Abstract
Purpose
We aimed to evaluate the synergistic effect of linezolid and fosfomycin on fosfomycin-sensitive and -resistant Enterococcus clinical isolates in vitro and in vivo and whether the emergence of fosfomycin resistance in Enterococcus is associated with changes in strain virulence, from the perspective of fitness cost.
Methods
The synergistic effect of linezolid and fosfomycin was studied via in vitro checkerboard and static time-kill assays, as well as based on the in vivo survival rate and hemolymph load of a Galleria mellonella infection model. Fosfomycin resistance was induced via a stepwise increase in concentration. Changes in the virulence of the strains after drug resistance were investigated using the G. mellonella infection model and reverse transcription quantitative polymerase chain reaction (RT-qPCR). In vitro and in vivo growth curves and competitive experiments were used to study the fitness cost of the strain. Finally, a static time-kill assay was performed to explore the effect of the combined medication.
Results
In vitro and in vivo data showed that linezolid combined with fosfomycin had a good synergistic effect on Enterococcus treatment. The G. mellonella infection model and RT-qPCR data showed that the virulence of the resistant strains was weakened to varying degrees. A survival curve and competition experimental data showed that this was related to the fitness cost of strains while acquiring resistance and negatively impacted linezolid treatment; however, the combination still showed a good synergistic effect in drug-resistant strains.
Conclusion
Linezolid combined with fosfomycin had a synergistic effect on both fosfomycin-sensitive and -resistant Enterococcus strains. Strains incur fitness costs as they develop drug resistance, which leads to a decrease in virulence. There is an interaction between fitness cost, virulence, and drug resistance, which indirectly affects drug treatment.
Introduction
Enterococcus is a gram-positive bacterium that can cause bacteremia, endocarditis, urinary tract infection, etc.Citation1–3 Because of its rapid adaptation to human hosts and the environment, it is the main cause of community and hospital infections.Citation4,Citation5 Moreover, owing to its resistance to almost all main first-line clinical antibiotics, such as cephalosporins, aminoglycosides, clindamycin, and trimethoprim-sulfamethoxazole, antimicrobial selection is currently difficult.Citation6–8 Linezolid is an oxazolidinone antibiotic that significantly affects the treatment of vancomycin-resistant Enterococcus (VRE) infection.Citation9 However, with the excessive use of antibiotics in hospitals in recent years, linezolid-resistant Enterococcus strains are gradually appearing in various places.Citation10 Drug combinations have been widely used to reduce the development of drug resistance. In vitro studies have shown that linezolid combined with fosfomycin has a good synergistic effect on clinical isolates of vancomycin-susceptible Enterococcus and VRE strains.Citation11,Citation12 However, the efficacy of this combination in the treatment of infection with fosfomycin-resistant Enterococcus has not been reported.
Galleria mellonella has been widely used to evaluate the pathogenicity of bacterial and fungal pathogens and determine the in vivo efficacy of established and novel antimicrobial agents.Citation13,Citation14 Because of its structural and functional similarity with the mammalian immune system, the infection process can be carried out in a certain temperature range (from 15 °C to over 37 °C).Citation12 The infection model of G. mellonella was studied using larval survival, bacterial load, and blood cell density.Citation15 In a previous study, Qi and Li et al observed a synergistic effect of drugs, mainly by observing the survival rate of larvae,Citation12,Citation16 but hemolymph load was used as an important supplement for single- and combined-drug therapy, which can more intuitively embody medicaments to the curative effect of organisms.Citation17,Citation18
When judging the synergistic effect, it is necessary to not only select susceptible strains but also study resistant strains and whether drug resistance will affect treatment. Meanwhile, a study on the treatment of enterococcal infection with fosfomycin by Mao et al using a static time-kill assay and an in vitro dynamic PK/PD model showed excellent bactericidal activity of fosfomycin in the first 4‒8 h; however, in the dynamic process of treatment, a duration of 6–8 h leads to the regeneration of resistant mutants, which affect the linezolid treatment.Citation19 Few reports have discussed fosfomycin resistance. The characteristics of easy resistance to fosfomycin were demonstrated by a static time-kill assay in vitro in previous studies, but the interaction between fosfomycin-resistant Enterococcus strains and the body and its related mechanisms have rarely been reported. The development of antibiotic resistance is often accompanied by fitness costs, defined as changes in competitiveness in antibiotic-free environments.Citation20,Citation21 This phenomenon often leads to the selection of winners, which may lead to the replacement of susceptible with resistant strains, thus leading to the proliferation of resistant strains. Competitive experiments provide the “gold standard” for studying fitness costs.Citation22 The fitness cost of Escherichia coli carrying resistant genes may be associated with the virulence of the strain, increasing the potential risk of infection, while indirectly impairing the therapeutic effect of colistin.Citation23 Thus, the difference in treatment may be related to this hypothesis, as shown by Wang’s study; VRE virulence gene expression (especially acm) and mortality of G. mellonella decreased with an increase in fosfomycin resistance, and virulence of the strain presumably decreased with an increase in fosfomycin resistance.Citation24 In addition, competition of Enterococcus strains to acquire fosfomycin resistance has not been reported yet. Studies on Enterococcus have mostly focused on in vitro rather than in vivo competition.
