Abstract
Purpose
This research investigated the dynamics of antibiotic resistance in Salmonella and the epidemiology of Salmonella infection in children. These data can aid in the prevention and control of the Salmonella epidemic and the diagnosis and treatment of salmonellosis.
Methods
In this study, we retrospectively reviewed and analysed data regarding epidemiology, clinical symptoms, Salmonella serotypes, and antibiotic resistance from the medical records of patients with Salmonella infections in Hangzhou Children’s Hospital from April 2006 to December 2021.
Results
A total of 2099 Salmonella isolates were identified during the 16-year study period, and 98.6% (2069) of the isolates were isolated from stool. About 84.5% (1773/2099) of the total Salmonella isolates were detected from May to October. The median age of the 2099 children with Salmonella infection was 1.4 years (17 months) (IQR: 0.9–2.8 years). In 1572 (74.9%) patients, the course of the disease was limited to uncomplicated gastroenteritis. S. Typhimurium (805/2099, 38.4%) was predominant, followed by S. Enteritidis (290/2099, 13.8%). The total number of serotypes and the number of less common serotypes are increasing. Nontyphoid Salmonella that cause invasive infections, including S. Typhimurium, S. Stanley, and S. Choleraesuis, accounted for 60.0% (18/30). The Salmonella strains were resistant to ampicillin, ampicillin-sulbactam, trimethoprim-sulfamethoxazole, ceftriaxone, and ciprofloxacin at percentages of 71.5%, 51.5%, 36.5%, 22.4%, and 14.7%, respectively. No imipenem-resistant strains were identified. 24.8% of the isolates exhibited multidrug resistance (MDR).
Conclusion
S. typhimurium and S. enteritidis were the dominant serotypes in children (<2 years) with salmonella-infected arrhoea in Hangzhou, China. Ongoing serotype monitoring should be necessitated and dynamic changes in serotypes should be carefully examined to prevent the sudden outbreak of foodborne illness. Salmonella exhibits a higher rate of resistance to common antibiotics, and the risk of multidrug resistance should not be ignored. Therefore, clinicians should administer antibiotics judiciously according to the results of drug sensitivity tests.
Introduction
Salmonella, a gram-negative bacillus belonging to the Enterobacterales family, is a major foodborne pathogen worldwide. The genus Salmonella consists of only two species: S. enterica and S. bongori. Salmonella enterica is divided into the following six subspecies: S. enterica subsp. enterica, S. enterica subsp. salamae, S. enterica subsp. arizonae, S. enterica subsp. diarizonae, S. enterica subsp. houtenae and S. enterica subsp. indica. The White-Kauffmann-Le Minor scheme is used to serotype Salmonella isolates based on the identification of surface O (somatic) and H (flagellar) antigenic epitopes. Over 2600 serotypes have been reported; S. enterica subsp. enterica comprises more than 1500 serotypesCitation1,Citation2 and is responsible for more than 99% of human salmonellosis cases,Citation3 such as those caused by Salmonella Typhi, Salmonella Paratyphi, Salmonella Typhimurium, and Salmonella Enteritidis.
Salmonella isolates with different serotypes vary in pathogenicity. S. Typhi and S. Paratyphi, classified as typhoidal Salmonella, can cause typhoid and paratyphoid and are usually associated with higher mortality.Citation4 Other serotypes, known as nontyphoidal Salmonella (NTS), usually cause self-limited acute gastroenteritis; however, they can cause invasive infections in immunocompromised infants and young children with malnutrition.Citation5 The report entitled “WHO Estimates of the Global Burden of Foodborne Diseases” shows that in 2010, the primary cause of nondiarrhoeal foodborne disease resulting in death was Salmonella Typhi (52,000). Most deaths due to foodborne diarrhoeal disease agents were due to nontyphoidal S. enterica, accounting for 59,000 deaths, and included 22,000 deaths due to invasive disease caused by this bacterium.Citation6 In recent years, the incidence of invasive nontyphoidal Salmonella infections (iNTSs) has increased annually. The serotypes most commonly associated with iNTS are S. Typhimurium, S. Enteritidis, and S. Dublin, and the serotype with the highest degree of invasiveness was found to be S. Dublin.Citation7,Citation8 Therefore, knowledge regarding the distribution of Salmonella serotypes is helpful for physicians to better grasp a patient’s condition.
The current recommendations for antimicrobial therapy for Salmonella infections include extended-spectrum cephalosporins and fluoroquinolones, and third-generation cephalosporins are the drugs of choice for the treatment of severe salmonellosis in children. With the widespread use of antibiotics in various fields, resistance to cephalosporins and fluoroquinolones along with first-line antimicrobial agents is increasing at an alarming rate, and some studies have found that antimicrobial-resistant NTS is associated with an increased risk of invasive illness or death.Citation9,Citation10 Therefore, surveillance of Salmonella antimicrobial resistance is important for physicians to formulate effective therapeutic plans to reduce the occurrence of complications.
In this study, we review the epidemiology of Salmonella infection, distribution of Salmonella serotypes and antimicrobial resistance of Salmonella among children infected with Salmonella from April 2006 to December 2021 to provide a basis for designing prevention and control strategies and reducing the incidence of infection by antimicrobial-resistant Salmonella.
