Abstract
We reported an HIV-naïve patient from a resource-limited area who was detected with multiple resistance sites associated with nucleoside reverse transcriptase inhibitors (NRTIs) and integrase strand transfer inhibitors (INSTIs) after the failure of the initial antiviral regimen dolutegravir/lamivudine (DTG/3TC) and subsequent Bictegravir/emtricitabine/tenofovir alafenamide (BIC/FTC/TAF). On May 8, 2021, a 53-year-old man was diagnosed with AIDS, Marneffei talaromycosis and fungal esophagitis, and was suspected of having tuberculosis (TB) in Guangxi, China. His baseline HIV RNA was 559,000 copies/mL and the CD4 count was 12 cells/µL, but resistance genotype testing was not performed. The patient remained immunosuppressed (CD4 count 3 cells/µL) after 12 weeks of initial antiviral treatment (ART) with DTG/3TC. After he was switched to BIC/FTC/TAF and started anti-TB treatment, the viral load (HIV RNA 163,200 copies/mL) was not effectively controlled, and there were multiple NRTIs drug-resistant mutations (D67N, K70R, M184V, T215V, K219Q) and INSTIs mutations (E138K, G140A, S147SG, Q148R). This suggested that in resource-limited areas, for HIV-naïve patients in advanced stages with active opportunistic infections, HIV RNA>500,000 copies/mL, and low CD4 count, baseline resistance testing and increased HIV RNA testing frequency should be recommended, DTG/3TC was not recommended as initiation, and opportunistic infections should be treated promptly. In addition, switching to other INSTIs was not recommended in the absence of resistance testing and ineffective use of DTG.
Introduction
In the 2020 International Antiviral Society-USA Panel (IAS-USA), 2020 European AIDS Clinical Society (EACS), 2021 US Department of Health and Human Services (DHHS) guidelines, and 2021 Chinese guidelines for the diagnosis and treatment of HIV/AIDS, dolutegravir/lamivudine (DTG/3TC) is the recommended first-line regimens in HIV-naïve patients,Citation1–4 but it is limited to use in patients with HIV RNA<500,000 copies/mL because of insufficient data. However, some inpatients with HIV RNA>500,000 copies/mL in the resource-limited areas also require 2 drug regimen for some reasons, such as multiple opportunistic infections or the use of other combination drugs or organ dysfunction. Therefore, multiple multicenter real-world studies were conducted in China to explore the effect of DTG/3TC on HIV-naïve patients with HIV RNA>500,000 copies/mL, and the results showed that the DTG/3TC could achieve a high viral suppression rate in these patients, even in patients with baseline HIV RNA>500,000 copies/mL and low CD4 countCitation5–7 (The first unit of this study also participated in the research of Dou et alCitation6).
The above studies suggested that DTG/3TC could be considered as a treatment regimen for HIV-naïve patients with HIV RNA>500,000 copies/mL. However, in a recent clinical application, we found that an HIV-naïve patient from a resource-limited area with high baseline HIV RNA, low CD4 count, and multiple opportunistic infections, whose HIV RNA was not effectively controlled after using DTG/3TC as the initial treatment regimen, and drug-resistant mutations were found at multiple sites related to NRTIs (D67N, K70R, M184V, T125V, K219Q) and INSTIs (E138K, G140A, S147SG, Q148R). This brought new challenges to the research and application of DTG/3TC.
