Abstract
Background
Carbapenem-resistant Enterobacteriaceae (CRE) colonization is associated with bacterial translocation, which can result in subsequent endogenous CRE infection. In the present study, we aim to investigate the colonization-related risk factors and molecular epidemiological characteristics of CRE in patients with acute leukemia.
Methods
From January 2021 to December 2021, acute leukemia patients were screened for CRE by fecal/perianal swabs. We identified the species, carbapenemase-encoding genes, and virulence genes of the colonizing strains and performed antimicrobial susceptibility tests and ERIC-PCR typing. Risk factors for CRE colonization were identified by univariate and multivariate analysis.
Results
We collected a total of 21 colonizing strains from 320 patients. All strains were resistant to meropenem. Klebsiella pneumoniae was the most abundant species, and ERIC-PCR typing showed low diversity. Univariate analysis showed that age, cephalosporins, penicillins, tigecyclines, and hematopoietic stem cell transplantation status were risk factors for CRE colonization; simultaneously discovered CRE strains played a dominant role in invasive infection of colonized patients. Logistic multivariate regression analysis showed that age, cephalosporins, and tigecyclines were independent risk factors for CRE intestinal colonization.
Conclusion
CRE colonization can increase the incidence of CRE infection in patients with acute leukemia. Early detection of CRE colonization through CRE screening is an important measure to control the spread of CRE.
Introduction
Carbapenem-resistant Enterobacteriaceae (CRE) infection is a major challenge in the field of global public health due to the limited treatment options for CRE isolates, high morbidity, and mortality.Citation1 It has been reported that patients with CRE colonization have at least a two-fold increased risk of infection than non-CRE colonized patients,Citation2 and CRE colonization is also one of the main sources of CRE transmission in hospitals and communities.Citation3,Citation4 The Centers for Disease Control and Prevention, the European Centers for Disease Control, and the World Health Organization emphasize that medical institutions must implement interventions to prevent an epidemic of CRE colonization.Citation5
In high-risk populations, such as those with hematological tumors and organ transplants and critically ill patients, the proportion of intestinal CRE colonization increases significantly. Prior studies reported an 8.8% intestinal CRE colonization rate in patients with hematological tumors.Citation6 Patients diagnosed with malignant hematological diseases may be more likely to have intestinal bacterial colonization due to loss of congenital immune function, changes in the intestinal flora, use of broad-spectrum antibiotics, and chemotherapy.Citation7 The intestinal colonizing bacteria can migrate to various organs and tissues of the body, leading to a higher risk of invasive infection, with a mortality rate of about 60–100% in high-risk patients.Citation8
Acute leukemia is the most common blood disorder. Concerning this particular type of leukemia, existing studies are largely limited by the lack of control groups, heterogeneous patient populations, and health systems; furthermore, clinical data on risk factors for CRE colonization or infection are scarce, and existing studies are only studied in blood diseasesCitation9 which lack of specificity.
The aim of this study was to demonstrate the importance of active CRE surveillance in the hematology department and verify the correlation between CRE colonization and subsequent CRE infection. The study also aimed to identify the microbiological parameters of the colonizing strains.
Methods
Research Setting and Ethics Statement
This study was conducted in Fujian Medical University Union Hospital in Fuzhou, Fujian province, from January 2021 to December 2021. In the department of hematology, we initiated CRE screening by fecal/perianal swabs. During the study period, a total of 320 inpatients with acute leukemia were recruited. We collected all screenings for CRE-colonizing strains from patients and divided them into colonized and non-colonized groups. Informed consent was obtained from all participants.
Bacterial Isolates Collection and Identification
Fecal/perianal swabs were collected and screened for CRE with Mueller-Hinton Agar (Antu Bio, China). Cultured isolates were identified by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOFMS; Bruker Daltonics Inc., Billerica, Massachusetts), and carbapenem (meropenem or imipenem) antimicrobial susceptibility testing was performed by the disk diffusion method to confirm CRE. Enterobacteriaceae that were resistant to meropenem or imipenem were classified as CRE.Citation10
Antibiotic Susceptibility Testing
According to the Clinical and Laboratory Standards Institute (CLSI) guidelines,Citation11 we used the microbroth dilution method to determine the minimum inhibitory concentrations (MIC) of cefepime, ceftazidime, cefoperazone/sulbactam, imipenem, meropenem, tobramycin, amikacin, aztreonam, minocycline, ciprofloxacin, levofloxacin, piperacillin-tazobactam, compound sulfamethoxazole, and tigecycline. Except for tigecycline, all antibiotic susceptibilities were based on the CLSI document standard. Tigecycline susceptibility was based on the US Food and Drug Administration criteria (susceptible, MIC≤2 mg/L; resistant, MIC≥8 mg/L).Citation12 Escherichia coli ATCC25922 and Pseudomonas aeruginosa ATCC27853 were used as quality control standards.