Materials and Methods
Source of Data
From April 2006 to December 2021, patients aged younger than 18 years and culture positive for Salmonella (repeated sampling was excluded) were identified from the records of the clinical microbiology laboratory at Hangzhou Children’s Hospital. The available information on age, sex, specimen source, clinical manifestations, Salmonella serotypes, and antimicrobial susceptibility was retrospectively reviewed and analysed. The calculation of age was based on the days from the date of birth to the date of presentation to the hospital divided by 365. In addition, the number of children who underwent stool culture for Salmonella during 2006–2021 was counted, repeated sampling was excluded, and the Salmonella isolation rate from stool was calculated.
Culture, Identification and Antibiotic Susceptibility Testing
Fresh faecal samples were inoculated on SS agar plates at 35 °C for 18–48 h, and suspicious colonies were inoculated on Kligler iron agar and chromogenic agar for biochemical identification. Whole blood (1 mL to 5mL) for bacterial culture was collected and inoculated into a paediatric bottle (BACT/ALERT PF, BioMérieux, France), and the culture was incubated in an automatic blood culture system (BACT/ALERT 3D) for 5 days or until it was rated positive. A colony from the positive blood culture was inoculated on blood agar and incubated at 36 °C for 18–24 h. Pus samples were cultured directly on blood agar at 36 °C for 18–48 h.
The identification and antibiotic susceptibility testing of Salmonella was performed using the VITEK 2 COMPACT automatic analysis system (BioMérieux, France), employing the GN and AST GN13 panels (BioMérieux, France), respectively. The susceptibility breakpoints that were used were those recommended by the Clinical and Laboratory Standards Institute (CLSI) guidelines for isolates subsequently obtained. The breakpoint criteria that were set in the analysis system were used to interpret the AST results, and the breakpoint criteria were updated in a timely manner when the CLSI guidelines changed. Standard strains (ATCC700323, ATCC25922, ATCC700327, and ATCC29213) were used for microbiological quality control. Isolates identified as the genus Salmonella were further investigated to detect the somatic (O) antigen and flagellar (H) antigen by slide agglutination with commercial antisera (Ningbo Tianrun Bio-Pharmaceutical Co., Ltd., Ningbo, China), and the serotype was determined according to the White-Kauffmann-Le Minor scheme. Normal saline was used as a control.
Definitions
We defined “non-invasive” cases as those in which Salmonella had been isolated from stool specimens and “invasive” cases as those in which Salmonella had been isolated from specimens such as blood, cerebrospinal fluid, other normally sterile fluids, pus and urine, which from other extraintestinal sites.
Multidrug resistance (MDR) was defined as resistance to three or more antibacterial classes, such as aminopenicillins (ampicillin), β-lactam combination agents (ampicillin-sulbactam, piperacillin-tazobactam), cephalosporins (ceftriaxone, ceftazidime, cefepime), monobactams (aztreonam), carbapenems (imipenem), dihydrofolate reductase inhibitors (trimethoprim-sulfamethoxazole), and fluoroquinolones (ciprofloxacin, levofloxacin).
Statistical Analysis
All data were entered into Microsoft Excel. SPSS 22.0 software was used for the statistical analysis. A chi-square test or Fisher’s exact test was used to analyse categorical data, and the multiple comparisons for percentages were verified based on Bonferroni methodology. Results with a p value of < 0.05 were considered statistically significant.
Results
Demographics, Monthly Prevalence and Clinical Presentation
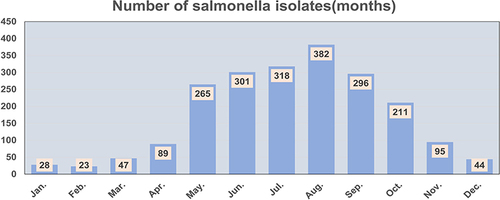
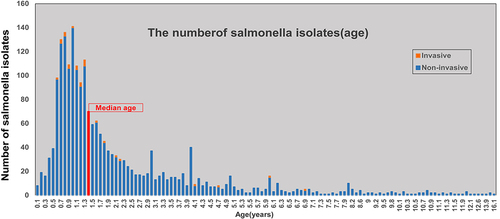
A total of 2099 Salmonella isolates were identified from April 2006, to December 2021; 2069 (98.6%) isolates were isolated from stool, 28 (1.3%) from blood and 2 (0.1%) from pus samples. The ward-to-outpatient ratio was 1:2.1 (ward, 667; outpatient, 1432). Salmonella isolation rates exhibited a 1.3:1 ratio for males to females (males, 1196; females, 903). The median age of the 2099 children with Salmonella infection was 1.4 years (17 months) (IQR: 0.9–2.8 years; range: 17 days-16.1 years). The number of Salmonella isolates detected increased with age before 1 year and decreased after 1 year. A total of 1400 children (66.7%) were < 2 years old, of whom 66.4% (929/1400) were 0.6–1.3 years (7–16 months) old. Invasive Salmonella infection was most common in children under 1.3 years (16 months) old (73.3%, 22/30) (). The number of Salmonella isolates detected was highest in August, followed by July, June, September, May and October, accounting for 84.5% (1773/2099) of the total ().
Figure 1 The number of Salmonella isolates from patients of different ages. The bar chart shows the number of Salmonella infection cases and the invasive or non-invasive status of infections among various age groups of patients in Hangzhou from April 2006 to December 2021. Red bar: The median age.
