Abstract
Long-term chemotherapy and immunosuppressants in acute myeloid leukaemia (AML) patients can result in a high risk of opportunistic infections. Rhizomucor pusillus is an opportunistic pathogen that exists in nature, but infection caused by R. pusillus is rare in the clinic. Notably, the sensitivity and detection time of conventional diagnostic tools for this fungus usually falls short of the needs of clinical diagnosis, resulting in treatment failure. Currently, metagenomics next-generation sequencing (mNGS) has played an important role in the detection of pathogens. Here, we report a case of R. pusillus pneumonia in a haematopoietic stem cell transplantation (HSCT) patient, detected by the mNGS method.
Background
Acute myeloid leukaemia (AML) is a common haematological disease that usually requires long-term chemotherapy and immunosuppression. Long-term chemotherapy and immunosuppression in AML patients can result in a high risk of opportunistic infections due to immune impairment, such as opportunistic fungal infections. Patients with AML are more likely to have mucormycotic infections than those with other haematologic malignancies.Citation1
Mucormycosis is the third most common invasive fungal disease, after aspergillosis and candidiasis, and Rhizomucor pusillus is one of its pathogensCitation2. The genus Rhizomucor exists in air, soil, water and decaying organic structures.Citation3 Infection caused by R. pusillus is rare in clinics but is often found in patients with immune deficiency, transplantation or underlying diseases.Citation4,Citation5 In addition, there are few effective antimicrobial agents for infections caused by this fungus. Amphotericin B is the drug of choice for initial therapy, while posaconazole and isavuconazole could be used for step-down therapy.
Notably, clinical symptoms, signs and imaging manifestations of R. pusillus are usually nonspecific, so its definitive diagnosis relies on a positive result of microorganism culture.Citation6 However, the sensitivity and detection time of conventional diagnostic tools usually do not meet the needs of clinical diagnosis, resulting in the treatment failure of the patients. In recent years, metagenomic next generation sequencing (mNGS) method has been widely recognized in clinic due to its rapid and sensitive advantages. It has played an important role in the detection of pathogens, especially in immunocompromised patients.
Here, we report a case of R. pusillus infection in a patient on long-term chemotherapy for AML who underwent HSCT. mNGS played an important role in the patient diagnosis.
Case Presentation
A 56-year-old male patient complaining of a high fever of 39 ℃ was diagnosed with acute myeloid leukaemia with karyotype 46 XY inv (p12q13) seven years ago in a local hospital. He subsequently received chemotherapy with idarubicin plus cytarabine (IA regimen, Idarubicin 8mg/m2/d from day 1 to day 3 and Cytarabine 100mg/m2/d from day 1 to day 7) over the past 7 years. With recurrent relapses due to suboptimal treatment, he was admitted to our hospital to receive a parental haploidentical HSCT in February 2021. At the same time, the patient was given prophylactic parental anti-infective treatment with venous transfusion of piperacillin tazobactam 4.5 g q8h.
Two months after HSCT, the patient developed frequent urination, urgent urination pain and haematuria. His body temperature was 36.9 ℃, his respiratory rate was 19 times/min, and his heart rate was 84 bpm. His white blood cell count (WBC) was 14.34×109/L, haemoglobin was 121 g/L and C-reactive protein (CRP) was 10.5 mg/L. Methylprednisolone 8 mg qd was given to prevent rejection, while intravenous (IV) levofloxacin 0.5 g qd with caspofungin (70 mg on day 1, then 50 mg once daily) was given to prevent infection. Pulmonary CT showed irregular nodules in the left upper lung (). Due to a positive test for cytomegalovirus DNA, the treatment regimen was switched to IV methylprednisolone (40 mg at 12-hour dosing intervals (q12h)) and foscarnet sodium (3 g q12h).
Figure 1 Pulmonary imaging manifestations of the patient. (A) Irregular nodules in the left upper lung. (B) Ground glass opacities and small nodules in the upper lobe of the right lung. (C) Diffuse ground glass opacities in both lungs. Lesions mentioned above were pointed by the green arrows.
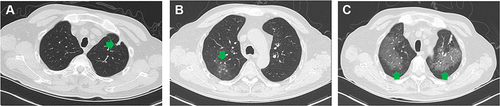
After two weeks of treatment, the highest body temperature of this patient was 38 ℃. The WBC was 2.74×109/L, and the CRP was 82 mg/L. Lung CT showed ground glass opacities and small nodules in the upper lobe of the right lung (). Galactomannan (GM test) and 1.3 3-β-D glucan (G test) were negative in serum samples. The treatment regimens were changed to IV meropenem (1g q8h) and oral posaconazole (200 mg tid). One week later, the patient developed white sticky sputum. Microscopic examination of the sputum by fluorescence staining and Gram staining showed fungal hyphae (). In the blood sample, sequences of the pathogenic fungus R. pusillus were detected by mNGS. And then the patient was started on IV amphotericin B 50 mg qd.