Molecular Detection of Resistance and Virulence Genes
For Klebsiella pneumoniae and Escherichia coli identified as CRE, the production of carbapenemases was determined by the modified Hodge experiment.Citation13 Polymerase chain reaction (PCR) was used to detect five common carbapenem-coding genes, including blaKPC, blaIMP, blaVIM, blaNDM, and blaOXA-48.Citation14 The virulence factors detected included type K capsule-specific genes (including three liver abscess-associated capsule serotypes; K1, K2, and K57) rmpA, rmpA2, iroN, allS, mrkD, iucA, aerobactin, magA, wcaG, traT, ecpA, iucD, fimH, ompT, iutA, hylA, afaC, and papC. The positive products were sequenced, and the sequencing results were compared using the basic local alignment search tool (BLAST) available at http://www.ncbi.nlm.nih.gov/BLAST.
DNA Fingerprint Technology
ERIC-1 and ERIC-2 primers were used to conduct ERIC-PCR in CRE-positive K. pneumoniae and E. coli. The ERIC-PCR conditions were adjusted according to the report published by Smith et al.Citation15 DNA fingerprint was analyzed using GelCompar II, version 6.5 (Applied Mathematics, NV, Keistraat, Belgium). A cutoff value of 80% similarity was applied to define the cluster. The similarity among species was evaluated by band-matching Dice coefficient, and the tree map of each species was drawn by the unweighted pair grouping method with arithmetic mean (UPGMA). According to the cluster diagram of the UPGMA system, same strains are defined as strains with > 97% similarity, and strains with < 95% similarity are defined as unrelated strains.
Statistical Analysis
Data were analyzed using IBM SPSS ver. 21.0 statistical software (IBM Co., Armonk, NY, USA). Frequency tables (n, %) for categorical variables and descriptive statistics (mean, median, standard deviation) for numerical variables were used. Comparisons of categorical variables were analyzed by the Chi square test. Logistic regression (Backward LR) methods (univariate, multivariate) were used to determine the risk factors for CRE colonization. Statistical significance was assigned to a P value of less than 0.05.
Results
Clinical Characteristics of CRE-Colonized Patients
A total of 21 CRE colonizing strains isolated from the intestines were collected from 320 patients; thus, the incidence of CRE colonization was 6.56%. The most common strain was K. pneumoniae (71.43%), followed by E. coli (28.57%). Among the 21 patients with CRE colonization, three (14.29%) had invasive CRE infection; two had K. pneumoniae secondary infection (66.67%), of which one patient had an invasive infection by K. pneumoniae and Acinetobacter baumannii; and one had E. coli secondary infection (33.33%).
Patients were divided into two groups: (1) patients with CRE colonization (n= 21), including patients with positive CRE screening tests, and (2) control group (n=299), including patients with negative CRE screening tests in the same period. In the colonization group and the control group, the proportion of males (76.19% and 54.85%, respectively) was higher than females (3.20: 1 and 1.21:1, respectively), and the number of deaths in males (100% and 55.56%, respectively) was higher than that of females (1: 0 and 1.25: 1, respectively) ().
Table 1 Comparison of Demographic Factors Among CRE and Non-CRE Groups
Risk Factors for Colonization
Risk factors for colonization were determined by assessing the effects of all independent variables that showed statistically significant differences (p<0.05) in comparative analyses of colonized and control patients. This final multivariable model also showed significant predictors for each group. Univariate analysis showed that age, cephalosporins, penicillins, tigecyclines, and hematopoietic stem cell transplantation status were risk factors for CRE colonization. In multivariate analysis, age (odds ratio [OR] 0.212; 95% confidence interval [CI]:0.056–0.807; p= 0.023), use of cephalosporins (OR 2.205; 95% CI:1.099–4.424; p=0.026), and tigecyclines (OR 1.680; 95% CI:1.170–3.311; p=0.011) were identified as independent risk factors for colonization (). In logistic regression analysis, CRE strains dominated aggressive infections in colonized patients ().