The disease course in 1572 (74.9%) patients was limited to uncomplicated gastroenteritis. A total of 10.1% (213/2099) of patients exhibited sepsis. Respiratory infections and convulsions were noted in 7.7% (162/2099) and 1.1% (23/2099) of patients, respectively. We compared the clinical presentation of invasive infection cases with that of non-invasive infection cases. The children with invasive infections were more likely to present with symptoms of respiratory infection and convulsions than the 2069 children with non-invasive infections ().
Table 1 Clinical Presentation of Children with Invasive Infections and Non-Invasive Infections
The Detection Rate of Salmonella Isolates from Stool
As 98.6% (2069/2099) of Salmonella isolates were detected in stool, an analysis of the annual Salmonella isolation rates from stool was performed. This analysis suggested that there was a sudden increase in the number of Salmonella isolates starting in 2014. The average detection rate from 2006 to 2013 was 3.77%, whereas that from 2014 to 2021 was 12.03%. The detection rates in the two periods were significantly different (χ2=464.796, P<0.001) ().
Table 2 The Annual Rates of Salmonella Isolation from Stool
Characteristics of Salmonella Serotype Distribution
Of the 2099 Salmonella isolates, 2070 were Salmonella enterica subsp. enterica (55 serotypes from 12 serogroups were identified), one was Salmonella enterica subsp. diarizonae and 28 were in the genus Salmonella but untyped. Group B (1248/2099, 59.46%) was the dominant serogroup, followed by Group D (427/2099, 20.34%).
The number of cases of Group B infection increased rapidly with age from 0.5 years (6 months) old and decreased from 0.8 years (10 months) old until 5 years old, which was followed by a plateau. The incidence of Group D infection was far lower than that of Group B before 2 years of age, but after 4 years of age, it was higher than that of Group B ( and ).
Table 3 Comparison of the Proportion of Group B and Group D and of S. Typhimurium and S. Enteritidis Isolates Before and After 4 Years of Age
Figure 3 The number of Salmonella Group B and Group D isolates by age in Hangzhou, China, from April 2006 to December 2021. The inset shows the number of S. Typhimurium and S. Enteritidis isolates by age.
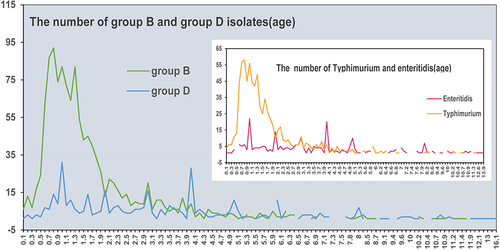
S. Typhimurium (805/2099, 38.4%) from Group B was the most prevalent serotype, and the proportion per year ranged from 18.7% (40/214) to 56.0% (75/134) between 2006 and 2021. S. Enteritidis (290/2099, 13.8%) from Group D, another common serotype, increased in prevalence significantly starting in 2013, and the average detection rate was 1.5% (3/202) from 2006 to 2012 (2 isolates in 2006 and 1 in 2012) and 15.1% (287/1897) from 2013 to 2021 (). The differences in distribution by age between S. Typhimurium and S. Enteritidis isolates were similar to those between Group B and Group D isolates ( and ).
Figure 4 Distribution of serogroups and serotypes by year. The different colours represent different serotypes. The figure shows the number of isolates.
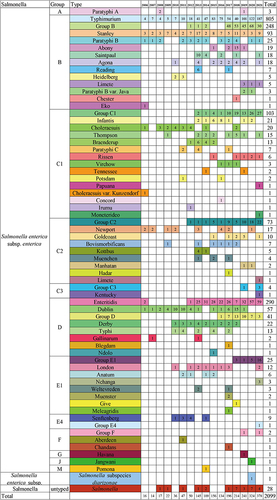
The rate of the detection of partially typed and untyped Salmonella isolates also increased; 25.0% (525/2099) of all Salmonella isolates were partially typed or untyped, and this percentage increased sharply starting in 2017, and the percentage ranged from 0–5.9% from 2006 to 2016 and rose to 28.3–46.7% from 2017 to 2021. The number of different kinds of serotypes increased, and less-common serotypes, such as S. Jangwani, S. Havana, S. Chandans, S. Meleagridis, S. Weltevreden, S. Aberdeen, S. Pomona, S. Irumu, S. Muenchen, S. Reading, S. Ndolo, S. Goldcoast, S. Concord, and S. Kentucky, were also identified ().
The Salmonella serogroups in 30 cases of invasive Salmonella infection were mainly Salmonella Group B (30.0%, 9/30) and Salmonella Group D (26.7%, 8/30). The Salmonella serotypes were dominated by NTS, including S. Typhimurium, S. Stanley, S. Choleraesuis, etc, which accounted for 60.0% (18/30); S. Typhi and S. Paratyphi accounted for 10.00% (3/30); and 30.00% (9/30) were untyped ().
Table 4 Distribution of Serogroups, Serotypes and NTS in Invasive Infection Cases
Antibiotic Susceptibility of Salmonella Isolates
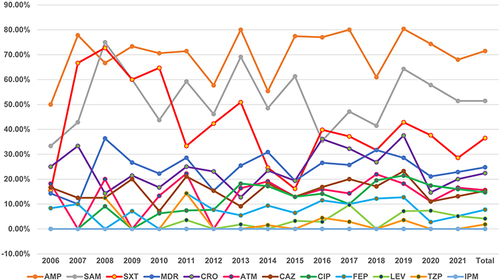
The antibiotic for which Salmonella had the highest rate of resistance was ampicillin (71.5%, 594/831), followed by ampicillin-sulbactam (51.5%, 425/826), trimethoprim-sulfamethoxazole (36.5%, 303/830) and ceftriaxone (22.4%, 155/692), and no imipenem-resistant isolates were identified. There was a rise in MDR and resistance to ampicillin, ampicillin-sulbactam, ceftriaxone, ceftazidime, cefepime, aztreonam, ciprofloxacin, and levofloxacin but a decline in resistance to trimethoprim-sulfamethoxazole and piperacillin-tazobactam from 2006 to 2021. Resistance to ciprofloxacin significantly increased in 2013, and the average rates of resistance to ciprofloxacin were 5.5% from 2009 to 2012 and 16.1% from 2013 to 2021 ().
