Abstract
Background
Millions were infected and many were dying because of the coronavirus disease 2019, since its emergence. The patients experience asymptomatic, mild, moderate, severe and critical disease with varying signs and symptoms. Decreased lymphocytes and abnormal liver and renal function tests are common among COVID-19 patients. Severe and critical cases show higher number of white blood cells, and neutrophils. However, studies showed different laboratory findings in different disease status. Therefore, this study investigated laboratory findings of COVID-19 admitted patients at Dilla University Referral Hospital treatment center, South Ethiopia.
Methods
A retrospective study design was conducted on 220 patients confirmed by real time polymerase chain reaction, and admitted to Dilla University Referral Hospital treatment center from September 2020 to July 2021. Data were collected from the patients’ record, and analyzed by GraphPad Prism version 8.0.1.244 software. Descriptive statistics were used to analyze the frequency while independent t-test was used to compare means of each parameter for each disease status.
Results
Of the 220 study cases, 120 (54.5%) were severe, 89 (40.5%) were moderate and 11 (5.0%) were mild. One hundred forty (71.1%) of the 197 laboratory tested cases, 87 (77.7%) of severe, and 49 (64.5%) of the moderate cases had neutrophils above normal range. However, 134 (68.0%) of them, 82 (73.2%) of severe and 49 (64.5%) of moderate cases showed decreased lymphocyte level. Most of the cases showed an increased level of aspartate transaminase, alanine transaminase, alkaline phosphatase, total bilirubin, and total calcium. There was statistically significant mean neutrophils (p=0.04), number of white blood cells (p= 0.02), and creatinine level (p=0.00) difference between severe and mild cases.
Conclusion
Most of the severe COVID-19 patients showed increased neutrophils, liver function tests; and decreased lymphocytes; suggesting higher inflammation and lymphopenia. Therefore, patients with severe and critical disease status require close follow-up.
Introduction
COVID-19 is caused by enveloped RNA viruses that infect humans and other animals, the SARS-CoV-2 virus.Citation1 The disease is spreading globally, and different COVID-19 variants are emerging in many countries around the world. Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2),Citation2 and Omicron (B.1.1.529) are the main variants that cause morbidity and mortality worldwide. The Delta variant is currently the dominant variant that is circulating globally.Citation3 The Omicron variant has many mutations that make the virus act differently from other variants.Citation4,Citation5 It spreads more easily than previous strains because it has more mutations than any other variant so far. Many of the mutations are in the spike proteins.Citation6 The SARS-CoV-2 virus transmit from an infected person’s mouth or nose within small liquid particles when they cough, sneeze, speak, sing, or breathe.Citation7 There have been 481,756,671 confirmed cases of COVID-19, including 6,127,981 deaths globally up to March 2022. 8,567,298 confirmed cases and 171,006 deaths in Africa.Citation8 The number of COVID-19 cases in Ethiopia is increasing in most parts of the country, in particular, Somali, Afar, Amhara, Oromia, and Tigray regions are more likely to be affected. The risk of COVID-19-related death is high in the country’s border parts, where health facilities are limited.Citation9
Most people infected with the virus experience mild to moderate respiratory illness and recover without requiring special support.Citation10 However, some may become seriously ill and need medical attention. Older people and those with chronic diseases like cardiovascular disease, diabetes, chronic respiratory diseases,Citation11 or cancer may develop serious illness.Citation7 The most prevalent symptoms are fatigue, shortness of breath, muscle pain, joint pain, headache, cough, chest pain, altered smell, altered taste, and diarrhea.Citation12,Citation13 The patients may also experience a range of clinical manifestations including asymptomatic, mild, moderate, severe or critical illness with varying signs and symptoms including, respiratory failure, septic shock, and multiple organ dysfunction.Citation14–16 Within 4 weeks after infection, most people infected with the SARS-CoV-2 virus develop neutralizing antibodies.Citation17 However, the strength and duration of the immune responses to the virus are not completely understood, and some evidence shows that they vary by age and the severity of the disease.Citation18 Like other viral infections, T cells play a vital role in generating early control of the virus, even though their role in the resolution or exacerbation of COVID-19, as well as their potential to provide long-term protection from reinfection with SARS-CoV-2, remains debated.Citation19 Specific T cells may even have a detrimental impact on the clinical outcome and contribute to long-term COVID-19 symptoms.Citation20 Reinfection with the virus can also occur; this indicates non durability of the immune response.Citation21,Citation22 The emergence of new variants with the ability to evade immune response may result in reinfection.Citation23–25 Preventive measures like wearing masks, vaccination and social distancing are very important, especially for patients who have underlying conditions, and older people.Citation10,Citation26
Results of blood tests of total white blood cells (WBCs), neutrophils, lymphocytes, and platelets, liver function, renal function tests, and other biomarkers have potential clinical value in COVID-19 patients.Citation27,Citation28 Reductions in leukocytes such as eosinophil and lymphocyte are common in Covid-19 infection, and severe lymphopenia is common among the deceased patients.Citation29 Aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), bilirubin and C-reactive protein levels,Citation30 and blood urea nitrogen (BUN) are found to be abnormal among COVID-19 patients, especially in severe cases.Citation31 Severe and critical cases show higher number of white blood cells, and neutrophil counts.Citation32–34 However, different studies showed different laboratory findings in different disease status,Citation35 that need further investigation. Therefore, this study attempted to describe laboratory findings in different disease status of COVID-19 admitted patients at Dilla University Referral Hospital treatment center, South Ethiopia.