Case Presentation
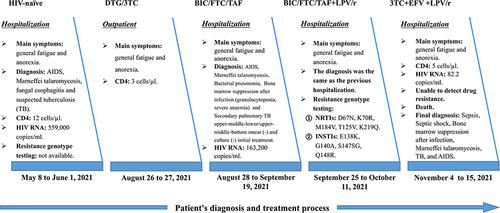
On 8 May 2021, a 53-year-old man was admitted to the hospital for treatment at the Fourth People’s Hospital of Nanning (Nanning, Guangxi, China) for Acquired Immunodeficiency Syndrome (AIDS), Marneffei talaromycosis and fungal esophagitis. The patient’s baseline HIV RNA was 559,000 copies/mL and the CD4 count was 12 cells/µL. Due to resource constraints, baseline resistance genotype testing was not performed. The patient self-reported that he had a history of prostitution more than 10 years ago, but had not had sex in the past five years, and denied a history of drug use and ART. In April 2021, he was suspected of having tuberculosis (TB) at the First Affiliated Hospital of Guangxi Medical University, but he had not been further diagnosed or received anti-TB treatment. During the hospitalization in our hospital, the chest imaging of the patient indicated that TB could not be excluded (atypical TB foci), but the sputum smear and culture were negative, and the sputum TB-DNA was also negative. Besides, after anti-fungal and bacterial treatment, the patient’s condition improved and the lung foci shrank, so it was still difficult to identify TB. For ART regimens, existing antiviral drugs in China include NRTIs (Tenofovir disoproxil (TDF), Zidovudine (AZT), 3TC), non-nucleoside reverse transcriptase inhibitors (NNRTIs) (Efavirenz (EFV), Nevirapine (NVP)), protease inhibitors (PIs) (Lopinavir/Ritonavir (LPV/r)), INSTIs (DTG, Raltegravir (RAL), BIC/FTC/TAF, DTG/3TC, Elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide fumarate (EVG/c/FTC/TAF)) and fusion inhibitors (FIs) (Albuvirtide (ABT)). Considering that the patient had a variety of opportunistic infections, TB could not be ruled out, the hemoglobin was 69g/L, and the clearance rate of endogenous creatinine was 41.93 mL/min, using TDF or AZT was not appropriate. If TB was later diagnosed, rifampicin was required, and rifampicin was contraindicated in combination with EVG/NVP/LPV/r/BIC. The patient’s current anti-fungal drugs (itraconazole) interact with EFV, NVP, and LPV/r. ABT is generally only used for special patients with drug resistance and inability to eat due to its high price. The only remaining treatment regimen, DTG/3TC, was shown to have high rates of viral suppression in HIV-naïve patients with HIV RNA>500,000 copies/mL in multiple multicenter real-world studies. It was also taken into account that despite the lack of baseline resistance data, no patients with treatment failure due to pretreatment integrase resistance were identified in Nanning. Therefore, DTG/3TC was selected as the initial treatment regimen for this patient.
The patient was discharged after treatment improved on 1 June 2021. After being discharged, he reported taking DTG/3TC regularly. However, at the end of July, he began to suffer from general fatigue and anorexia. When he returned to the hospital on 26 August for a follow-up visit, his CD4 count was 3 cells/µL. Considering that the patient had only received ART for 3 months, it was not a treatment failure, and the poor immune status might be due to a previous serious opportunistic infection that had not recovered CD4. However, considering the patient’s high baseline HIV RNA and low CD4, on 27 August, we adjusted the treatment regimen to BIC/FTC/TAF, which is less restrictive than DTG/3TC in the guidelines.
From 28 August to 19 September 2021, he was re-hospitalized due to general fatigue and anorexia, and was diagnosed as “1. Marneffei talaromycosis 2. AIDS 3. Bacterial pneumonia 4. Bone marrow suppression after infection (granulocytopenia, severe anaemia) 5. Secondary pulmonary TB upper-middle-lower/upper-middle-bottom smear (-) and culture (-) initial treatment.” On 17 September 2021, we found that the patient’s HIV RNA was 163,200 copies/mL, which was most likely caused by the failure of anti-HIV treatment or non-adherence or uncontrolled opportunistic infections. Therefore, he was switched to BIC/FTC/TAF + LPV/r. At the same time, after anti-fungal, anti-bacterial, anti-TB (isoniazid, ethambutol, pyrazinamide, and levofloxacin) and other symptomatic treatment, he was improved and was discharged.