Table 2 Univariate Analysis and Multivariate Logistic Regression Analysis of Risk Factors for CRE Colonization in Colonized versus Non-Colonized Patients
Table 3 Univariate Analysis of the Risk of Development of Invasive Clinical CRE Infection in the Sample
Antimicrobial Resistance Characteristics
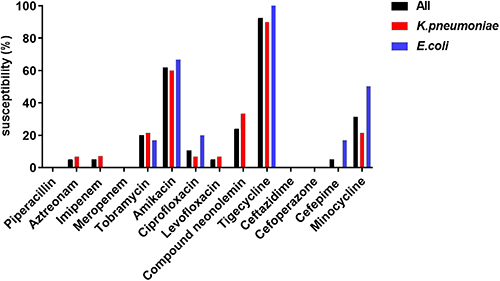
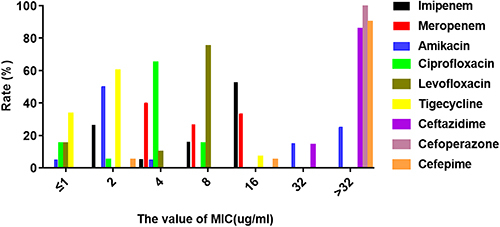
The results of drug susceptibility testing for CRE colonizing strains are shown in and . Overall, the sensitivity rate of CRE to tigecycline was the highest (93.21%), followed by amikacin (61.90%). Compared with K. pneumoniae, E. coli was more sensitive to minocycline (50.00% vs 21.43%), ciprofloxacin (20.00% vs 6.67%), and cefepime (16.67% vs 0).
Detection of Resistance and Virulence Genes
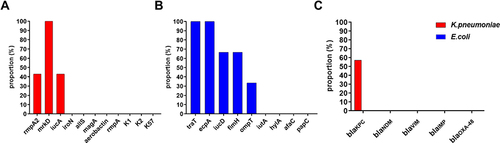
In K. pneumoniae, 57.14% of the isolates produced carbapenemases. BlaKPC was the only carbapenem resistance gene detected. The most common virulence factor was mrkD (100%), followed by rmpA2 (42.86%) and iucA (42.86%). In E. coli, the most common virulence factors were traT(100%) and ecpA(100%), followed by iucD (66.67%), fimH (66.67%), and ompT (33.33%). No carbapenem resistance genes were detected in blaKPC, blaIMP, blaVIM, blaNDM, and blaOXA-48 (). Patients with more virulence factors had a worse prognosis, with Eastern Cooperative Oncology Group (ECOG) scores in the range of 3–5, and most patients with fewer virulence factors had a better prognosis, with a score of about 2.
ERIC-PCR Typing of Colonizing Isolates
ERIC-PCR typing showed 8 and 4 fingerprint patterns for K. pneumoniae and E. coli strains, respectively. In K. pneumoniae, 77.78% of the isolates showed a separate ERIC profile, and 22.22% of the isolates had the same pattern. In E. coli, 60.00% of the isolates showed a separate ERIC profile, and 40.00% of the isolates had the same pattern.
Discussion
In this study, the colonization rate of CRE in patients with acute leukemia was 6.56%. Komurcu et al collected rectal swabs from 57 patients with neutropenia and found a CRE colonization rate of 8.8%.Citation6 Moreover, the literature shows a CRE colonization rate of 8.8% to 18.9% in long-term hospitalized patients and 28% in transplant patients.Citation16,Citation17 The lower intestinal CRE colonization rate found in this study could be explained by: (1) differences in CRE colonization rate in different regions; (2) inconsistency of disease range; and (3) differences in the site of detection.