Figure 5 Changes in antimicrobial resistance by year.
The antibiotic resistance profiles of the four main serotypes (Typhimurium, Enteritidis, Stanley, Dublin) were analysed and compared. We found that the four serotypes had the greatest rate of resistance to ampicillin, with resistance rates of 82.3%, 84.7%, 52.9%, and 87.5%, respectively, but the resistance rate of Stanley isolates was significantly lower than those of Typhimurium, Enteritidis, and Dublin isolates. The rates of ampicillin-sulbactam resistance were followed by the rates of ampicillin resistance in the four serotypes, which were 55.9%, 70.9%, 41.2%, and 54.2%, respectively; among them, the resistance rate of Enteritidis isolates was significantly higher than those of Typhimurium and Stanley isolates. The rates of resistance to trimethoprim-sulfamethoxazole in the Typhimurium, Stanley, and Dublin isolates were 44.4%, 34.3%, and 24.0%, respectively, while that in the Enteritidis isolates was 8.2%, which was significantly lower than those of Typhimurium and Stanley isolates. The rates of resistance to ciprofloxacin in the four strains were 19.6%, 1.8%, 8.6%, and 0.0%, respectively, and there were statistically significant differences between the rates in the Typhimurium and Enteritidis isolates. Compared to Enteritidis, Stanley, and Dublin, there were more Typhimurium isolates with MDR, but no significant difference was found among them ().
Table 5 The Antibiotic Resistance Profiles of Four Serotypes
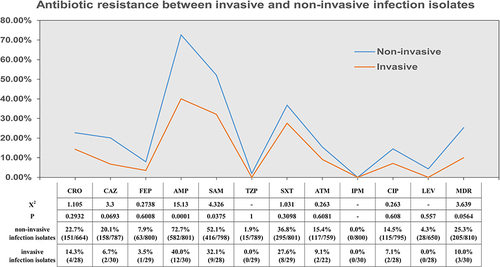
The number of Salmonella isolates with MDR and rates of resistance to ceftriaxone, ceftazidime, cefepime, ampicillin, ampicillin-sulbactam, piperacillin-tazobactam, trimethoprim-sulfamethoxazole, aztreonam, ciprofloxacin, and levofloxacin observed in non-invasive isolates were higher than those observed in invasive isolates, but there were only statistically significant differences for ampicillin and ampicillin-sulbactam resistance ().
Figure 6 Analysis of antibiotic resistance in invasive and non-invasive infection isolates.
Discussion
The current study was conducted over a period of fifteen years and nine months. Salmonella was detected in more than 2000 patients. Nearly one-third of the patients were admitted, as reflected by the ward-to-outpatient department ratio. Gastroenteritis was more common in children with non-invasive Salmonella infection, while respiratory infections and convulsions were more likely to be present in children with invasive infections. Nonspecific presentations, such as sepsis and respiratory symptoms, are frequently present in children; in such circumstances, health care workers may commence inappropriate treatment, which may result in delayed or ineffective antimicrobial treatment.Citation11,Citation12
Children under five years of age are at particular risk of developing Salmonella infection, especially when children are under 2 years of age.Citation7 The case fatality ratio among cohorts of children with iNTS disease across Africa has been reported to be 20 to 28% and is highest among children <2 years of age.Citation12 The majority of Salmonella infections in this study were observed in infants aged <2 years and were concentrated in those aged 7–16 months, with the peak number of infections occurring in those aged 1 year. Invasive Salmonella infection is more common in infants under 16 months of age. Potential reasons for increased susceptibility to Salmonella infections include numerous factors, such as gastric hypoacidity, immature endogenous bowel flora, immature gut-associated lymphoid tissue or a lack of serogroup-specific maternal antibodies; other factors include switching from breast feeding to bottle feeding and adding supplementary food, such as meat and eggs, to the diet in addition to crawling habits and the tendency of children to place their fingers or fomites inside the mouth.Citation13,Citation14
Consistent with the results of other reports,Citation14–18 the proportion of boys infected with Salmonella was found to be higher than that of girls, and the majority of Salmonella cases occurred in summer and autumn. There is no definitive reason for this phenomenon. One of the possible reasons why boys are more likely to be exposed to pathogens than girls is because they are more active. The association between season and infection is biologically plausible because Salmonella growth occurs between 5.2 and 46.2 °C and within an ideal temperature range of 35–43 °C.Citation19 In the summer, the hot and humid climate facilitates microbial proliferation, so food is more vulnerable to bacterial contamination. In addition, people prefer to eat raw and cold food in summer. All of these factors may increase the risk of Salmonella infection.
