire. According to the risk assessment criteria of the Joint Prevention and Control Mechanism of Chinese Government, the risk of COVID-19 epidemic in local areas (street, town, or city) are divided into three levels. Low risk was defined as no confirmed cases or no new cases within previous 14 days. Medium risk was defined as new cases within previous 14 days (less than 50 confirmed cases), or cumulatively more than 50 cases (no clusters of cases) within previous 14 days. High risk was defined as more than 50 cases, or clusters of cases within previous 14 days.
This research complies with the Declaration of Helsinki, and was approved by the Medical Ethics Committee of Children’s Hospital of Nanjing Medical University. Informed consent was obtained from all participants. Data used in this work were impersonal and anonymous.
Statistical Analysis
Descriptive data were presented as mean ± standard deviation (SD) or frequency (%). The parents were divided into two groups based on willingness to get COVID-19 vaccination. To analyze the differences between two groups, independent-sample t-test was conducted for continuous variables, and chi-square test for categorical variables. A two-sided P value <0.05 indicated significance. All analyses were performed in the SPSS version 23.0 (IBM Inc., Armonk, New York, USA).
Results
A total of 868 children (455 boys [52.4%] and 413 girls [47.6%]) were recruited in this study, with a mean age of 3.17 (SD 2.68) years, a mean height of 95.63 (SD 21.25) centimeters, and a mean weight of 17.07 (SD 9.63) kilograms (). Of them, 65 (7.5%) were delivered as preterm births and 290 (33.4%) as cesarean births; 122 (14.1%) had fallen ill in previous one month; 692 (79.7%) were living in low-risk areas, 49 (5.6%) in middle-risk areas, and the other 127 (14.6%) in high-risk areas.
Table 1 Demographic Characteristics and Physical Conditions of Children
The majority of parents (76.0%, n=660) were willing to vaccinate their children (willing group). The mean age of children from the willing group (3.31±2.81 years) was older than that from the unwilling group (2.73±2.13 years) (P=0.018). Consistently, the mean height of children from the willing group (96.49±21.71 centimeters) was larger than that from the unwilling group (92.91±19.47 centimeters) (P=0.034). A higher proportion of children from the unwilling group (20.2%) had been sick within previous one month, compared with that from the willing group (12.1%) (P=0.030). There were no statistical differences in gender, weight, preterm birth rate, cesarean delivery rate, and local COVID-19 epidemic risk between two groups.
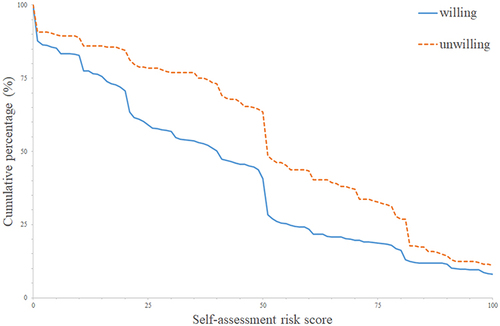
provides parents-scored risks of vaccinating children against COVID-19. The total mean risk score was 43.43 (SD 31.37). The risk score in the unwilling group (53.76±30.60) was higher than that in the willing group (40.18±30.93). shows the distribution of risk scores of two groups. The primary reason for refusing vaccination was the worry about safety (53.8%, n=112); 50 parents (24%) refused for the scant information of COVID-19 vaccines in children, 14 (6.7%) for the worry about low efficacy, 13 (6.3%) for vaccine refusal in general, and 19 (9.1%) for no reasons ().
Table 2 Parents’ Self-Scored Risks of COVID-19 Vaccination for Children
Table 3 Reasons for Refusing COVID-19 Vaccination for Children
Figure 1 Distribution of risk scores in both willing and unwilling groups. The blue solid curve shows the risk score and cumulative percentage in the willing group, and the orange dotted curve shows that in the unwilling group.
A health worker introduced benefits and risks of vaccination to the parents in the unwilling group on telephone. The parents were asked to complete the questionnaire again voluntarily. As shown in , 50 parents (24.0%) changed their attitudes. Of those who remained unwilling, 85 (53.8%) refused for unsafety, and 32 (20.3%) for unfamiliarity, 11 (7.0%) for uncertain efficacy, 11 (7.0%) for vaccine refusal, and 19 (12.0%) for no reasons. Similarly in the parents who changed their attitudes, most had refused for unsafety (n=27, 54.0%) and unfamiliarity (n=18, 36.0%), 3 (6.0%) for low efficacy, and 2 (4.0%) for vaccine hesitancy.