Therefore, in this study, we explored the synergistic effect of linezolid combined with fosfomycin on fosfomycin-susceptible strains via checkerboard and static time-kill assays. The synergistic effect of the drug combination was further verified based on the survival rate and hemolymph load of the G. mellonella infection model. Through artificial induction of fosfomycin-resistant strains, the virulence of susceptible bacteria and corresponding resistant bacteria was studied using the G. mellonella infection model and quantitative real-time PCR. Growth curves and competition experiments were used to measure the fitness costs of the strain. Finally, NO.22 was selected for the static time-kill assay to study the influence of the fitness cost of strains on drug therapy.
Materials and Methods
Strains, Medium, and Antibiotics
The strains were derived from 19 clinical isolates of Enterococcus, mainly collected from patients’ urine and blood samples at the First Affiliated Hospital of Anhui Medical University in 2020. They were part of the routine hospital laboratory procedure. This study was approved by the institutional review board of the First Affiliated Hospital of Anhui Medical University. All strains were identified using the automated Vitek-2 system (Marcy l’Etoile BioMérieux, France). ATCC 51299 was used as the quality control strain.
Linezolid and fosfomycin were purchased from the National Institute for Food and Drug Control of China (Beijing). Glucose-6-phosphate (G-6-P) was purchased from Sigma-Aldrich. Mueller‒Hinton broth (MHB) (Oxoid, England) and MH agar (MHA) (Oxoid, England) were used for the susceptibility, checkerboard, and time-kill assays.
In vitro Sensitivity Test and Checkerboard Assay
The minimal inhibitory concentrations (MICs) of linezolid and fosfomycin were determined via the agar dilution method in accordance with the Clinical and Laboratory Standards Institute (CLSI) Standard Methods guidelines.Citation25 Briefly, 0.1 mL of antibiotics and 0.1 mL of a final bacterial inoculum of 5×105 colony-forming units (CFU)/mL made from fresh broth were placed in each well. The antibiotic concentration was diluted using the doubling dilution method, and the fosfomycin agar plate also included G-6-P at a final concentration of 25 mg/L. The plates were incubated at 37 °C for 18–24 h. The MIC was defined as the lowest drug concentration without visible colony growth. According to the CLSI 2021 guidelines,Citation25 drug resistance (R) was defined as MIC ≥ 8 mg/L linezolid and MIC ≥ 32 mg/L fosfomycin. MIC assays were performed in triplicate for each strain. ATCC 29212 was used as the quality control strain.