From 25 September to 11 October 2021, he was hospitalized for a third time due to general fatigue and anorexia, with the same diagnosis as before. On 29 September, we sent the patient’s blood samples to Guangzhou Hailite Biotechnology Co., Ltd., China for HIV resistance genotype testing (Sanger sequencing. The testing fee was funded by the scientific research funds). The results showed that the virus type was CRF01-AE, and the NRTIs-related sites D67N, K70R, M184V, T125V, and K219Q were mutated, suggesting that the virus strain was resistant to abacavir (ABC), AZT, stavudine (d4T), didanosine (ddI), FTC, 3TC, and TDF. The INSTIs-related sites E138K, G140A, S147SG, and Q148R were also mutated, indicating that the virus strain was resistant to BIC, cabotegravir (CAB), DTG, EVG, and RAL. However, there was no drug resistance to PIs and NNRTIs (). According to the resistance test results, the ART regimen was adjusted to 3TC + EFV + LPV/r. For the choice of NRTIs, among the three drugs (TDF, AZT, 3TC) available in China, TDF and AZT were not appropriate considering that the patient’s endogenous creatinine clearance rate was 14.94 mL/min and hemoglobin was 44g/L. Although the patient had M184V mutation, 3TC might still have certain antiviral ability. After comprehensive consideration, we chose 3TC.
Table 1 HIV Drug Resistance Genotype Test Results
The patient was admitted to the hospital for a fourth time on 4 November 2021, due to general fatigue and anorexia. During the hospitalization, his CD4 count was 5 cells/µL and HIV RNA was 82.2 copies/mL; the drug resistance test could not be performed due to the low HIV RNA. The patient died on 15 November 2021, due to an aggravated opportunistic infection. The final diagnoses were Sepsis, Septic shock, Bone marrow suppression after infection, Marneffei talaromycosis, TB, and AIDS. Throughout treatment, the patient stated that he had taken antiviral drugs regularly, which was also confirmed by his family. The diagnosis and treatment process related to this patient was shown in .
Discussion
Because of resource constraints in this case’s region, baseline resistance testing is not available for all HIV-naïve patients, even high-risk patients. This is also a dilemma in many resource-constrained regions. In this case, we usually make decisions based more on existing references and clinical experience. In terms of the patient’s initial treatment regimen as reported in this case report. GEMINI 1 and GEMINI 2, two duplicate, Phase III, randomized, double-blind, multicentre, parallel-group, non-inferiority studies, showed that DTG/3TC was not inferior to the current standard three-drug therapy (DTG + TDF/FTC), and its safety was consistent with the results of previous studies.Citation8,Citation9 More importantly, the GEMINI study also showed that in PLHIV with a baseline HIV RNA>500,000 copies/mL, DTG/3TC and DTG+TDF/FTC had similar efficacy and no drug resistance occurred.Citation10 STAT, a phase IIIb, multicentre, open-label, single-arm study, showed favorable suppression rates with DTG/3TC as a rapid-start treatment regimen in patients with baseline HIV RNA>500,000 copies/mL.Citation11 Multiple multicenter real-world studies in southern China have also shown that DTG/3TC has a high viral inhibition rate in HIV-naïve patients with HIV RNA>500,000 copies/mL. Our hospital was involved in one of those studies. Another point was that, despite the lack of baseline resistance data, no patients in the Nanning were found to have failed treatment due to pretreatment integrate resistance. In this regard, combined with the patient’s condition and current resource conditions, we chose DTG/3TC as the initial treatment regimen for this patient.