Materials and Methods
Data Collection
This study was conducted at the Dilla University Referral Hospital, South Ethiopia. A retrospective study design was conducted for 220 real time polymerase chain reaction (RT-PCR) confirmed COVID-19 patients, who were admitted to Dilla University Referral Hospital treatment center from September 2020 to July 2021. All patients were confirmed for COVID-19 by RT-PCR testing their nasal and pharyngeal swabs samples. All COVID-19 positive cases were admitted to the treatment center. Therefore, we included all the admitted patients’ records except the incomplete ones, and used the baseline data that were recorded at the time of admission. 220 complete records of admitted patients were included. Socio-demographic, disease status and Laboratory findings data were collected from the patients’ record. Trained general practitioners and nurses who were working at treatment center collected the data from the patients’ record.
Data Analysis
Data was entered and analyzed by GraphPad Prism version 8.0.1.244 software. Descriptive statistics were used to analyze frequency and percentage of the parameters while independent t-test was used to compare means of each parameter for each disease status (mild, moderate and severe).
Definitions
Study patients are classified into severe, moderate and mild according to the WHO criteria. Accordingly, mild cases were symptomatic patients without evidence of viral pneumonia or hypoxia, moderates were the cases with clinical signs of non-severe pneumonia while severe cases were the cases with clinical signs of severe pneumonia like fever, cough, dyspnea and respiratory rate >30 breaths /minute or SPO2<90%.
Ethical Approval
Ethical approval and waiver consent was obtained from the Institutional Review Board (IRB) of the Dilla University College of Medicine and Health sciences under the protocol unique number of duirb/002/21-10 before the data collection. The consent to participate in the study was not applicable because the study was retrospective and was conducted on the data collected from the patients’ medical records. The privacy and confidentiality of their personal information were protected according to ethical principles for medical research involving human subjects of the World Medical Association Declaration of Helsinki.
Result
Socio-Demographic Factors
Out of 220 study cases, 113 (51.4%) of them were above 50 years of age, and the mean age was 47 years. Most of the cases, 123 (55.9%), were male, and housewife accounted for the largest number of cases 47 (21.4%) followed by unemployment 41 (18.6%), Government employees 31 (14.1%), farmers 30 (13.6%), self-employed 28 (12.7%), merchants 20 (9.1%) and students (6.8%) ().
Table 1 Socio-Demographic Factors of Hospitalized COVID-19 Patients (n=220) to Dilla University Referral Hospital, December 2021
Disease Status
Of the 220 admitted patients, 120 (54.5%) were severe, 89 (40.5%) were moderate, and 11 (5.0%) were mild. One hundred sixty-seven (75.9%) cases were improved and 49 (22.3%) of them died, while 4 (1.8%) cases were transferred out. Out of the 167 improved cases, 80 (47.9%) were 50+ years, 58 (34.7%) were between 30–49 years, and 29 (17.4%) were <30 years, and 96 (57.5%) were male while 71 (42.5%) were female. Eighty-six (51.5%) were moderate, 70 (41.9%) were severe and 11 (6.6%) cases were mild. Of the 49 died cases, 29 (59.2%) cases were beyond the age of 50+ years, while 11 (22.4%) were between the age group of 30–49 years and 9 (18.4%) were <30 years age, and males accounted for 28 (57.1%) while females were 21 (42.9%), and 48 (98%) cases were severe and 1 (2%) was moderate ().
Table 2 Disease Status of Admitted COVID-19 Confirmed Cases (n=220) to Dilla University Referral Hospital, December 2021
Laboratory Findings
Normal, Increased, or Decreased Laboratory Findings
Of the 197 Neutrophil, Lymphocyte, Platelet, WBC, red blood cells (RBC) and hematocrit (Hct) tested COVID-19 cases, 140 (71.1%) had neutrophils above normal range while 51 (25.9%) showed within normal range and 6 (3%) below the normal range. Likewise, of the 112 severe cases, 87 (77.7%) of them showed increased Neutrophils, 21 (18.8%) within the normal range and 4 (3.6%) decreased Neutrophil number. Among 76 moderate cases, 49 (64.5%) showed increased Neutrophils, 25 (32.9%) within normal range and 2 (2.6%) decreased Neutrophils. Therefore, most of severe and moderate cases showed increased Neutrophils level. However, 134 (68.0%) of the cases showed decreased Lymphocytes, 3 (1.5%) of the cases had Lymphocytes above normal range and 60 (30.5%) within normal range. Similarly, of the 112 severe cases, 82 (73.2%) showed decreased Lymphocyte level, 3 (2.7%) had above normal range and 27 (24.1%) within normal range. Out of 76 moderate cases, 27 (35.5%) had normal Lymphocytes number while 49 (64.5%) had decreased number. Therefore, severe and moderate cases showed decreased Lymphocyte number. Most of the cases showed normal platelet number; 142 (72.1%) of the total tested cases, 77 (68.8%) of the severe cases, 58 (76.3%) moderate cases and 5 (83.3%) mild cases respectively.
Ninety-three (47.2%) of the total tested cases showed normal number of WBC, 86 (43.7%) of them showed increased WBC; 55 (49.1%) of the severe cases showed normal, 49 (43.8%) increased number, while 32 (42.1%) of moderate cases showed normal, and 35 (46.1%) showed increased number of WBC. Most of the tested cases showed normal number of RBC: 159 (80.7%) of the total tested, 94 (83.9%) of the severe, 56 (73.7%) of the moderate and 6 (100.0%) of the mild cases respectively. Similarly, most of them showed normal Hct level: 123 (62.4%) of the total tested, 72 (64.3%) of the severe, 41 (53.9%) of the moderate and 5 (83.3%) of the mild cases respectively.