The synergistic effects of linezolid and fosfomycin at different concentrations were evaluated via a checkerboard assay. The concentration of linezolid ranged from 0.03125 mg/L to 8 mg/L, and that of fosfomycin ranged from 0.5 mg/L to 256 mg/L. Each strain was inoculated into a 96-well plate to obtain suitable suspension (105 CFU/mL) with a final volume of 200 µL (25 mg/L G-6-P) and incubated at 37 °C for 18–22 h. All experiments were repeated in triplicate. The fractional inhibitory concentration index (FICI) was defined as follows: FICI = (drug A combined MIC/Drug A alone MIC)/(drug B combined MIC/Drug B alone MIC). The effect of FICI on Enterococcus was explained as follows: FICI ≤ 0.5, synergistic effect; 1 < FICI ≤ 4, no difference; and FICI > 4, antagonistic effect.Citation26
Static Time-Kill Assays
The static time-kill assays followed the method described above.Citation27 The initial vaccination of bacteria in the 10-mL MHB system was ~1 × 106 CFU/mL, the designed concentrations of linezolid were 2 mg/L and 4 mg/L, and those of fosfomycin were 128 mg/L and 256 mg/L. The bacterial suspension was incubated at 37 °C with moderate shaking, and CFU were counted at 0, 2, 4, 6, 8, 10, and 24 h. Three replicates were performed for each strain. The synergistic effect was defined as a reduction of more than 2 log10 CFU/mL at 24 h compared to that of the most active single drug.
Galleria Mellonella Infection Model
G. mellonella (Tianjin, China) was used to study the effect of linezolid combined with fosfomycin on strain NO.22. As previously described,Citation28 Enterococcus was centrifugally precipitated in overnight cultures using phosphate buffer saline (PBS) to adjust the concentration, using a 20-μL Hamilton syringe (Hamilton, Shanghai, China) to enter the last left front leg of larvae, followed by inoculation of 10 μL of different bacterial suspensions (106, 107, and 108 CFU/larva), and the concentration that caused 80% mortality in larvae at 24 h was determined; the bacterial colony count was used to confirm the consistency of the inoculum. After 2 h of infection, we applied antibiotics (linezolid 5 and 10 mg/kg, fosfomycin 100 and 200 mg/kg) alone or in combination (linezolid 5 mg/kg + fosfomycin 100 mg/kg, linezolid 10 mg/kg + fosfomycin 200 mg/kg); 10 mg/kg of linezolid and 200 mg/kg of fosfomycin were based on human doses, and larvae injected with PBS alone were used as controls.Citation29 We selected 16 larvae from each group weighing 250–350 mg without gray spots. The number of dead caterpillars was recorded every 24 h for 120 h. When the body blackens or shrivels and there is no movement and no response to touch, they are considered dead. At 120 h, the hemolymph of surviving larvae was collected, the bacterial load was counted, and the treatment effect was completed in triplicate. The primary outcome of the insect model was to assess the rate and extent of death of G. mellonella using Kaplan–Meier analysis and Log rank test.
In vitro Induction of Fosfomycin-Resistant Strains
In vitro artificial induction of drug-resistant strains was carried out in accordance with the above method.Citation30 Briefly, the concentrations were increased stepwise to induce drug-resistant strains in vitro. First, the strain was incubated overnight in 1/2 × MIC (64 mg/L) drug-containing MHB, and the centrifuged strain was added to 1 × MIC (128 mg/L) drug-containing medicine broth for 24 h to gradually increase the concentration of fosfomycin up to 1024 mg/L. After incubation for 24 h, the bacterial liquid was uniformly spread on an MHA plate containing 1024 mg/L fosfomycin, and the bacterial liquid was placed in a 37 °C incubator overnight. Then, the selected drug-resistant mutants were confirmed using an antibacterial agent-free medium, and serial passages were passaged 20 times. Finally, the MIC was determined via strain analysis using linezolid and fosfomycin.
Survival of G. mellonella
To study whether strain virulence changes correspondingly after acquired drug resistance, the above methods were used to inject 108 CFU/mL strains into G. mellonella and observe the survival rate.
Relative Quantification of Virulence Gene Expression
RNA from the parent and drug-resistant strains was extracted using RNAex Pro Reagent (Agbio Co., Hunan, China) in accordance with the manufacturer’s instructions. cDNA was synthesized using Evo M-MLV RT Master Mix (Agbio Co., Hunan, China). Real-time PCR was performed using SYBR® Green Pro Taq HS Premix II (Rox Plus) (Agbio.co, Hunan, China) on a fluorescence quantitative PCR instrument (Roche LightCycler 96, Switzerland). The target gene expression levels were normalized to that of the housekeeping gene (gyrb) mRNA and determined via the 2–ΔΔCT calculation method, where CT is the threshold cycle. The primers used in this study are listed in Supplementary Table 1.