Table 4 Attitudes of Unwilling Parents Before or After Online Consultation
Discussion
In our study, we found that almost three quarters of parents accepted vaccination for children against COVID-19, compared to previously reported 89% in England,Citation12 80% in New ZealandCitation13 73% in China,Citation14 65% in the USA,Citation15 64% in South KoreaCitation16 and 36% in Turkey.Citation17 Children’s age was related to parents’ willingness. The average age of children from the willing group was older than that from the unwilling group, and the average height demonstrated the same trend. In a study conducted in a pediatric emergency department, parents’ willingness increased with children’s age.Citation15 In addition, children’s recent physical condition also affected parents’ decision on vaccination. The parents whose children had been sick in the previous one month were less willing to accept vaccination for children. Previous studies reported the significant hesitancy among the parents of children with chronic diseases.Citation15,Citation17 It may reflect the concerns of parents about the unsafety of vaccines.
Not surprisingly, the parents in the unwilling group rated the risk of vaccination with a higher score. Safety concern was the chief factor that influenced parents’ attitudes towards vaccination for children, followed by inadequate information about children’s SARS-CoV-2 vaccines. A study reported that the inactivated vaccine CoronaVac was well tolerated and safe in children and adolescents aged 3–17 years; and most adverse reactions were mild and moderate in severity.Citation11 To promote the vaccination for children, parents should be informed of adequate medical knowledge, especially the benefits and risks of vaccines. It is reported that CoronaVac can induce stronger humoral responses in children aged 3–17 years than in adults aged 18–59 years and the elderly aged over 60 years.Citation11 In the present study, a small group of parents worried about effectiveness of vaccination, suggesting that the long-term immunogenicity and protective effect of vaccines in real-world remain to be proven.
About one quarter of parents changed their attitudes from unwilling to willing, after a telephone consultation about the benefits and risks of vaccination for children. The benefits include preventing COVID-19 and the deterioration into severe illness among individuals, as well as building an immune barrier in a population. The risks are mainly side effects that have been reported. It is reported that face-to-face consultation may enhance parents’ intention to get their children vaccinated.Citation18–20 Parents view health workers as an important source of information.Citation21,Citation22 Here, we found that telephone consultation could strengthen parents’ willingness. It is reported that parents also need general information about vaccination risks, not just its benefits.Citation23 The tailored health education is more effective to let parents accept vaccination for children, compared to untailored health education.Citation20,Citation21,Citation24,Citation25
This study has some limitations. First, the mean age of the children was 3.17 years, making them less representative of older children. Second, in the online questionnaire survey, the parents’ self-reported results may not reflect their actual vaccination behavior. Additionally, a convenience sampling approach may result in biased estimates. Third, although we collected a relative larger sample, the power of this single-center cohort study is still limited.
Conclusion
In conclusion, our findings suggest that children’s age and physical condition are related to parents’ attitudes to vaccinating children against COVID-19. The major concerns are unsafety and unfamiliarity. Parents view health workers as a reliable source of vaccine information. A successful consultation with health workers to understand the benefits and risks of vaccination can increase parents’ willingness. This study is innovative in evaluating parents’ attitudes towards COVID-19 vaccination for children in China. Our findings could be referenced in establishing policies for COVID-19 vaccination for children in the future.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank all volunteers who participated in this study and also thank the investigators contributed to the site work of this study.