Most of the AST tested cases showed increased level: 46 (75.4%) of the total 61 tested cases, 31 (79.5%) of severe cases, 13 (65.0%) of the moderate cases and 2 (100.0%) of the mild cases. Similar increased finding was seen in ALT; 37 (61.7%) of the total, 26 (66.7%) of severe and 10 (52.6%) of the moderate cases respectively. Which is also similar for ALP in which 26 (78.8%) of the total, 14 (70.0%) of the severe, 11 (91.7%) of the moderate and 1 (100.0%) of the mild cases respectively showed increased level. All the direct bilirubin tested cases showed increased level; while most of the total bilirubin tested cases also showed increased level: 7 (70.0%) of the total, 3 (75.0%) of the severe and 4 (66.7%) of the moderate cases respectively.
Most of the total and severe cases showed normal level of creatinine (Cr) 58 (44.3%) and 44 (50.6%) respectively, while it seems increased in moderate cases 18 (46.2%). Similarly, most of the cases showed normal level of blood urea nitrogen (BUN): 94 (71.8%) of the total tested, 59 (67.8%) of the severe, 30 (76.9%) of the moderate and 4 (80.0%) of the mild cases respectively. Thirteen (38.2%) of the sodium (Na) tested cases had below normal, 12 (35.3%) above normal and 12 (35.3%) within normal range respectively, and in severe cases 10 (35.8%) below normal, and 9 (32.1%) above and 9 (32.1%) within normal ranges respectively. Most of the cases showed normal potassium (K) level; 20 (60.6%) of the total tested, 17 (60.7%) of the severe, 2 (50.0%) of the moderate and 1 (100.0%) of the mild cases respectively. 9 (28.1%) of the total chloride (Cl) tested cases, 8 (29.6%) of severe cases and 1 (25.0%) of moderate cases showed increased level. 12 (37.5%) of the total tested cases, 9 (33.3%) of severe cases, 2 (50.0%) of the moderate cases and 1 (100.0%) of the mild cases showed normal level, while 11 (34.4%) of the total tested, 10 (37.0%) of the severe and 1 (25.0%) of the moderate cases showed decreased level. Most of the total calcium (Ca) tested cases showed increased level 15 (78.9%) of the total tested, 13 (76.5%) of the severe and 2 (100.0%) of the moderate cases respectively. Similarly, 6 (75.0%) of the total tested, 2 (66.7%) of the severe and 3 (75.0%) of the moderate cases showed increased level of high-density lipoprotein (HDL). 6 (75.0%) of the total tested, 2 (50.0%) of the moderate cases showed increased level, and 2 (25.0%) of total tested, 3 (100.0%) of the severe and 2 (50.0%) of the moderate cases showed normal level of low-density lipoprotein (LDL). 5 (62.5%) of the total tested, 2 (66.7%) of the severe and 2 (50.0%) of the moderate cases showed increased level of Triglycerides. Similarly, most of the total cholesterol tested cases showed increased level, which means 6 (75.0%) of the total tested, 3 (100.0%) of the severe and 3 (75.0%) of the moderate cases respectively ().
Table 3 Laboratory Findings of Admitted COVID-19 Cases to Dilla University Referral Hospital, December 2021
Laboratory Findings in Different Disease Status
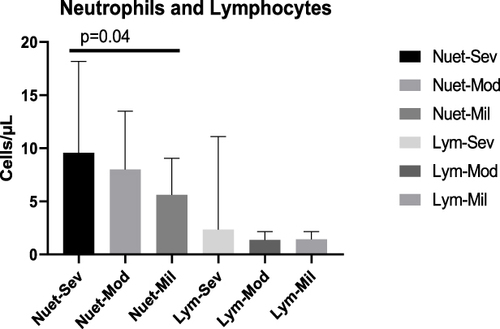
The mean laboratory finding was calculated for each test in different disease status and was stated as mean ± standard deviation (SD). Accordingly, the mean Neutrophil for severe cases was 9.556 ± (8.622) x109 /L while it was 7.992 ± (5.499) x109 /L for moderate cases. So, there is no statistically significant mean Neutrophil difference between severe and moderate cases, p= 0.1308. It was also 5.612 ± (3.423) x109 /L for mild cases, and there was statistically significant mean Neutrophil difference between severe and mild cases, p=0.04, however there was no mean Neutrophil difference in moderate and mild cases, p= 0.16.
The mean Lymphocytes for severe cases was 2.333 ± (8.755) x109 /L, and 1.370 ± (0.7894) x109 /L for moderate cases, while it was 1.415 ± (0.7469) x109 /L for mild cases. There was no statistically significant mean Lymphocytes difference between severe and moderate cases (p=0.25) and severe and mild cases (p=0.30), and moderate and mild cases (p=0.89) ().
Figure 1 The mean number of Neutrophils and Lymphocytes of COVID-19 cases in severe, moderate and mild disease status. There was a significant mean number of Neutrophils differences between severe and mild cases (p=0.04).
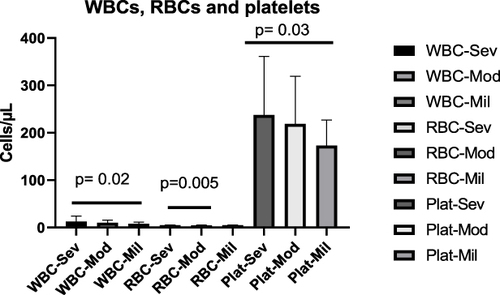
The mean number of WBCs for severe cases was 12.56 ± (11.58) x109 /L, 10.14 ± (5.664) x109 /L for moderate cases and 7.875 ± (3.508) x109 /L for mild cases. There was a significant mean number of WBC difference between severe and mild cases (p= 0.02), however, there was no significant mean number difference between severe and moderate (p= 0.06), and moderate and mild cases (p= 0.19).
The mean number of RBCs was 4.735 ± (0.8264) x1012/L for severe, 4.379 ± (0.8391) x1012/L for moderate and 4.768 ± (0.4005) x1012/L for mild cases. Therefore, there was a significant mean RBC number difference between severe and moderate cases (p=0.005); however, no significant differences between severe and mild (p=0.86), and moderate and mild cases (p= 0.07).