In our study, we found that the detection rate of Salmonella in stool increased sharply in 2013, and the annual detection rate was similar through 2021 and much higher than that from 2006 to 2012. In patients with Salmonella infection, stool testing typically demonstrates the presence of leucocytes.Citation20 Since 2013, physicians in our hospital have referred to the results of stool testing to determine whether it is necessary to perform stool culture. Therefore, the detection rate was greatly improved.
The dynamics of the Salmonella Group B and Group D isolation rates among different age groups of patients were similar to those observed in a previous report.Citation11,Citation21 In our study, children younger than 4 years were more likely to have Group B strain infection than older children were, and infections caused by Group D strains were more common than those caused by Group B strains in patients older than 4 years. Furthermore, S. Typhimurium (Group B) and S. Enteritidis (Group D) infections showed the same trend, which was similar to the result of another previous study.Citation22 However, S. Typhimurium remains the most prevalent serotype, and its prevalence rises annually; many reports are in concordance with our findings, such as those in Ningbo, Zhejiang, China,Citation23 southern India,Citation24 Africa,Citation25 Australia,Citation16 Thailand and Laos provinces.Citation26 However, studies from Shanghai, China,Citation27 and the United StatesCitation28 reported that S. Enteritidis was more common than S. Typhimurium. A study from Kolkata, India, showed that S. Guorthington was the predominant serotype, followed by S. Enteritidis and S. Typhimurium. The variation in serotype distribution in a region may be associated with the presence of a local animal reservoir or food source.
In our report, S. Enteritidis incidence increased significantly starting in 2013, whereas there were only three isolates observed from 2006 to 2012. The reason for this increased incidence may be that some isolates were misidentified as other serotypes due to use of commercial antisera or other factors from 2006 to 2012.Citation29 The less common serotypes, such as S. GoldcoastCitation30 and S. Hadar,Citation31 have gradually increased in incidence in recent years. The most frequently isolated Salmonella serotypes in European countries, such as S. Java, S. Jangwani, S. Manhattan, and S. Pomona are associated with exotic pets,Citation32 which could be due to international travel, human migration, and food, animal feed, and livestock trade.Citation33 In addition, the number of partially typed and untyped Salmonella isolates also increased. This result may be due to the quality of commercial antisera, the lack of flagellar antigens in Salmonella isolates, and the increase in the incidence of less common serotypes.Citation29 To solve this problem, we took different measures, such as increasing the use of commercial antisera from different manufacturers, using soft agar plates to induce flagellum expression, and training staff members.
The rising prevalence of iNTS disease has become acutely apparent in recent decades, and the Salmonella serotypes most commonly associated with iNTS are S. Typhimurium, S. Enteritidis, and S. Blin.Citation7 In our study, the Salmonella serogroups in 30 cases of invasive Salmonella infection were mainly Group B and Group D. The Salmonella serotypes were dominated by NTS, but there was no dominant serotype in iNTS infection. We will continue to focus on these strains that develop invasive infections and study them in-depth by genome sequencing methods.
The results of our report revealed higher rates of antimicrobial resistance to conventional antibiotics (ampicillin, ampicillin-sulbactam and trimethoprim-sulfamethoxazole), which were similar to the rates observed in other regions of ChinaCitation15,Citation22,Citation23,Citation34–36 but higher than those in Vietnam,Citation37 Kolkata,Citation14 the United States and Europe,Citation38 and Addis Ababa.Citation39 Due to the high rate of resistance, first-line antibiotics are no longer used for the treatment of Salmonella infections,Citation12 which may be related to the declining trend of antimicrobial resistance to trimethoprim-sulfamethoxazole that was observed in our study.
Third-generation cephalosporins are the standard antimicrobial option for invasive Salmonella infections in children. A worrisome development in children is the increased resistance to third-generation cephalosporins in China. A report showed that the rate of resistance to ceftriaxone has increased from 9.1% to 20.4% in recent years.Citation40 Our study revealed that the rate of resistance to ceftriaxone was 22.4%. The highest rate of resistance to ceftriaxone was found to be 34.9% in nearby regions.Citation23 The main mechanisms underlying resistance to third-generation cephalosporins in Salmonella have been attributed to extended-spectrum β-lactamase (ESBL) genes obtained via horizontal transfer, and CTX-M enzymes include the most common ESBLs that are prevalent worldwide.Citation41 A previous study by our team showed that the rate of ESBL gene detection in our hospital was 16.38%, and CTX-M was the most common ESBL gene.Citation42
Our report suggested a continuous and slow increase in the rate of resistance to some antimicrobial agents, with only a significant increase in the rate of resistance to ciprofloxacin in 2013 because the CLSI lowered the ciprofloxacin susceptibility breakpoints in 2013, with the new MIC being applied only to Salmonella spp. From 2013 to 2016 in our hospital, the rate of drug resistance to ciprofloxacin was 16.1%; this rate was higher than those observed in other studies. Although fluoroquinolones are not recommended for use in children due to their adverse effects on cartilage,Citation43 the higher rate of resistance to ciprofloxacin reported among paediatric Salmonella isolates in China should not be ignored.Citation44 The increasing frequency of ciprofloxacin resistance has been associated with an increasing prevalence of plasmid-mediated quinolone resistance (PMQR), which provides only low-level resistance that, by itself, does not exceed the clinical breakpoint for susceptibility but nonetheless facilitates the selection of microbes with higher-level resistance and renders infections by pathogens containing PMQR harder to treat.Citation45 In addition, there is an interesting finding derived from the data shown in the figure displaying the changes in antimicrobial resistance by year (): the rates of resistance to all antibiotics in 2020 were lower than those in 2019, and whether this was related to the coronavirus disease 2019 (COVID-19) pandemic is unknown.