References
- Tanne JH. Covid-19: FDA authorises Pfizer vaccine for children 12–15. BMJ. 2021;n1204. doi:10.1136/bmj.n1204
- Rubens JH, Akindele NP, Tschudy MM, Sick-Samuels AC. Acute covid-19 and multisystem inflammatory syndrome in children. BMJ. 2021;n385. doi:10.1136/bmj.n385
- Schaffer DeRoo S, Pudalov NJ, Fu LY. Planning for a COVID-19 vaccination program. JAMA. 2020;323(24):2458. doi:10.1001/jama.2020.8711
- Ke R, Sanche S, Romero-Severson E, Hengartner N. Estimating the reproductive number R0 of SARS-CoV-2 in the United States and eight European countries and implications for vaccination. Epidemiology. 2020. doi:10.1101/2020.07.31.20166298
- Velavan TP, Pollard AJ, Kremsner PG. Herd immunity and vaccination of children for COVID-19. Int J Infect Dis. 2020;98:14–15. doi:10.1016/j.ijid.2020.06.065
- Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(2):181–192. doi:10.1016/S1473-3099(20)30843-4
- Wu Z, Hu Y, Xu M, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(6):803–812. doi:10.1016/S1473-3099(20)30987-7
- Tanriover MD, Doğanay HL, Akova M, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, Phase 3 trial in Turkey. Lancet. 2021;398(10296):213–222. doi:10.1016/S0140-6736(21)01429-X
- Jara A, Undurraga EA, González C, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021;385(10):875–884. doi:10.1056/NEJMoa2107715
- Ranzani OT, Hitchings MDT, Dorion M, et al. Effectiveness of the CoronaVac vaccine in older adults during a gamma variant associated epidemic of covid-19 in Brazil: test negative case-control study. BMJ. 2021;n2015. doi:10.1136/bmj.n2015
- Han B, Song Y, Li C, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy children and adolescents: a double-blind, randomised, controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(12):1645–1653. doi:10.1016/S1473-3099(21)00319-4
- Bell S, Clarke R, Mounier-Jack S, Walker JL, Paterson P. Parents’ and guardians’ views on the acceptability of a future COVID-19 vaccine: a multi-methods study in England. Vaccine. 2020;38(49):7789–7798. doi:10.1016/j.vaccine.2020.10.027
- Jeffs E, Lucas N, Walls T. CoVID ‐19: parent and caregiver concerns about reopening New Zealand schools. J Paediatr Child Health. 2021;57(3):403–408. doi:10.1111/jpc.15234
- Zhang KC, Fang Y, Cao H, et al. Parental acceptability of COVID-19 vaccination for children under the age of 18 years: cross-sectional online survey. JMIR Pediatr Parent. 2020;3(2):e24827. doi:10.2196/24827
- Goldman RD, Yan TD, Seiler M, et al. Caregiver willingness to vaccinate their children against COVID-19: cross sectional survey. Vaccine. 2020;38(48):7668–7673. doi:10.1016/j.vaccine.2020.09.084
- Choi SH, Jo YH, Jo KJ, Park SE. Pediatric and parents’ attitudes towards COVID-19 vaccines and intention to vaccinate for children. J Korean Med Sci. 2021;36(31):e227. doi:10.3346/jkms.2021.36.e227
- Yılmaz M, Sahin MK. Parents’ willingness and attitudes concerning the COVID‐19 vaccine: a cross‐sectional study. Int J Clin Pract. 2021;75(9). doi:10.1111/ijcp.14364
- Kaufman J, Ryan R, Walsh L, et al. Face-to-face interventions for informing or educating parents about early childhood vaccination. Cochrane Database Syst Rev. 2018;2018(5). doi:10.1002/14651858.CD010038.pub3
- Williams SE. What are the factors that contribute to parental vaccine-hesitancy and what can we do about it? Hum Vaccin Immunother. 2014;10(9):2584–2596. doi:10.4161/hv.28596
- Glanz JM, Kraus CR, Daley MF. Addressing parental vaccine concerns: engagement, balance, and timing. PLoS Biol. 2015;13(8):e1002227. doi:10.1371/journal.pbio.1002227
- Du F, Chantler T, Francis MR, et al. Access to vaccination information and confidence/hesitancy towards childhood vaccination: a cross-sectional survey in China. Vaccines. 2021;9(3):201. doi:10.3390/vaccines9030201
- Olson O, Berry C, Kumar N. Addressing parental vaccine hesitancy towards childhood vaccines in the United States: a systematic literature review of communication interventions and strategies. Vaccines. 2020;8(4):590. doi:10.3390/vaccines8040590
- Ames HM, Glenton C, Lewin S. Parents’ and informal caregivers’ views and experiences of communication about routine childhood vaccination: a synthesis of qualitative evidence. Cochrane Database Syst Rev. 2017;2017(4). doi:10.1002/14651858.CD011787.pub2
- Gowda C, Schaffer SE, Kopec K, Markel A, Dempsey AF. A pilot study on the effects of individually tailored education for MMR vaccine-hesitant parents on MMR vaccination intention. Hum Vaccin Immunother. 2013;9(2):437–445. doi:10.4161/hv.22821
- Kempe A, Daley MF, McCauley MM, et al. Prevalence of parental concerns about childhood vaccines. Am J Prev Med. 2011;40(5):548–555. doi:10.1016/j.amepre.2010.12.025