The mean number of Platelets was 237.5 ± (123.7) x109 /L for severe, 218.9 ± (100.3) x109 /L for moderate, and 173.2 ± (53.64) x109 /L for mild cases. There was a significant mean platelets number difference between severe and mild cases (p= 0.03). However, there was no significant mean platelets number differences between severe and moderate (p= 0.26), and moderate and mild cases (p= 0.10) ().
Figure 2 The mean number of WBCs, RBCs and platelets of COVID-19 cases in severe, moderate and mild disease status. There were a significant mean number of WBCs differences between severe and mild cases (p=0.02), mean RBCs differences between severe and moderate cases (0.005), and mean platelets differences between severe and mild cases (p=0.03).
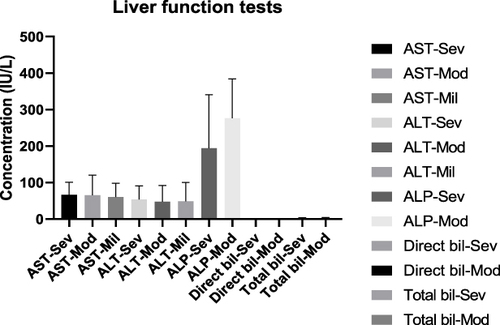
The mean AST level in severe cases was 66.56 ± (34.28) U/L, 65.58 ± (55.22) U/L for moderate cases, 60.60 ± (37.62) U/L for mild cases. It was 53.77 ± (37.45) U/L for severe, 47.63 ± (44.17) U/L for moderate and 48.70 ± (51.90) U/L for mild cases for mean ALT level. The mean ALP concentration was 194.1 ± (146.6) U/L for severe, 276.4 ± (107.7) U/L for moderate cases. The mean direct bilirubin concentration for severe cases was 0.6525 ± (0.2277) mg/dL, 1.045 ± (1.139) mg/dL for moderate cases. It was 2.210 ± (1.328) mg/dL for severe and 2.415 ± (2.596) mg/dL for moderate cases for a total bilirubin level. There was no statistically significant mean AST concentration differences between severe and moderate (p=0.94), severe and mild (P= 0.86), and moderate and mild cases (P= 0.89). Similarly, there was no statistically significant mean ALT concentration difference between severe and moderate, severe and mild, and moderate and mild cases (P= 0.61, P= 0.91, P= 0.98 respectively). There was also no statistically significant mean ALP concentration difference between severe and moderate cases (P= 0.08). Similar non-significant values were seen in direct bilirubin and total bilirubin concentration between severe and moderate cases (p=0.45, p=0.87) respectively. However, there was a trend towards decrease in mean ALP level in severe cases ().
Figure 3 The mean level of AST, ALT, ALP, direct bilirubin and total bilirubin of COVID-19 cases in severe, moderate and mild disease status.
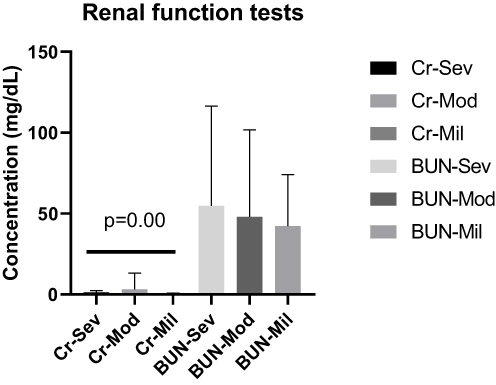
The mean Cr concentration for severe cases was 1.351 ± (1.135) mg/dL, 3.239 ± (9.992) mg/dL for moderate and 0.7320 ± (0.1126) mg/dL for mild cases. The mean concentration of BUN in severe cases was 54.66 ± (61.82) mg/dL, 47.90 ± (53.96) mg/dL for moderate and 42.22 ± (31.87) mg/dL for mild cases. There was statistically significant mean Cr concentration difference between severe and mild cases (p=0.00). However, there was no statistically significant mean Cr concentration difference between severe and moderate cases (p= 0.24); moderate and mild (P=0.12); severe and moderate (P=0.54); severe and mild (p=0.46) and moderate and mild cases (P=0.74) for BUN concentration ().
Figure 4 The mean level of Cr and BUN of COVID-19 cases in severe, moderate and mild disease status. There was a significant mean Cr concentration differences between severe and mild cases (p=0.00).
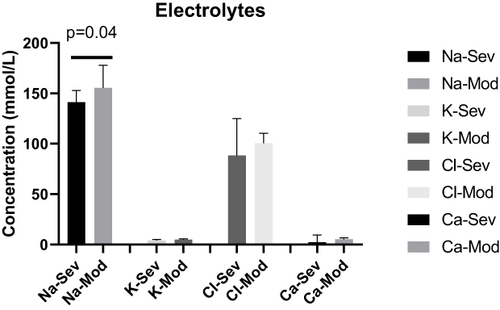
Mean concentration of Na for severe cases was 141.3 ± (11.63) mmol/L, 155.4 ± (22.55) mmol/L for moderate, and it was 4.018 ± (0.8260) mmol/L for severe, 4.825 ± (0.6752) mmol/L for moderate for K, 88.27 ± (36.62) mmol/L for severe, 100.6 ± (9.761) mmol/L for moderate cases for Cl, and 5.342 ± (4.071) mmol/L for severe and 5.300 ± (1.697) mmol/L moderate cases for total Ca. There was statistically significant mean Na concentration difference (p=0.04) between severe and moderate cases; however, there was no significant mean concentration difference for K (p=0.07), for Cl (p=0.17), for Ca (p=0.98) between severe and moderate cases ().