Bacterial Growth Curve in vitro and in vivo
The parental strain NO.22 and the drug-resistant strain NO.22R were selected, and fresh overnight cultured strains were diluted to 1×106 CFU/mL in 10 mL MHB and placed in a 37 °C incubator for shaking. Ten microliters of the bacterial solution were extracted from the culture system at 0, 4, 8, 12, 24, and 48 h and coated on an agar plate with a sterile L-shaped plastic coated rod. The samples were incubated at 37 °C for 24 h and counted. In the in vivo experiment, the hemolymph of infected larvae was collected at the same time points as above, diluted, and spotted.
In vitro and in vivo Competition Experiments
As mentioned previously,Citation31 a competition experiment of the strains was carried out. Briefly, the mixed bacterial liquid of NO.22 and NO.22R (1:1) was cultured under the above conditions, and cultured bacteria were sucked at 0, 4, 8, 12, 24, and 48 h. The CFU of the culture solution was counted on an agar plate and an agar plate containing 1 × MIC fosfomycin for 24 h. Fitness was calculated using a competition experiment, and the relative fitness (W) of the mutant to wild type was calculated as follows:
Where RF is the number of resistant bacteria at the end of culture, RI is the number of resistant bacteria before culture, SF is the number of susceptible bacteria at the end of culture, and SI is the number of susceptible bacteria before culture. W < 1 indicates that the competitive fitness of S is better than that of R, W = 1 indicates that the competitive fitness of S and R is similar, and W > 1 indicates that the competitive fitness of R is better than that of S.Citation32
To further study the competition between the mutant and parent body, the same volume of NO.22 and NO.22R suspensions was adjusted with PBS to a bacterial concentration with lethality of 80% and injected into the larvae. The hemolymph was collected at the same time point as in vitro, and the hemolymph was uniformly spread on a common agar plate and an agar plate containing 1 × MIC fosfomycin and incubated at 37 °C for 24 h. A colony count was performed to calculate the load of hemolymph fluid in each node.
Static Time-Kill Assay of Fosfomycin-Resistant Strain
NO.22R was selected for static time-kill assay. The concentrations of linezolid were 2 mg/L and 4 mg/L and those of fosfomycin were 2048 mg/L and 4096 mg/L.
Statistical Analysis
All statistical analyses were performed using GraphPad Prism, version 8.0 (GraphPad Software, Inc., San Diego, CA, United States). One-way ANOVA was used to evaluate significant differences in mRNA expression. Survival analyses were performed using Kaplan–Meier survival curves, and significant differences between groups were tested using the Log rank test. Statistical significance was set at P < 0.05.
Results
MIC results and Checkerboard Assay
The susceptibility and FICI data of the 19 clinical isolates of linezolid and fosfomycin are shown in . The MIC assay for linezolid showed that 89.5% (17/19) of Enterococcus isolates were susceptible, 5.26% (1/19) were intermediate, and 5.26% (1/19) were resistant. In the determination of the fosfomycin MIC, 57.9% (11/19) of Enterococcus were susceptible, 36.8% (7/19) were intermediate, and 5.26% (1/19) were resistant. The FICI value of the experimental strains revealed that the combination of linezolid and fosfomycin had a synergistic or additive effect on 68.4% (13/19) of the tested strains in vitro, and there was no antagonism between the two drugs. Among them, NO.22, NO.23, and NO.24 showed a better synergistic effect on linezolid and fosfomycin than the other strains; therefore, three strains were selected as the main experimental objects.