Intestinal colonization of CRE at any stage is a risk factor for CRE infection.Citation18 It was reported that the incidence of infection increased in patients with CRE colonization, ranging from 7.6% to– 86.4%.Citation18–20 In this study, the infection rate was 14.29% in colonized patients and 2.68% in non-colonized patients, which was statistically significant by logistic regression analysis (p=0.012). In the colonization group, strains of CRE infection were mainly K. pneumoniae (66.67%, 2/3), followed by E. coli (33.33%, 1/3).
In the present study, we found decreased patient age, previous use of cephalosporins, and tigecyclines to be risk factors for CRE colonization among acute leukemia patients. Among the modifiable risk factors, the use of drugs is the most noteworthy. The reasons for this may be that: (1) cephalosporins and tigecyclines are often used as substitutes for carbapenem antibiotics, and the symbiotic gastrointestinal flora changes in the presence of broad-spectrum antibiotics, resulting in the selection of organisms with antibiotic resistance and (2) plasmids that cause carbapenem resistance usually carry additional genes that make them resistant to other antibiotics; therefore, the use of these antibiotics may be related to CRE carrier.Citation9 These findings highlight the importance of antimicrobial management interventions aimed at limiting the unnecessary use of multiple antibiotics, especially in high-risk patients, including those hospitalized in the hematology department. Future studies should further determine the potential modifiable risk factors for CRE and evaluate the role of cephalosporins and tigecyclines in the treatment of CRE infection in acute leukemia.
The vast majority of K. pneumoniae and E. coli strains were resistant to multiple antibiotics. The colonizing and infective strains showed similar drug resistance characteristics. In terms of antibiotics, previous studies have shown that the drugs effective in the treatment of CRE infection are tigecyclines and aminoglycosides.Citation21,Citation22 Correspondingly, the CRE strain isolated from fecal/perianal swabs in this study was highly sensitive to tigecycline and aminoglycosides and can be used as an effective drug for treatment combined with anti-infection.
We analyzed the microbiological parameters of colonizing strains to determine the characteristics of CRE-colonizing strains and their correlation with prognosis. K. pneumoniae was the most common strain of CRE colonizing the intestinal tract. Approximately half of the CRE-colonizing strains tested in our study do not seem to produce carbapenemases. The dominant carbapenemase group of K. pneumoniae strains was blaKPC. Compared with other carbapenem genes, blaKPC had greater virulence and transmission. The common carbapenem resistance genes of blaKPC, blaIMP, blaVIM, blaNDM, and blaOXA-48 were not detected in E. coli. In addition, this study tested virulence factors to explore the correlation between virulence factors and prognosis. It was found that compared with other patients with a lower virulence factor carrier rate, patients with more virulence factors had a worse prognosis, with an ECOG score in the range of 3–5.
Similar to previous studies, we also studied the clonal correlation between colonizing isolates, and 71.43% of the isolates showed a separate ERIC map. The uniqueness of ERIC patterns indicates non-clonal transmission. In contrast, 28.57% of the isolates had the same pattern, indicating that the sources of transmission were similar. Genetic analysis using ERIC-PCR showed that most colonizing strains had no genetic relationship in cloning, indicating that the transmission of these strains was not due to clonal outbreaks. The non-clonality between strains also correspondingly explains the low detection rates of blaKPC and blaNDM.
Human intestines are the storage place and source of infection for all kinds of drug-resistant bacteria and genes. Feces can pollute the surrounding environment and lead to the spread of drug-resistant bacteria. Thus, patients with CRE colonization are considered to be important hosts for horizontal transmission of carbapenem-resistant strains in hospital environments.Citation23 The medical team is gradually looking for multifaceted plans to control the spread of CRE in hospitals to achieve the best interventions.Citation24 This study examined the relevant data on colonization and infection rates and proved the importance of active CRE monitoring in the implementation of early preventive measures. We propose a significant point: CRE colonization and colonization infection are known risk factors that can be changed through the implementation of antibiotic allocation plans.