Carbapenems remain one of the last resorts for the treatment of severe Salmonella infections. Although all isolates were observed to be sensitive to imipenem in this study, NDM-1-producing Salmonella has been isolated from a child in China.Citation46 Continuous surveillance of resistance to carbapenems is necessary to preserve their efficacy in the treatment of NTS and other infections.
The resistance profiles differed among different serotypes. We compared the antibiotic resistance profiles of the four main serotypes (Typhimurium, Enteritidis, Stanley, and Dublin), and the results showed that S. Typhimurium had higher resistance to some antibiotics than other serotypes, whereas the serotypes showed no significant difference in resistance to ceftriaxone, which was similar to the results of previous studies.Citation15,Citation47
A study in the United States reported that blood isolates were more likely to be resistant to a first-line antimicrobial agent.Citation28 Therefore, we compared the antibiotic resistance of stool isolates and nonstool isolates. Non-invasive isolates were more abundant than invasive isolates, but there were no statistically significant differences except for in the rates of resistance to ampicillin and ampicillin-sulbactam. Although MDR/hyper pathogenic phenotypes have emerged, such as Salmonella enterica serotype Typhimurium phage type DT104 (DT104)Citation48 and Salmonella Typhimurium ST313,Citation49, the relationship between the virulence and antibiotic resistance of Salmonella is worth further study.
Our study showed that the number of Salmoenlla isolates with MDR increased from 14.3% in 2006 to 22.86% in 2021. It may not be possible to compare different regions because antibiotic use may vary, but the prevalence of isolates with MDR has steadily risen worldwide. Hence, the emergence of isolates with MDR and increasing resistance to important antibiotics suggest that prevention measures and ongoing surveillance of antibiotic resistance are needed to control infections.
Conclusion
In conclusion, the most common symptom of Salmonella infection was diarrhoea caused by nontyphoid Salmonella in children (mainly under two years old) in Hangzhou, China. Routine stool results obtained from children with diarrhoea are recommended to determine the need for Salmonella testing.
S. Typhimurium and S. Enteritidis were the dominant serotypes in children (<2 years) with salmonella-infected diarrhoea. Therefore, more care should be taken to ensure children’s food safety, especially during the summer and fall. Furthermore, the prevalence of less common serotypes has gradually increased in recent years, so we should continue to strengthen the surveillance of Salmonella serotypes and pay attention to the dynamic changes in serotypes to prevent the sudden outbreak of foodborne illness.
The antibiotic resistance rates of Salmonella to third-generation cephalosporins and fluoroquinolones showed an upwards trend. Ceftriaxone in particular, with a resistance rate of 22.4%, may not be suitable for empirical use until the sensitivity is established. In addition, the rate of MDR is more than 20%, suggesting that prevention measures and ongoing surveillance of antibiotic resistance are needed to control infections.
There were some shortcomings to this study. First, only one isolate typing method was used, and many isolates failed to be typed, which had some influence on the analysis of the results. Methods based on molecular typing techniques will be used in the next study. Second, not all isolates underwent antibiotic sensitivity testing, and there was no investigation of the mechanisms underlying resistance; this will be our next focus and direction of research.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the Declaration of Helsinki and obtained approval from the Medical Ethics Committee at Hangzhou Children’s Hospital, China. All isolates were the result of regular laboratory procedures, and no patient-identifiable information was acquired. Therefore, informed consent was not needed.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
The authors thank all patients and colleagues for helping to collect research data.
Additional information
Funding
References
- Patrick ADG, Weill FX. Antigenic formulae of the salmonella serovars. WHO Collaborating Center for Reference and Research on Salmonella; 2007.
- Issenhuth-Jeanjean S, Roggentin P, Mikoleit M, et al. Supplement 2008–2010 (no. 48) to the White-Kauffmann-Le Minor scheme. Res Microbiol. 2014;165(7):526–530. doi:10.1016/j.resmic.2014.07.004
- Lamas A, Miranda JM, Regal P, et al. A comprehensive review of non-enterica subspecies of Salmonella enterica. Microbiol Res. 2018;206:60–73. doi:10.1016/j.micres.2017.09.010
- Buckle GC, Walker CL, Black RE. Typhoid fever and paratyphoid fever: systematic review to estimate global morbidity and mortality for 2010. J Glob Health. 2012;2(1):010401. doi:10.7189/jogh.01.010401
- Uche IV, Maclennan CA, Saul A, Systematic A, Baker S. Review of the incidence, risk factors and case fatality rates of invasive nontyphoidal Salmonella (iNTS) disease in Africa (1966 to 2014). PLoS Negl Trop Dis. 2017;11(1):e0005118. doi:10.1371/journal.pntd.0005118
- World Health O. WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007–2015[M]. Geneva: World Health Organization; 2015.