Table 1 MICs of Antimicrobial Agents Against Nineteen Strains of Enterococcus
In vitro Static Time-Kill Assays
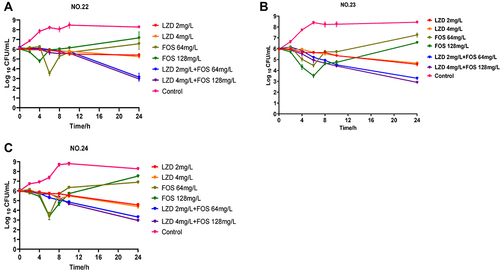
The results of the static time-kill assay are shown in and . Linezolid at 2 mg/L and 4 mg/L had similar therapeutic effects and could cause ~1 log10 value of bacterial killing in 24 h. For all strains, all concentrations of fosfomycin (64 mg/L and 128 mg/L) resulted in 1–2 log10 bacterial kills in 4–8 h. However, at 8–12 h, all strains regrew after fosfomycin treatment, although the final population density was still slightly lower (~1 log10 CFU/mL) than that of the untreated control group. In contrast, all combination groups resulted in sustained bacterial killing at 24 h of treatment, and the total bacterial count did not exceed 4 log10 values at 24 h. Among them, the low-dose combination group (2 mg/L LZD + 64 mg/L FOS) showed an excellent bactericidal effect, and there was no significant difference in the therapeutic effect compared with the high-dose combination group (4 mg/L LZD + 128 mg/L FOS). Compared with the initial colony count (∆log10CFU0–24h), changes in bacterial counts after 24 h of treatment in the low-dose combination groups were −2.99 ± 0.3, −2.73 ± 0.07, and −2.77 ± 0.06 log10 values, respectively. The final results showed that the low-dose combination groups had additive effects on all three strains, with −2.35, −1.35, and −1.38 log10 values, of which NO.22 was the most significant, showing a synergistic effect.
Table 2 ∆logCFU0–24h Values of Linezolid and Fosfomycin as Monotherapy and in Combination
Galleria mellonella Infection Model
To verify the therapeutic effect of the combination of linezolid and fosfomycin against Enterococcus in vivo, we used a G. mellonella infection model. As shown in , exploration of the preliminary concentration showed that 1×108 CFU/mL was 80% of the lethal dose. Almost the entire control group with injected strains died within 120 h, while the survival rate was significantly improved by drug treatment (P < 0.05). As shown in , the survival rate in the monotherapy group was significantly lower than that in the combination therapy group (P < 0.05), and there were slight differences between the monotherapy groups. The mortality in the high-dose linezolid group was similar to that in the low-dose group. The fosfomycin monotherapy group showed excellent antibacterial efficacy in vivo, with similar survival rates in the fosfomycin high-dose monotherapy (200 mg/kg) and low-dose combination therapy groups (5 mg/kg + 100 mg/kg), although not as good as high-dose linezolid combined with fosfomycin (10 mg/kg + 200 mg/kg). Although the combination of high-dose linezolid and fosfomycin was superior to the low-dose combination, no significant differences were observed.
Figure 2 In vivo assays using G.mellonella model. (A) exploration of the concentration of 50% lethal bacteria (B) survival curves of infected G.mellonella larvae treated with with linezolid and fosfomycin at different concentrations alone or in combination.(C) Haemolymph CFU burden of G. mellonella after infection with NO.22 followed by antibiotic treatment. CFU/mL values are shown for the drug treatment groups in relation to the mean CFU/mL value for the control group, indicating the reduction in log10 CFU/mL due to treatment. Data expressed as mean ± standard deviation of three independent experiments (*P<0.05, **P<0.01).
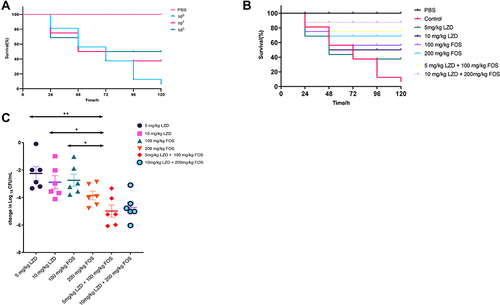
shows the hemolymph load of larvae treated with either monotherapy or combined therapy; the bacterial burden of the latter was significantly lower than that of the former (P < 0.05), and the efficacy of linezolid was similar. Fosfomycin, as shown in previous survival data, significantly reduced the bacterial load compared with linezolid (P < 0.05). The effect of high-dose fosfomycin monotherapy was similar to that of low-dose combined therapy and caused a decrease of −3.85 and −4.98 log10 values, respectively, compared to the control group. Both combination groups could achieve significant bactericidal effects, but high-dose combination therapy improved survival rates; however, the bactericidal effect was slightly inferior to that of the low-dose combination group. Overall, this suggests that linezolid combined with fosfomycin has a synergistic effect in the treatment of Enterococcus infection in vivo.