To understand the risk factors of intestinal CRE colonization in patients with acute leukemia, the significance of actively detecting intestinal CREs in high-risk populations is as follows: first, when intestinal CRE colonization develops into CRE infection, we can guide the clinical development of appropriate initial anti-infective treatment according to the drug resistance of the colonizing CRE and use sensitive antibiotics as soon as possible to reduce mortality; second, we can take timely prevention and control measures in patients with intestinal CRE colonization to avoid hospital transmission; third, active CRE screening is limited to CRE outbreaks or high-risk groups (such as close contact with CRE carriers and local epidemics), because active screening of CRE in non-epidemic areas consumes manpower and is difficult to implement widely. At present, most studies have focused on risk factor analysis for CRE infection, and the research on intestinal CRE colonization is mostly focused on patients with basic diseases, such as Intensive Care Unit (ICU); thus, there are few studies on the risk factors of intestinal CRE colonization in hematological diseases from home and abroad. Patients with hematological diseases are at high risk of intestinal CRE colonization. This study is a targeted study of patients with acute leukemia, a large group of blood diseases.
The limitations of this study are that it was a single-center study and was conducted only in hematological wards. Because the limitations of the patient population may lead to a lack of generalizability of the findings, the prevalence of CRE colonizing strains and risk factors may not be extended to other institutions or departments. In addition, due to the limitations of a retrospective study, we could not acquire the strains after infection to compare the microbiological characteristics of infective strains and colonizing strains.
Conclusion
In summary, implementing CRE screening may be an effective measure to reduce CRE infection and transmission, and better antibiotic treatment regimens can help reduce CRE colonization rates.
Ethics Approval and Informed Consent
All procedures of this study involving humans (individuals, medical records, human samples, clinical isolates and human cell lines) were reviewed and approved by the Medical Ethics Committee of Fujian Medical University Union Hospital (2022KY101). We confirm that this study was conducted in accordance with the Declaration of Helsinki.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgment
The authors would like to thank those who participated in this study.
References
- Borer A, Saidel-Odes L, Riesenberg K, et al. Attributable mortality rate for carbapenem-resistant Klebsiella pneumoniae bacteremia. Infect Control Hosp Epidemiol. 2009;30(10):972–976. doi:10.1086/605922
- Dickstein Y, Edelman R, Dror T, Hussein K, Bar-Lavie Y, Paul M. Carbapenem-resistant Enterobacteriaceae colonization and infection in critically ill patients: a retrospective matched cohort comparison with non-carriers. J Hosp Infect. 2016;94(1):54–59. doi:10.1016/j.jhin.2016.05.018
- Salomao MC, Freire MP, Boszczowski I, et al. Increased risk for carbapenem-resistant enterobacteriaceae colonization in intensive care units after hospitalization in emergency department. Emerg Infect Dis. 2020;26(6):1156–1163. doi:10.3201/eid2606.190965
- Dutcher L, Lautenbach E. A deeper dive: implications of identifying more of the carbapenem-resistant Enterobacteriaceae iceberg. J Infect Dis. 2020;221(11):1743–1745. doi:10.1093/infdis/jiz290
- Magiorakos AP, Burns K, Rodriguez Bano J, et al. Infection prevention and control measures and tools for the prevention of entry of carbapenem-resistant Enterobacteriaceae into healthcare settings: guidance from the European Centre for Disease Prevention and Control. Antimicrob Resist Infect Control. 2017;6(1):113. doi:10.1186/s13756-017-0259-z
- Komurcu B, Tukenmez Tigen E, Toptas T, et al. Rectal colonization with multidrug-resistant gram-negative bacteria in patients with hematological malignancies: a prospective study. Expert Rev Hematol. 2020;13(8):923–927. doi:10.1080/17474086.2020.1787145