- Balasubramanian R, Im J, Lee JS, et al. The global burden and epidemiology of invasive non-typhoidal Salmonella infections. Hum Vaccin Immunother. 2019;15(6):1421–1426. doi:10.1080/21645515.2018.1504717
- Mughini-Gras L, Pijnacker R, Duijster J, et al. Changing epidemiology of invasive non-typhoid Salmonella infection: a nationwide population-based registry study. Clin Microbiol Infect. 2020;26(7):941 e9–941 e14. doi:10.1016/j.cmi.2019.11.015
- Helms M, Simonsen J, Molbak K. Quinolone resistance is associated with increased risk of invasive illness or death during infection with Salmonella serotype Typhimurium. J Infect Dis. 2004;190(9):1652–1654. doi:10.1086/424570
- Varma JK, Molbak K, Barrett TJ, et al. Antimicrobial-resistant nontyphoidal Salmonella is associated with excess bloodstream infections and hospitalizations. J Infect Dis. 2005;191(4):554–561. doi:10.1086/427263
- Chen HM, Wang Y, Su LH, et al. Nontyphoid salmonella infection: microbiology, clinical features, and antimicrobial therapy. Pediatr Neonatol. 2013;54(3):147–152. doi:10.1016/j.pedneo.2013.01.010
- Crump JA, Sjolund-Karlsson M, Gordon MA, et al. Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive salmonella infections. Clin Microbiol Rev. 2015;28(4):901–937. doi:10.1128/CMR.00002-15
- Thompson CN, Phan VT, Le TP, et al. Epidemiological features and risk factors of Salmonella gastroenteritis in children resident in Ho Chi Minh City. Vietnam Epidemiol Infect. 2013;141(8):1604–1613. doi:10.1017/S0950268812002014
- Jain P, Chowdhury G, Samajpati S, et al. Characterization of non-typhoidal Salmonella isolates from children with acute gastroenteritis, Kolkata, India, during 2000–2016. Braz J Microbiol. 2020;51(2):613–627. doi:10.1007/s42770-019-00213-z
- Qu M, Lv B, Zhang X, et al. Prevalence and antibiotic resistance of bacterial pathogens isolated from childhood diarrhea in Beijing, China (2010–2014). Gut Pathog. 2016;8:31. doi:10.1186/s13099-016-0116-2
- Parisi A, Crump JA, Stafford R, et al. Increasing incidence of invasive nontyphoidal Salmonella infections in Queensland, Australia, 2007–2016. PLoS Negl Trop Dis. 2019;13(3):e0007187. doi:10.1371/journal.pntd.0007187
- Harb A, O’dea M, Hanan ZK, et al. Prevalence, risk factors and antimicrobial resistance of Salmonella diarrhoeal infection among children in Thi-Qar Governorate, Iraq. Iraq Epidemiol Infect. 2017;145(16):3486–3496. doi:10.1017/S0950268817002400
- Olsen SJ, Bishop R, Brenner FW, et al. The changing epidemiology of salmonella: trends in serotypes isolated from humans in the United States, 1987–1997. J Infect Dis. 2001;183(5):753–761. doi:10.1086/318832
- Milazzo A, Giles LC, Zhang Y, et al. Heatwaves differentially affect risk of Salmonella serotypes. J Infect. 2016;73(3):231–240. doi:10.1016/j.jinf.2016.04.034
- Wen SC, Best E, Non-typhoidal NC. Salmonella infections in children: review of literature and recommendations for management. J Paediatr Child Health. 2017;53(10):936–941. doi:10.1111/jpc.13585
- Lo HY, Lai FP, Yang YJ. Changes in epidemiology and antimicrobial susceptibility of nontyphoid Salmonella in children in southern Taiwan, 1997–2016. J Microbiol Immunol Infect. 2020;53(4):585–591. doi:10.1016/j.jmii.2018.06.004
- Liang Z, Ke B, Deng X, et al. Serotypes, seasonal trends, and antibiotic resistance of non-typhoidal Salmonella from human patients in Guangdong Province, China, 2009–2012. BMC Infect Dis. 2015;15:53. doi:10.1186/s12879-015-0784-4
- Ke Y, Lu W, Liu W, et al. Non-typhoidal Salmonella infections among children in a tertiary hospital in Ningbo, Zhejiang, China, 2012–2019. PLoS Negl Trop Dis. 2020;14(10):e0008732. doi:10.1371/journal.pntd.0008732
- Jacob JJ, Solaimalai D, Muthuirulandi Sethuvel DP, et al. A nineteen-year report of serotype and antimicrobial susceptibility of enteric non-typhoidal Salmonella from humans in Southern India: changing facades of taxonomy and resistance trend. Gut Pathog. 2020;12:49. doi:10.1186/s13099-020-00388-z
- Kariuki S, Revathi G, Kariuki N, et al. Invasive multidrug-resistant non-typhoidal Salmonella infections in Africa: zoonotic or anthroponotic transmission? J Med Microbiol. 2006;55(Pt 5):585–591. doi:10.1099/jmm.0.46375-0
- Sinwat N, Angkittitrakul S, Coulson KF, et al. High prevalence and molecular characteristics of multidrug-resistant Salmonella in pigs, pork and humans in Thailand and Laos provinces. J Med Microbiol. 2016;65(10):1182–1193. doi:10.1099/jmm.0.000339
- Ran L, Wu S, Gao Y, et al. Laboratory-based surveillance of nontyphoidal Salmonella infections in China. Foodborne Pathog Dis. 2011;8(8):921–927. doi:10.1089/fpd.2010.0827