Figure 2 The morphology of R. pusillus. Hyphae forms of R. pusillus in sputum stained with Gram stain (A) Original magnification X 1000) and fluorescent stain (B), Original magnification X 400). R. pusillus colony grown in 28 ℃ PDA medium for 7 days. (C) Lactophenol cotton blue (LPCB) staining of the R. pusillus colony. (D), Original magnification X 400).
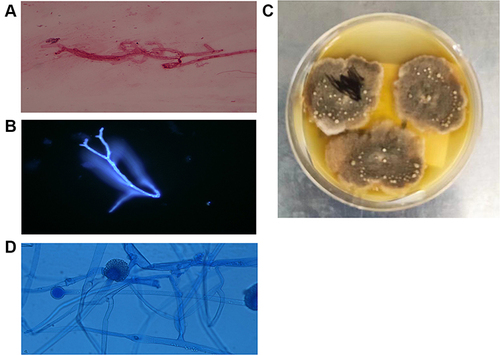
The subsequent sputum culture results revealed the presence of fungal growth 7 days after the positive result of mNGS. The fungus grew at 28 ℃ on SDA (Emmons’ modification) and on SDA with chloramphenicol, but not on Mycosel agar. The colony showed a brownish and woolly appearance (). Fungal morphology on staining with lactophenol cotton blue (LPCB) showed some stolon mycelia and globose sporangium terminating a branch (). The sporangium spores were spherical. The fungus was unsuccessfully identified by morphology and MALDI-TOF-MS (Sysmex-bioMérieux, Marcy l’Etoile, France). The mould was later identified as R. pusillus by PCR with primers (ITS1: 5′-TCCGTAGGTGAACCTGCGG-3′; ITS4: 5′-TCCTCCGCTTATTGATATGC-3′).
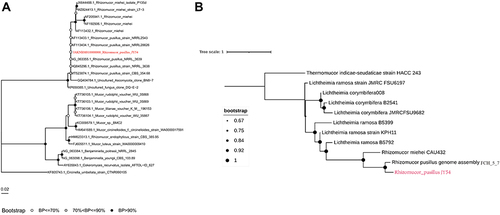
This isolate was subjected to whole-genome sequencing (WGS) using Illumina MiniSeq (https://www.illumina.com) for further characterization. WGS identified R. pusillus on the basis of 18S rRNA phylogenetic tree analysis performed using CLCbio (QIAGEN, https://www.qiagen.com), which showed that the microorganism clustered closely with strains R. pusillus () and very far from other Mucor species. To verify the results, we further performed single-nucleotide polymorphism phylogenetic tree analysis. This isolate was closely related to the reference genome R. pusillus FCH5.7 (GenBank accession no. FWWN00000000) according to Geneious (BioMatters, https://www.geneious.com) (), indicating that this rare fungus is R. pusillus.
Figure 3 Comparisons of the R. pusillus isolate and reference sequences. (A) All 18S rRNA reference sequences and genomes were obtained from the NCBI database. Muscle (V3.8.31) was used for multisequence comparison of 18S rRNA sequences. IQTree (V 1.6.12) was used to draw the evolutionary tree. (B) All reference genome sequences used were obtained from the NCBI database. Nucmer (V 3.1) was used to compare the whole-genome sequences, Mummer (V 3.23) was used to compare the large-fragment sequences in the genome, and FastTree (V 2.1.11) was used to draw the whole-genome phylogenetic tree after data integration. Finally, ITOL was used to visualize the figure.
Antifungal susceptibility testing was performed using a microdilution technique (CLSI M38-A2) (Clinical and Laboratory Standards Institute, 2008).Citation7 The results showed that this strain had high minimum inhibitory concentration (MIC) values in response to many antifungal drugs but had low MICs to amphotericin B and posaconazole ().
Table 1 Anti-Fungal Susceptibility of the Clinical Isolate R. pusillus JY54
One week later, the patient’s symptoms continued to worsen, with haemorrhagic sputum. Lung CT showed diffuse ground glass opacities in his lungs and suspected alveolar haemorrhage (). With the continuous deterioration of symptoms, the patient’s family decided to give up treatment, and the patient was discharged.