- Weinstock DM, Conlon M, Iovino C, et al. Colonization, bloodstream infection, and mortality caused by vancomycin-resistant enterococcus early after allogeneic hematopoietic stem cell transplant. Biol Blood Marrow Transplant. 2007;13(5):615–621. doi:10.1016/j.bbmt.2007.01.078
- Trecarichi EM, Pagano L, Martino B, et al. Bloodstream infections caused by Klebsiella pneumoniae in onco-hematological patients: clinical impact of carbapenem resistance in a multicentre prospective survey. Am J Hematol. 2016;91(11):1076–1081. doi:10.1002/ajh.24489
- van Loon K, Voor In ‘t Holt AF, Vos MC. A systematic review and meta-analyses of the clinical epidemiology of carbapenem-resistant Enterobacteriaceae. Antimicrob Agents Chemother. 2018;62(1). doi:10.1128/AAC.01730-17
- Castagnola E, Tatarelli P, Mesini A, et al. Epidemiology of carbapenemase-producing Enterobacteriaceae in a pediatric hospital in a country with high endemicity. J Infect Public Health. 2019;12(2):270–274. doi:10.1016/j.jiph.2018.11.003
- Institute CaLS. Performance Standards for Antimicrobial Susceptibility Testing. 28th ed. CLSI supplement M100. PA: Clinical and Laboratory Standards InstituteWayne; 2018
- Huang PH, Chen WY, Chou SH, et al. Risk factors for the development of colistin resistance during colistin treatment of carbapenem-resistant Klebsiella pneumoniae infections. Microbiol Spectr. 2022;10(3):e0038122. doi:10.1128/spectrum.00381-22
- Tamma PD, Simner PJ. Phenotypic detection of carbapenemase-producing organisms from clinical isolates. J Clin Microbiol. 2018;56(11). doi:10.1128/JCM.01140-18
- Kiaei S, Moradi M, Hosseini Nave H, et al. Emergence of co-existence of blaNDM with rmtC and qnrB genes in clinical carbapenem-resistant Klebsiella pneumoniae isolates in burning center from southeast of Iran. Folia Microbiol (Praha). 2019;64(1):55–62. doi:10.1007/s12223-018-0630-3
- Smith JL, Drum DJ, Dai Y, et al. Impact of antimicrobial usage on antimicrobial resistance in commensal Escherichia coli strains colonizing broiler chickens. Appl Environ Microbiol. 2007;73(5):1404– 1414. doi:10.1128/AEM.01193-06
- Prasad N, Labaze G, Kopacz J, et al. Asymptomatic rectal colonization with carbapenem-resistant Enterobacteriaceae and Clostridium difficile among residents of a long-term care facility in New York City. Am J Infect Control. 2016;44(5):525–532. doi:10.1016/j.ajic.2015.11.021
- Zaidah AR, Mohammad NI, Suraiya S, et al. High burden of carbapenem-resistant Enterobacteriaceae (CRE) fecal carriage at a teaching hospital: cost-effectiveness of screening in low-resource setting. Antimicrob Resist Infect Control. 2017;6(1):42. doi:10.1186/s13756-017-0200-5
- Freire MP, Oshiro IC, Pierrotti LC, et al. Carbapenem-resistant Enterobacteriaceae acquired before liver transplantation: impact on recipient outcomes. Transplantation. 2017;101(4):811–820. doi:10.1097/TP.0000000000001620
- Collingwood A, Blostein F, Seekatz AM, et al. Epidemiological and microbiome associations between Klebsiella pneumoniae and vancomycin-resistant Enterococcus colonization in intensive care unit patients. Open Forum Infect Dis. 2020;7(1):ofaa012. doi:10.1093/ofid/ofaa012
- Kim YK, Song SA, Lee JN, et al. Clinical factors predicting persistent carriage of Klebsiella pneumoniae carbapenemase-producing carbapenem-resistant Enterobacteriaceae among patients with known carriage. J Hosp Infect. 2018;99(4):405–412. doi:10.1016/j.jhin.2017.10.017
- Ni W, Han Y, Liu J, et al. Tigecycline treatment for carbapenem-resistant Enterobacteriaceae infections: a systematic review and meta-analysis. Medicine. 2016;95(11):e3126. doi:10.1097/MD.0000000000003126
- Demirlenk YM, Gucer LS, Ucku D, et al. A meta-analysis for the role of aminoglycosides and tigecyclines in combined regimens against colistin- and carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Eur J Clin Microbiol Infect Dis. 2022;41(5):761–769. doi:10.1007/s10096-022-04429-0
- Macesic N, Gomez-Simmonds A, Sullivan SB, et al. Genomic surveillance reveals diversity of multidrug-resistant organism colonization and infection: a prospective cohort study in liver transplant recipients. Clin Infect Dis. 2018;67(6):905–912. doi:10.1093/cid/ciy199
- Hussein K, Rabino G, Eluk O, et al. The association between infection control interventions and carbapenem-resistant Enterobacteriaceae incidence in an endemic hospital. J Hosp Infect. 2017;97(3):218–225. doi:10.1016/j.jhin.2017.07.018