Figure 5 The mean level of Na, K, Cl and Ca of COVID-19 cases in severe, moderate and mild disease status. There was a significant mean Na concentration differences between severe and moderate cases (p=0.04).
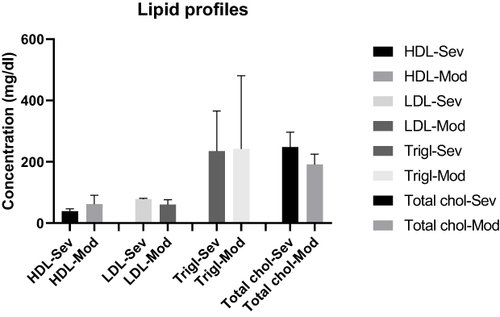
The mean HDL concentration for severe cases was 39.33 ± (7.638) mg/dL, 62.00 ± (28.97) mg/dL for moderate cases, and 78.33 ± (3.055) mg/dL for severe cases and 60.75 ± (15.37) mg/dL for moderate cases for LDL, while it was 234.7 ± (131.3) mg/dL for severe, and 242.3 ± (238.3) mg/dL for moderate cases for Triglycerides. It was 248.3 ± (48.42) mg/dL for severe and 191.5 ± (33.79) mg/dL for moderate cases for total cholesterol level. There was no statistically significant mean concentration difference in severe and moderate cases for HDL, LDL, triglycerides and total cholesterol level (p=0.21, P=0.10, p=0.96, and p=0.17 respectively) ().
Figure 6 The mean level of HDL, LDL, triglycerides and total cholesterol of COVID-19 cases in severe, moderate and mild disease status.
Discussion
Most of the tested cases showed increased Neutrophil number, and the same result was also found in most of the severe and moderate cases. However, most of the severe and moderate cases showed decreased Lymphocyte number. The number of lymphocytes also decreased for total Lymphocyte tested COVID-19 cases, 68.0% (134/197) of the cases showed decreased Lymphocytes. These findings are consistent with the study done in China that stated dramatically increased neutrophil counts found in severe COVID-19 patients in comparison to the other groups. This may be due to high inflammation in severe COVID-19 infected cases that increases an inflammatory cytokines and chemokine, and complement 5a (C5a) that attract and enhance the number of Neutrophils in the lung as well as in the circulation. However, the lymphocyte counts persisted at lower values in severe COVID-19 patients,Citation36 which may be due to the virally infected Lymphocytes are targeted by other immune cells that directly kill the infected Lymphocytes hence decreasing the number in the circulation. It is also in line with the study that showed 70.2% of the COVID-19 cases had low circulating lymphocyte count, of which 64.1% were severe cases,Citation37 which is also in line with the studies that showed 63% (26/40) of the patients showed lymphopenia,Citation38 patients with severe and fatal disease had significantly decreased lymphocyte counts compared to non-severe disease.Citation39 Our study also showed that there was no statistically significant mean Neutrophil difference between severe and moderate cases, p= 0.13, and there was statistically significant mean Neutrophil difference between severe and mild cases, p=0.04, however there was no mean Neutrophil difference in moderate and mild cases, p= 0.16. This may be due to the increasing number of Neutrophils in all disease statuses which could possibly be higher in severe cases due to higher inflammation. It was in line with the study that stated significantly higher neutrophil in severe COVID-19 compared to non-severe COVID-19.Citation40 There was no statistically significant mean Lymphocytes difference between severe and moderate cases (p=0.25) and severe and mild cases (p=0.30), and moderate and mild cases (p=0.89), which likely be due to a comparable decrease in the number of Lymphocytes in different disease status.
Most of the severe, moderate and mild cases shown normal number of platelet count (within normal range). Which is discordant with meta-analysis study findings that stated the association of thrombocytopenia with severe COVID-19 illness.Citation41 This may be due to the sample size and socio-demographic factors difference. However, we had found a significant mean platelets number difference between severe and mild cases (p= 0.03). There was a slight increase in the platelet number in the severe cases than the mild cases, which may be due to higher inflammation in severe cases than mild cases. However, there was no significant mean platelets number differences between severe and moderate (p= 0.26), and moderate and mild cases (p= 0.10).
Ninety-three (47.2%) of the total tested cases showed normal number of WBC, 86 (43.7%) of them showed increased and 18 (9.1%) of them showed decreased number, and 55 (49.1%) of the severe cases showed normal, 49 (43.8%) increased number and 8 (7.1%) decreased, while 32 (42.1%) of moderate cases showed normal, and 35 (46.1%) showed increased number of WBC and 9 (11.8%) of them showed decreased number, while 2 (33.3%) of the mild cases showed normal, increased and decreased number of WBCs; which was different from the study done in China that stated at the time of admission, 23% patients presented a leukocyte count below the normal range, 12% with a leukocyte count above the normal range,Citation42 which may be due to the sample size and socio-demographic factors differences. So, in our study, the number of WBCs in the total and severe cases was normal, while it seems increased in moderate cases for most of the patients; which was different from the study stated patients with severe and fatal disease had significantly increased WBC count compared to non-severe disease.Citation39 There was a significant mean number of WBC difference between severe and mild cases (p= 0.02), however, there was no significant mean number difference between severe and moderate (p= 0.058), and moderate and mild cases (p= 0.19). It was in line with the study that stated significantly higher leukocyte in severe COVID-19 as compared to non-severe COVID-19 cases.Citation40 This may be due to an increase in the number of Neutrophils in severe than mild cases, that accounts 40–70% of total WBCs.