Discussion
Despite its low pathogenicity in humans compared to other agents of mucormycosis, R. pusillus infection has a high mortality rate (over 50%).Citation6 The clinical manifestations of pulmonary mucormycosis lack specificity and are characterized mainly by fever, dyspnoea, and chest pain.Citation8 When R. pusillus invades blood vessels, it is prone to form pulmonary embolism, pulmonary infarction or pulmonary aneurysm. It can lead to massive haemoptysis and even suffocation when it invades large blood vessels. Some studies on animals have revealed the pathophysiological processes of R. pusillus infection.Citation9 In this case, the patient had haemoptysis, which we suspected was related to the invasion of pulmonary blood vessels by R. pusillus. Currently, the combination of antifungal medication and surgery is the best treatment method. Most azoles are ineffective against mucormycosis, with the exception of posaconazole.Citation10 In this study, this strain had high MIC values in response to multiple azole drugs but had low MICs to amphotericin B and posaconazole, indicating that these two drugs can be treatment options for infections caused by R. pusillus. Of note, some evidence shows that posaconazole is effective in the treatment of mucormycosis,Citation11 but our patient still developed pulmonary infections with R. pusillus after prophylactic use, suggesting that there still seems to be a risk of treatment failure in immunodeficient patients. In addition, this patient was treated with caspofungin as an antifungal prophylaxis because candidiasis and aspergillosis are the most common invasive fungal infections after HSCT,Citation12 but R. pusillus can be selected for due to its resistance and can cause severe infections when caspofungin is used as a preventive treatment.Citation13 Our case further supports this result.
Notably, R. pusillus infection is progressive and rapidly invasive, leading to a short period from diagnosis to death.Citation14 Given the rapid progression of the disease, early diagnosis and timely antifungal treatment are crucial for the best outcomes. However, current diagnostic tools rarely meet the needs of clinical rapid diagnosis of R. pusillus infection. Among the traditional methods, direct microscopic examination of fungi is simple and is applicable to a variety of clinical samples. It can quickly find positive results, but it can also be false negative and has a low sensitivity. Special staining techniques, such as Grocott’s Hexamine-Silver Borate Stain, are complex and not easy to implement due to operational/technological complexity and the high level of personnel experience needed. Fungal culture is an important diagnostic method for fungal infection and is of great value in diagnosing infection and confirming pathogenic fungal species. The cultured fungi can also be used for drug sensitivity detection in vitro to guide clinical drug selection. The drawbacks are that fungal culture is time-consuming, has a low positive rate and cannot easily distinguish between colonization and infection. Moreover, the identification of the fungi after culture is also difficult. In this study, although we eventually cultured R. pusillus, it was unsuccessfully identified by morphology and MALDI-TOF-MS technology, which made it difficult to correctly treat.
In addition, serological antigen testing is suitable for the early diagnosis of invasive fungal infections and is a useful supplement to traditional mycological tests. Different serological examination methods have different clinical significance and applicable fungal infection types. In our case, the patient had negative G and GM tests. Serological detection methods of R. pusillus are limited, making this pathogenic fungus hard to find.
In recent years, mNGS has played an important role in the detection of pathogens, especially in immunocompromised patients.Citation15 In some difficult clinical cases that are not diagnosed by routine examination methods, mNGS tests can be performed when an infection cannot be ruled out clinically. However, mNGS results should be interpreted with caution because of the high rate of false-positive results due to contamination.Citation16,Citation17
In this case, R. pusillus was not identified by some traditional methods, but the sequences of R. pusillus were quickly detected by mNGS in blood samples of this patient, indicating that this method is much faster at identifying R. pusillus infection than traditional methods. Although mNGS is not currently the first-line diagnostic method, it still has some unique advantages in detecting pathogens, especially complicated coinfections as well as some infections that are difficult to cultureCitation18,Citation19. Thus, the mNGS method is expected to allow the early diagnosis of these invasive fungal infections.
Conclusions
Infection caused by R. pusillus is rare, but its high mortality poses a great threat to immunocompromised patients. It is important for clinicians to use multiple methods, such as mNGS, as soon as possible to detect pathogens to increase the chances of patient survival.
Nucleotide Sequence Accession No
The complete genome sequences of the R. pusillus JY54 isolate reported here have been deposited at DDBJ/ENA/GenBank under the accession no JAKNBM000000000. The version described in this paper is the first version JAKNBM000000000.
Ethics Approval
This study was conducted in accordance with the Declaration of Helsinki and had been reviewed and approved by the Research Ethics Committee of the Zhejiang Provincial People’s Hospital (QT2022038). The wife of this patient provided consent for publication of the clinical details, and written informed consent was obtained.
Disclosure
The authors declare that they have no conflicts of interest.
Acknowledgments
We thank doctor Lieming Bao for his technical assistance.