After 12 weeks of regular ART, the patient remained in still a severely immunosuppressed state (CD4 count 3 cells/µL). We considered that the drug resistance barrier of DTG/3TC was unlikely to be breached in a short period. The patient’s poor immune status might be related to previous severe opportunistic infections. In addition, due to the limitations of DTG/3TC use in multiple guidelines, BIC/FTC/TAF with fewer limitations may be a more appropriate initial treatment regimen. After changing the patient’s ART regimen to BIC/FTC/TAF, the patient’s HIV RNA remained as high as 163,200 copies/mL. Combining the patient’s reported good adherence (not documented) during treatment and the patient’s presence of drug-resistant mutations related to NRTIs and INSTIs, where INSTIs mutation sites were high-level drug-resistant mutations related to DTG and BIC, it could be determined that the patient failed ART.
For the NRTIs mutation sites, the patient reported that he had not received ART before and had not used AZT or d4T. However, the mutation sites D67N, K70R, T215V, and K219Q related to these two antiviral drugs were found in the drug resistance test. These four mutation sites are thymidine analogue mutations (TAMs), and TAMs are closely related to M184V. The near-uniform development of M184V during most virological failures blunts the effects of the TAMs on AZT, d4T, and TDF susceptibility, but is associated with further reductions in susceptibility to ABC and ddI.Citation12 An increase in the number of TAMs is related to a gradual decrease in drug sensitivity of all NRTIs.Citation13 It has been seen that, if a patient develops M184V after receiving DTG/3TC treatment, the number of TAM-related mutations may decrease, but the result is that there are still multiple TAM-related mutation sites. This might suggest that the patient was likely to be an NRTIs transmissible drug resistance patient, that was, the patient had D67N, K70R, T215V, K219Q, and M184V before NRTIs-related drug treatment. The presence of baseline M184V might have weakened the role of 3TC in DTG/3TC or FTC in BIC/FTC/TAF. In addition, the patient had other high-risk factors for failure of DTG/3TC and BIC/FTC/TAF, such as delayed TB treatment, extreme immunodeficiency, high HIV RNA, and multiple coinfections at baseline. It might be the combination of the above factors that led to the resistance mutations associated with DTG and BIC. However, since the lack of HIV RNA and resistance results after DTG/3TC, we could not identify which integrase inhibitor was associated with the treatment failure that led to resistance.
Another point that should be considered is that E138K, G140A, Q148R are common resistance mutation sites for DTG, BIC, RAL, and EVG.Citation12 Therefore, It was highly likely that the patient concealed a previous treatment history of NRTIs (AZT or d4T) and INSTIs (RAL or EVG).
Conclusion
Globally, cases of INSTIs transmission resistance are still rare,Citation14–16 especially those with high levels of drug resistance at multiple sites. More importantly, we have seen that in Guangxi, China, resource constraints have affected the choice of treatment options for patients, resulting in patients failing to receive timely and effective ART. Therefore, for HIV-naïve patients in advanced stages with active opportunistic infections, HIV RNA>500,000 copies/mL, and low CD4 count, baseline resistance testing and increased HIV RNA testing frequency should be recommended, DTG/3TC was not recommended as initiation, and opportunistic infections should be treated promptly. In addition, switching to other INSTIs was not recommended in the absence of resistance testing and ineffective use of DTG.
Ethics Approval and Consent to Participate
Written informed consent was obtained from the patient and his family. The Ethics Committee of the Nanning Infectious Disease Hospital Affiliated to Guangxi Medical University & The Fourth People’s Hospital of Nanning approved this study.
Informed Consent for Publication
Written informed consent was obtained from the patient’s family for the publication. The patient’s family provided written informed consent to participate in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
Additional information
Funding
References
- Saag MS, Gandhi RT, Hoy JF., et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2020 recommendations of the international antiviral society-USA panel. JAMA. 2020;324(16):1651–1669. doi:10.1001/jama.2020.17025
- Society EAC. EACS guidelines (Version 11.1). Available from: https://www.eacsociety.org/media/final2021eacsguidelinesv11.0_oct2021.pdf. Accessed July 27, 2022.
- Services UDoHaH. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Available from: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/AdultandAdolescentGL.pdf. Accessed July 27, 2022.