Most of the tested cases showed normal number of RBC: 80.7% of the total tested, 83.9% of severe, 73.7% of the moderate and all the 6 mild cases respectively. Similarly, most of them showed normal Hct level: 62.4% of the total tested, 64.3% of the severe, 53.9% of the moderate and 83.3% of the mild cases respectively. Therefore, the number of RBCs and Hct in severe, moderate and mild cases seems normal for most of the cases. There was a significant mean RBC number difference between severe and moderate cases (p=0.005); however, no significant differences between severe and mild (p=0.86), and moderate and mild cases (p= 0.07). This was in line with the study in Bangladesh that showed no significant difference in Hct and total RBC count was observed among severe, moderate and mild cases,Citation43 even though, it was discordant in case of number of RBCs in severe and moderate cases in which we found significant difference.
All the direct bilirubin tested cases showed increased level, while most of the total bilirubin tested cases also showed increased concentration: 70.0% of the total, 75.0% of the severe and 66.7% of the moderate cases respectively, which was in line with meta-analysis study that stated the severity of COVID-19 increases with the level of total bilirubin increases,Citation44 which is also in line with the study in United States of America that stated there was elevated level of bilirubin in COVID-19 infected cases,Citation45 which may be due to liver dysfunction due to cytokines storm and inflammation. Non-significant differences were seen in direct bilirubin and total bilirubin concentration between severe and moderate cases (p=0.44 and p=0.87 respectively).
Most of the AST tested cases showed increased level: 75.4% of the total 61 tested cases, 79.5% of severe cases, 65.0% of the moderate cases and both of the mild cases. Similar increased finding was seen in ALT; 61.7% of the total, 66.7% of severe and 52.6% of the moderate cases respectively, which is also similar for ALP in which 78.8% of the total, 70.0% of the severe, 91.7% of the moderate and the single tested mild cases respectively showed increased level. Therefore, most of the tested cases shown increased level of AST, ALT and ALP which likely is due to inflammation of the liver as the liver is the site of some of the inflammatory cytokines and other inflammatory mediators production. Our study is in line with the study in China that stated a total of 27% patients exhibited varying degrees of liver dysfunction, with ALT or AST levels above the normal range.Citation42 Which is also in line with meta-analysis study that stated the liver dysfunction was found in a larger number of severe patients as compared to the mild patients, and the severity of COVID-19 increases, the level of AST, ALT, and ALP increase.Citation44 Which was also similar with study in Pakistan that showed AST and ALT were all significantly increased in severe COVID-19 cases.Citation46 In our study we found no statistically significant mean AST concentration differences between severe and moderate (p=0.94), severe and mild (P= 0.86), and moderate and mild cases (P= 0.89). Similarly, there was no statistically significant mean ALT concentration difference between severe and moderate, severe and mild, and moderate and mild cases (P= 0.61, P= 0.91, P= 0.98 respectively). There was also no statistically significant mean ALP concentration difference between severe and moderate cases (P= 0.08).
Most of the total and severe cases showed normal level of creatinine 58 (44.3%) and 44 (50.6%) respectively, while it seems increased in 18 (46.2%) moderate cases. Similarly, most of the cases showed normal level of BUN: 94 (71.8%) of the total tested, 59 (67.8%) of the severe, 30 (76.9%) of the moderate and 4 (80.0%) of the mild cases respectively. Our study was different from the study in China that stated renal dysfunction occurred in 32 (7%) patients, who showed elevated blood urea nitrogen or serum creatinine levels.Citation42 Which was also true for other study in China that stated the prevalence of elevated BUN and elevated serum creatinine at admission was 6.29% and 5.22%, respectively,Citation47 study in Pakistan that showed creatinine was significantly increased in severe COVID-19 cases.Citation46 However, our study was in line with other study in China that showed serum creatinine was not abnormally elevated in all the patients, and BUN was abnormally elevated in only few (25.0%) of the patients, and other study that showed the abnormally elevated rates of serum creatinine and BUN are quite low.Citation6 Though it was different from the study that stated kidney function tests findings were significantly elevated in patients with both severe and fatal COVID-19,Citation39 we found a statistically significant mean Cr concentration difference between severe and mild cases (p=0.00). However, there was no statistically significant mean Cr concentration difference between severe and moderate cases (p= 0.24), moderate and mild (P=0.12); severe and moderate (P=0.54), severe and mild (p=0.46) and moderate and mild cases (P=0.74) for BUN concentration.
Na level seems slightly decreased for the severe and total tested cases, while most of the cases showed normal K level; 60.6% of the total tested, 60.7% of the severe, 50.0% of the moderate and a single mild cases respectively. Which was similar with the meta-analysis study findings that stated Na was significantly lower in patients with severe COVID-19; different from the study in China that stated severe low K level were 18%, low K level were 37%, and normal K level patients were 46% among COVID-19 cases,Citation48 which may be due to the sample size difference. It was also discordant with other study in which it stated K was significantly lower in COVID-19 patients with severe disease status,Citation49 study in Italy that showed majority of patients (90.7%) experienced a mild decrease in serum K level.Citation50 There was statistically significant mean Na concentration difference (p=0.04) between severe and moderate cases, however, there was no significant mean concentration difference for K (p=0.07) between severe and moderate cases.
There was no significant mean concentration difference for Cl between severe and moderate cases (p=0.17), which was similar with the study that stated for Cl, no statistical differences were observed between patients with severe and non-severe COVID-19.Citation49 However, most of the Ca tested cases shown increased level of the concentration. There was no significant mean concentration difference for Ca (p=0.98) between severe and moderate cases, which was discordant with the study that stated for calcium, a statistically significant lower concentration was noted in patients with severe COVID-19,Citation49 and study in China stated both mild/moderate and severe critical cases showed low calcium level in the early stage of viral infection.Citation51 This difference may be due to the sample size and socio-demographic factors difference.