- Angelo KM, Reynolds J, Karp BE, et al. Antimicrobial Resistance Among Nontyphoidal Salmonella Isolated From Blood in the United States, 2003–2013. J Infect Dis. 2016;214(10):1565–1570. doi:10.1093/infdis/jiw415
- Hendriksen RS, Mikoleit M, Carlson VP, et al. WHO Global Salm-Surv external quality assurance system for serotyping of Salmonella isolates from 2000 to 2007. J Clin Microbiol. 2009;47(9):2729–2736. doi:10.1128/JCM.02437-08
- Scavia G, Ciaravino G, Luzzi I, et al. A multistate epidemic outbreak of Salmonella Goldcoast infection in humans, June 2009 to March 2010: the investigation in Italy. Euro Surveill. 2013;18(11):20424. doi:10.2807/ese.18.11.20424-en
- Deshpande A, Curran ET, Jamdar S, et al. Historical outbreak of Salmonella hadar. J Hosp Infect. 2015;91(2):171–175. doi:10.1016/j.jhin.2015.05.014
- Woodward DL, Khakhria R, Johnson WM. Human salmonellosis associated with exotic pets. J Clin Microbiol. 1997;35(11):2786–2790. doi:10.1128/jcm.35.11.2786-2790.1997
- Hendriksen RS, Vieira AR, Karlsmose S, et al. Global monitoring of Salmonella serovar distribution from the World Health Organization Global Foodborne Infections Network Country Data Bank: results of quality assured laboratories from 2001 to 2007. Foodborne Pathog Dis. 2011;8(8):887–900. doi:10.1089/fpd.2010.0787
- Li Y, Xie X, Xu X, et al. Nontyphoidal salmonella infection in children with acute gastroenteritis: prevalence, serotypes, and antimicrobial resistance in Shanghai. China Foodborne Pathog Dis. 2014;11(3):200–206. doi:10.1089/fpd.2013.1629
- Tian L, Zhu X, Chen Z, et al. Characteristics of bacterial pathogens associated with acute diarrhea in children under 5 years of age: a hospital-based cross-sectional study. BMC Infect Dis. 2016;16:253. doi:10.1186/s12879-016-1603-2
- Shen H, Chen H, Ou Y, et al. Prevalence, serotypes, and antimicrobial resistance of Salmonella isolates from patients with diarrhea in Shenzhen. China BMC Microbiol. 2020;20(1):197. doi:10.1186/s12866-020-01886-5
- Parisi A, Le Thi Phuong T, Mather AE, et al. Differential antimicrobial susceptibility profiles between symptomatic and asymptomatic non-typhoidal Salmonella infections in Vietnamese children. Epidemiol Infect. 2020;148:e144. doi:10.1017/S0950268820001168
- Michael GB, Schwarz S. Antimicrobial resistance in zoonotic nontyphoidal Salmonella: an alarming trend? Clin Microbiol Infect. 2016;22(12):968–974. doi:10.1016/j.cmi.2016.07.033
- Kebede R, Alemayehu H, Medhin G, et al. Nontyphoidal Salmonella and their antimicrobial susceptibility among Diarrheic Patients Attending Private Hospitals in Addis Ababa, Ethiopia. Biomed Res Int. 2021;2021:6177741. doi:10.1155/2021/6177741
- Guogang L, Jiajun L, Sheng Z. Epidemiological investigation and drug resistance analysis of children salmonella infection in Dongyang region. China Mod Doctor. 2018;2018(06):135–137+141.
- Guo L, Zhao Y. Global spread and molecular characterization of CTX-M-producing Salmonella typhimurium isolates. Antibiotics. 2021;10(11). doi:10.3390/antibiotics10111417
- Meina Y, Xiaoyu L, Di L, et al. Discussion on the extended-spectrum beta-lactamases-producing genotypes and antimicrobial resistance patterns of Salmonella strains isolated from children in Hangzhou. Chin J Health Lab Tec. 2017;27(04):594–597.
- Patel K, Goldman JL. Safety Concerns Surrounding Quinolone Use in Children. J Clin Pharmacol. 2016;56(9):1060–1075. doi:10.1002/jcph.715
- Kaijie G, Junwen Y, Jing J, et al. Salmonella strains isolated from children’s Hospital affiliated to Zhengzhou University in 2015–2017. Chin J Nosocomiol. 2019;19(01):120–124.
- Jacoby GA, Strahilevitz J, Hooper DC, Tolmasky M, Alonso JC. Plasmid-mediated quinolone resistance. Microbiol Spectr. 2014;2(5):5. doi:10.1128/microbiolspec.PLAS-0006-2013
- Huang J, Deng S, Ren J, et al. Characterization of a blaNDM1harboring plasmid from a Salmonella enterica clinical isolate in China. Mol Med Rep. 2017;16(2):1087–1092. doi:10.3892/mmr.2017.6733
- Zhang H, Pan F, Zhao X, et al. Distribution and antimicrobial resistance of enteric pathogens in Chinese paediatric diarrhoea: a multicentre retrospective study, 2008–2013. Epidemiol Infect. 2015;143(12):2512–2519. doi:10.1017/S0950268814003756
- Rasmussen MA, Carlson SA, Franklin SK, et al. Exposure to rumen protozoa leads to enhancement of pathogenicity of and invasion by multiple-antibiotic-resistant salmonella enterica bearing SGI1. Infect Immun. 2005;73(8):4668–4675. doi:10.1128/IAI.73.8.4668-4675.2005
- Van Puyvelde S, Pickard D, Vandelannoote K, et al. An African Salmonella Typhimurium ST313 sublineage with extensive drug-resistance and signatures of host adaptation. Nat Commun. 2019;10(1):4280. doi:10.1038/s41467-019-11844-z