- Aids, Hepatitis C Professional Group SoIDCMA, Chinese Center for Disease C. [Chinese guidelines for diagnosis and treatment of HIV/AIDS (2021 edition)]. Zhonghua Nei Ke Za Zhi. 2021;60(12):1106–1128. Chinese. doi:10.3760/cma.j.cn112138-20211006-00676
- Zhao F, Rao M, Chen W, et al. Dolutegravir plus lamivudine dual therapy in treatment naïve HIV-1 patients: preliminary data from real-world. Asia-Pacific AIDS & Co-Infections Conference (APACC); June 17–19; 2021; 29.
- Dou Y, Li Y, Hong Z, et al. The efficacy of DTG+3TC in naive HIV patients: the real world data from Southern China. 18th European AIDS Conference; October 27–30; 2021; PE2/19.
- Dou. Y, lv J, Su L, et al. Efficacy of DTG+3TC in naive HIV/AIDS patients with HIV RNA>500,000 copies/mL. Asia-Pacific AIDS & Co-Infections Conference (APACC); June 16–18; 2022; 64.
- Cahn P, Madero JS, Arribas J, et al. Durable efficacy of dolutegravir (DTG) plus lamivudine (3TC) in antiretroviral treatment-naïve adults with HIV-1 infection – 3-year results from the GEMINI studies. Presented at HIV Glasgow; 2020. Available from: https://www.gsk.com/en-gb/media/press-releases/viiv-healthcare-announces-dolutegravir-plus-lamivudine-three-year-data-confirming-long-term-viral-suppression/. Accessed July 27, 2022.
- Ait-Khaled M, Sierra Madero J, Estrada V, et al. Impact of treatment adherence on efficacy of dolutegravir plus lamivudine and dolutegravir plus tenofovir disoproxil fumarate/emtricitabine: pooled analysis of the GEMINI-1 and GEMINI-2 clinical studies. HIV Res Clin Pract. 2021;16:1–6.
- Van W, Man CY, Jörg S, et al. Durable efficacy of Two-Drug Regimen (2DR) of Dolutegravir (DTG) plus Lamivudine (3TC) in antiretroviral treatment-naïve adults with HIV-1 infection at 96 weeks: subgroup analyses in the GEMINI studies. Open Forum Infect Dis. 2019;6:45.
- Rolle CP, Berhe M, Singh T, et al. Dolutegravir/lamivudine as a first-line regimen in a test-and-treat setting for newly diagnosed people living with HIV. AIDS. 2021;35:1957–1965. doi:10.1097/QAD.0000000000002979
- Shafer RW. Rationale and uses of a public HIV drug-resistance database. J Infect Dis. 2006;Suppl 194(s1):S51–S58. doi:10.1086/505356
- Whitcomb JM, Parkin NT, Chappey C, et al. Broad nucleoside reverse-transcriptase inhibitor cross-resistance in human immunodeficiency virus type 1 clinical isolates. J Infect Dis. 2003;188(7):992–1000. doi:10.1086/378281
- Fokam J, Takou D, Semengue ENJ, et al. First case of Dolutegravir and Darunavir/r multi drug-resistant HIV-1 in Cameroon following exposure to Raltegravir: lessons and implications in the era of transition to Dolutegravir-based regimens. Antimicrob Resist Infect Control. 2020;9(1):143. doi:10.1186/s13756-020-00799-2
- Lai J, Liu Y, Han X, et al. Low frequency of integrase inhibitor resistance mutations among therapy-naïve HIV patients in Southeast China. Drug Des Devel Ther. 2021;15:889–894. doi:10.2147/DDDT.S286863
- Alvarez M, Casas P, de Salazar A, et al. Surveillance of transmitted drug resistance to integrase inhibitors in Spain: implications for clinical practice. J Antimicrob Chemother. 2019;74(6):1693–1700. doi:10.1093/jac/dkz067