Most of the severe and moderate, and total cases shown increased level of HDL and total cholesterol level, while most of the severe, and 50% of the moderate cases shown increased level of LDL and Triglycerides. These findings were different from the study in Pakistan that stated all parameters decreased gradually with COVID-19 disease severity (LDL, HDL, Triglycerides and total cholesterol),Citation46 and meta-analysis findings that stated significantly decreased levels of total cholesterol, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) in the severe group when compared with the non-severe group,Citation52 which may be due to the sample size and socio-demographic factors difference. There was no statistically significant mean concentration difference between severe and moderate cases for HDL, LDL, triglycerides and total cholesterol level (p=0.21, P=0.10, p=0.96 and p=0.17 respectively).
Limitation
Since our study was a retrospective study, we used secondary data, and low sample size, and we analyzed only baseline data. So, further study should be done with full follow up data.
Conclusion
We found increased number of Neutrophils in most of the COVID-19 cases, and the same result was found in most of the severe and moderate cases. There was statistically significant mean Neutrophil difference between severe and mild cases, even though there was no statistically significant mean Neutrophil difference between severe and moderate cases. The number of Lymphocytes was decreased in most of the severe and moderate cases, and there was no statistically significant mean Lymphocytes difference between different disease statuses. The number of WBCs in the total and severe cases was normal, and there was a significant mean number of WBC difference between severe and mild cases. Biomarkers like AST, ALT, and ALP showed increased in severe cases, and most of the bilirubin tested cases showed increased concentration. However, renal function tests were normal for all cases, and Na level seems slightly decreased for the severe and total cases, while most of the cases showed normal K level. The level of Ca concentration was increased in most of the cases though there was no significant mean concentration difference between severe and moderate cases. In conclusion, increased number of neutrophils and level of liver function tests; and decreased number of lymphocytes suggest higher inflammation and lymphopenia in severe cases. Therefore, patients with severe and critical disease status require close follow up.
Abbreviations
ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; BUN, blood urea nitrogen; Ca, calcium; Cl, chloride; COVID-19, coronavirus disease 2019; Cr, creatinine; Hct, hematocrit; HDL, high density lipoprotein; HIV, human immuno-virus; IRB, Institutional Review Board; K, potassium; LDH, lactate dehydrogenase; LDL, high density lipoprotein; Na, sodium; RBC, red blood cell; RT-PCR, real time polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SNNPR, Southern Nations, Nationalities, and Peoples Region; WBC, white blood cell.
Data Sharing Statement
The datasets used during this study are available from the corresponding author when required.
Ethics Approval
Ethical approval and waiver consent was obtained from the Institutional Review Board (IRB) of the Dilla University College of Medicine and Health sciences under the protocol unique number of duirb/002/21-10, and the permission from treatment center record room for data collection.
Consent
Not applicable as the study was retrospective.
Author Contributions
All authors made a significant contribution to the work, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest in relation to this work.
Acknowledgments
We would like to thank the Dilla University Referral Hospital COVID-19 treatment center and Patients’ record room staffs for their cooperation.
Additional information
Funding
References
- World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it. Braz J Implantol Health Sci. 2020;2(3):620–632.
- Abdool Karim SS, de Oliveira T, Loots G. Appropriate names for COVID-19 variants. Science. 2021;371(6535):1215.
- Parums DV. Revised World Health Organization (WHO) terminology for variants of concern and variants of interest of SARS-CoV-2. Med Sci Monit. 2021;27:e933622–1.
- Del Rio C, Omer SB, Malani PN. Winter of Omicron—the evolving COVID-19 pandemic. JAMA. 2022;327(4):319–320.
- Madhi SA, Kwatra G, Myers JE, et al. Population immunity and Covid-19 severity with Omicron variant in South Africa. N Engl J Med. 2022;386:1314–1326.
- Zhang X, Wu S, Wu B, et al. SARS-CoV-2 Omicron strain exhibits potent capabilities for immune evasion and viral entrance. Signal Transduct Target Ther. 2021;6(1):1–3.
- WHO report. Available from: https://www.who.int/health-topics/coronavirus#tab=tab_1. Accessed August 3, 2022.
- WHO report. Available from: https://covid19.who.int/. Accessed August 3, 2022.
- Alene KA, Gelaw YA, Fetene DM, et al. COVID-19 in Ethiopia: a geospatial analysis of vulnerability to infection, case severity and death. BMJ Open. 2021;11(2):e044606.
- Snider B, Patel B, McBean E. Asymptomatic cases, the hidden challenge in predicting COVID-19 caseload increases. Infect Dis Rep. 2021;13(2):340–347.
- Leulseged TW, Maru EH, Hassen IS, et al. Predictors of death in severe COVID-19 patients at millennium COVID-19 care center in Ethiopia: a case-control study. Pan Afr Med J. 2021;38:351.
- Aiyegbusi OL, Hughes SE, Turner G, et al. Symptoms, complications and management of long COVID: a review. J R Soc Med. 2021;114(9):428–442.
- Struyf T, Deeks JJ, Dinnes J, et al. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID‐19. Cochrane Database Syst Rev. 2021;(2). doi:10.1002/14651858.cd013665
- NIH COVID Treatment guidelines: clinical spectrum of SARS-CoV-2 infection. 2021.
- Health NIo. Clinical spectrum of SARS-CoV-2 infection. Retrieved April. 2021;12:2021.
- Efrati S, Catalogna M, Abu Hamed R, et al. Early and long term antibody kinetics of asymptomatic and mild disease COVID-19 patients. Sci Rep. 2021;11(1):1–9.
- Spellberg B, Nielsen TB, Casadevall A. Antibodies, immunity, and COVID-19. JAMA Intern Med. 2021;181(4):460–462.
- World Health Organization. COVID-19 Natural Immunity: Scientific Brief, 10 May 2021. World Health Organization; 2021.