Additional information
Funding
References
- Noorifard M, Sekhavati E, Jalaei Khoo H, Hazraty I, Tabrizi R. Epidemiology and clinical manifestation of fungal infection related to Mucormycosis in hematologic malignancies. J Med Life. 2015;8(SpecIss 2):32–37.
- Toda Y, Nagai Y, Abe N, Hata T, Ohno HJT. Disseminated Rhizomucor pusillus infection in a patient with acute myeloid leukemia successfully treated with extensive surgical debridement and long-term liposomal amphotericin B. Tenri Med Bull. 2018;21(1):30–40. doi:10.12936/tenrikiyo.21-004
- Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13(2):236–301. doi:10.1128/CMR.13.2.236
- Kimura M, Udagawa S, Makimura K, Satoh K, Toyazaki N, Ito H. Isolation and identification of Rhizomucor pusillus from pl eural zygomycosis in an immunocompetent patient. Med Mycol. 2009;47(8):869–873. doi:10.3109/13693780903059485
- Hutter RV. Phycomycetous infection (mucormycosis) in cancer patients: a complication of therapy. Cancer. 1959;12(2):330–350. doi:10.1002/1097-0142(195903/04)12:2<330::AID-CNCR2820120217>3.0.CO;2-F
- Dien Bard J, Mangahis A, Hofstra TC, Bender JM. First case report of bloodstream infection by Rhizomucor pusillus in a child with hemophagocytic lymphohistiocytosis. Med Mycol Case Rep. 2014;5:20–23.
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard. 2nd ed. Wayne, PA: CLSI; 2008.
- Danion F, Aguilar C, Catherinot E, et al. Mucormycosis: new developments into a persistently devastating infection. Semin Respir Crit Care Med. 2015;36(5):692–705. doi:10.1055/s-0035-1562896
- Desmidt M, De Laender P, De Groote D, et al. Rhizomucor pusillus mucormycosis combined with chlamydiosis in an African grey parrot (Psittacus erithacus erithacus). Vet Rec. 1998;143(16):447–448. doi:10.1136/vr.143.16.447
- Garner D, Machin K. Investigation and management of an outbreak of mucormycosis in a paediatric oncology unit. J Hosp Infect. 2008;70(1):53–59. doi:10.1016/j.jhin.2008.05.017
- Clark NM, Grim SA, Lynch JP 3rd. Posaconazole: use in the prophylaxis and treatment of fungal infections. Semin Respir Crit Care Med. 2015;36(5):767–785. doi:10.1055/s-0035-1562902
- Morris SK, Allen UD, Gupta S, Richardson SE. Breakthrough filamentous fungal infections in pediatric hematopoetic stem cell transplant and oncology patients receiving caspofungin. Can J Infect Dis Med Microbiol. 2012;23(4):179–182. doi:10.1155/2012/957973
- Kivivuori SM, Karikoski R, Koukila-Kähkölä P, Anttila VJ, Saarinen-Pihkala UM. Zygomycosis presenting a major clinical challenge: case report on Rhizomucor pusillus infection in a stem-cell-transplant recipient. Mycopathologia. 2011;172(3):241–245. doi:10.1007/s11046-011-9424-8
- Freifeld AG, Iwen PC. Zygomycosis. Semin Respir Crit Care Med. 2004;25(2):221–231. doi:10.1055/s-2004-824905
- Zhou H, Larkin PMK, Zhao D, et al. Clinical impact of metagenomic next-generation sequencing of bronchoalveolar lavage in the diagnosis and management of pneumonia: a multicenter prospective observational study. J Mol Diagn. 2021;23(10):1259–1268. doi:10.1016/j.jmoldx.2021.06.007
- Gu W, Miller S, Chiu CYJ. Clinical metagenomic next-generation sequencing for pathogen detection. Annu Rev Pathol. 2019;14(1):1. doi:10.1146/annurev-pathmechdis-012418-012751
- Jing C, Chen H, Liang Y, et al. Clinical evaluation of an improved metagenomic next-generation sequencing test for the diagnosis of bloodstream infections. Clin Chem. 2021;67(8):1133–1143. doi:10.1093/clinchem/hvab061
- Wu WH, Hui TC, Wu QQ, et al. Pneumocystis jirovecii and Legionella pneumophila coinfection in a patient with diffuse large B-cell lymphoma: a case report. World J Clin Cases. 2021;9(28):8595–8601. doi:10.12998/wjcc.v9.i28.8595
- Wu W, Fan W, Zhou Z, et al. Liver abscess caused by Fusobacterium nucleatum: a case report. Jundishapur J Microbiol. 2021;14(4):e116591. doi:10.5812/jjm.116591