- Chen Z, John Wherry E. T cell responses in patients with COVID-19. Nat Rev Immunol. 2020;20(9):529–536.
- Karlsson AC, Humbert M, Buggert M. The known unknowns of T cell immunity to COVID-19. Sci Immunol. 2020;5(53):eabe8063.
- WHO. What We Know About the COVID-19 Immune Response. WHO; 2020.
- Iwasaki A. What reinfections mean for COVID-19. Lancet Infect Dis. 2021;21(1):3–5.
- Prevost J, Finzi A. The great escape? SARS-CoV-2 variants evading neutralizing responses. Cell Host Microbe. 2021;29(3):322–324.
- Jabbari P, Rezaei N. With risk of reinfection, is COVID-19 here to stay? Disaster Med Public Health Prep. 2020;14(4):e33–e.
- West J, Everden S, Nikitas N. A case of COVID-19 reinfection in the UK. Clin Med (Northfield Il). 2021;21(1):e52.
- Benoni R, Campagna I, Panunzi S, et al. Estimating COVID-19 recovery time in a cohort of Italian healthcare workers who underwent surveillance swab testing. Public Health. 2021;196:52e8.
- An X-S, Li X-Y, Shang F-T, et al. Clinical characteristics and blood test results in COVID-19 patients. Ann Clin Lab Sci. 2020;50(3):299–307.
- Liu Y, Du X, Chen J, et al. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect. 2020;81(1):e6–e12.
- Tan L, Wang Q, Zhang D, et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5(1):1–3.
- Wang Y, Liu S, Liu H, et al. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73(4):807–816.
- Cotton DM, Liu L, Vinson DR, et al. Clinical characteristics of COVID‐19 patients evaluated in the emergency department: a retrospective cohort study of 801 cases. J Am Coll Emerg Physicians Open. 2021;2(4):e12538.
- Xie Y, Wang Z, Liao H, Marley G, Wu D, Tang W. Epidemiologic, clinical, and laboratory findings of the COVID-19 in the current pandemic: systematic review and meta-analysis. BMC Infect Dis. 2020;20(1):1–12.
- Middleton EA, He X-Y, Denorme F, et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136(10):1169–1179.
- Moradi EV, Teimouri A, Rezaee R, et al. Increased age, neutrophil-to-lymphocyte ratio (NLR) and white blood cells count are associated with higher COVID-19 mortality. Am J Emerg Med. 2021;40:11–14.
- Stegeman I, Ochodo EA, Guleid F, et al. Routine laboratory testing to determine if a patient has COVID‐19. Cochrane Database Syst Rev. 2020;(11). doi:10.1002/14651858.CD013787
- Wang J, Li Q, Yin Y, et al. Excessive neutrophils and neutrophil extracellular traps in COVID-19. Front Immunol. 2020;2020:2063.
- Zhang H-J, Qi G-Q, Gu X, et al. Lymphocyte blood levels that remain low can predict the death of patients with COVID-19. Medicine. 2021;100(28):e26503.
- Wu W, Wang A, Liu M. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506.
- Henry BM, De Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58(7):1021–1028.
- Soraya GV, Ulhaq ZS. Crucial laboratory parameters in COVID-19 diagnosis and prognosis: an updated meta-analysis. Med Clin (Barc). 2020;155(4):143–151.
- Zong X, Gu Y, Yu H, Li Z, Wang Y. Thrombocytopenia is associated with COVID-19 severity and outcome: an updated meta-analysis of 5637 patients with multiple outcomes. Lab Med. 2021;52(1):10–15.
- Wang L, Cheng X, Dong Q, et al. The characteristics of laboratory tests at admission and the risk factors for adverse clinical outcomes of severe and critical COVID-19 patients. BMC Infect Dis. 2021;21(1):1–8.
- Layla KN, Yeasmin S, Azad AB, et al. Red blood cell profile in patients with mild, moderate and severe COVID-19. IMC J Med Sci. 2021;15(2):26–31.
- Zhao X, Lei Z, Gao F, Xie Q, Jang K, Gong J. The impact of coronavirus disease 2019 (COVID-19) on liver injury in China: a systematic review and meta-analysis. Medicine. 2021;100(4). doi:10.1101/2020.05.03.20089557
- Hundt MA, Deng Y, Ciarleglio MM, Nathanson MH, Lim JK. Abnormal liver tests in COVID‐19: a retrospective observational cohort study of 1827 patients in a major US hospital network. Hepatology. 2020;72(4):1169–1176.
- Malik J, Ishaq U, Laique T, et al. Effect of COVID-19 on lipid profile and its correlation with acute phase reactants. medRxiv. 2021. doi:10.1101/2021.04.13.21255142
- Liu Y-M, Xie J, Chen -M-M, et al. Kidney function indicators predict adverse outcomes of COVID-19. Med. 2021;2(1):38–48. e2.
- Chen D, Li X, Song Q, et al. Assessment of hypokalemia and clinical characteristics in patients with coronavirus disease 2019 in Wenzhou, China. JAMA Ntwk Open. 2020;3(6):e2011122–e.
- Lippi G, South AM, Henry BM. Electrolyte imbalances in patients with severe coronavirus disease 2019 (COVID-19). Ann Clin Biochem. 2020;57(3):262–265.
- Alfano G, Ferrari A, Fontana F, et al. Hypokalemia in Patients with COVID-19. Clin Exp Nephrol. 2021;25(4):401–409.
- Zhou X, Chen D, Wang L, et al. Low serum calcium: a new, important indicator of COVID-19 patients from mild/moderate to severe/critical. Biosci Rep. 2020;40(12):BSR20202690.
- Mahat RK, Rathore V, Singh N, et al. Lipid profile as an indicator of COVID-19 severity: a systematic review and meta-analysis. Clin Nutr ESPEN. 2021;45:91–101.