In vitro Induction of Resistance of Fosfomycin
The susceptibility of induced strains was measured by the method described above. The sensitivity of the resistant strain did not change when it was passed 20 times on the blank plate, indicating good stability of the bacteria, and the MICs are shown in .
Table 3 MICs of Antimicrobial Agents Against Four Strains
Survival of Galleria mellonella Infection Model
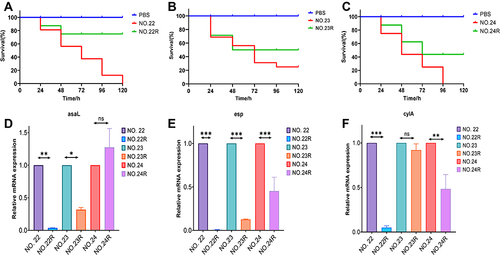
To determine the effect of fosfomycin resistance on the virulence of Enterococcus faecalis, we used the G. mellonella infection model to test virulence. The induced drug-resistant strains were inoculated with G. mellonella at a density of 10 µL and 1×108 CFU/mL. A comparison of the survival rate curves showed that the virulence of the three strains before and after drug resistance was significantly downregulated (P < 0.05), As shown in , which NO.22R was the most representative. The survival rate of this strain was significantly increased by 75%. shows that NO.23R, the survival rate increased by only 31.25%, and showed that NO.24R increased by 56.25%.
Figure 3 Virulence analysis of fosfomycin-resistant mutants (A–C). The Galleria mellonella infection model was used to explore the difference in pathogenicity between baseline isolates (NO.22, NO.23, NO.24) and fosfomycin-resistant mutants (NO.22, NO.23, NO.24). (D–F) The relative mRNA expression levels of virulence genes (D) asaL, (E) esp, (F) cylA were compared between baseline isolates and fosfomycin-resistant isolates mutants. (*P < 0.05, **P < 0.01 and ***P < 0.001).NO.22R, isolate resistant to NO.22; NO.23R, isolate resistant to NO.23; NO.24R, isolate resistant to NO.24.
Relative Quantification of Virulence Gene Expression
Parental and drug-resistant strains also differ in the expression of virulence genes. Virulence genes of the strains were downregulated after drug resistance. As illustrated in , compared with that of the parental strains, the expression of asaL in NO.22R and NO.23R decreased by 0.03 and 0.31 times respectively, while that of NO.24R increased by 1.27 times. depicted the expression of esp decreased by 0.005, 0.12, and 0.29 times, respectively, and showed that of cylA decreased by 0.07, 0.86, and 0.48 times, respectively. Compared to the parental strain, there were significant differences (P < 0.05). This corresponded to the survival data of the G. mellonella infection model, indicating a correlation between resistance to fosfomycin and the release of virulence factors. Increasing exposure to fosfomycin causes a decrease in virulence factors.
Growth Curves of Fosfomycin-Resistant Strain
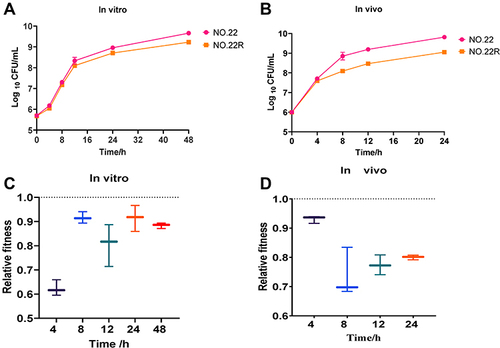
As shown in , in vitro data showed that the growth rate of drug-resistant strain NO.22 was slower than that of the parental strain, but it was flat with no significant change. shows that the growth rate of drug-resistant strains in vivo was slower than that in the in vitro culture, but there was no significant difference in the growth rate of the parental strains (P > 0.05).
Figure 4 Fitness cost analysis of fosfomycin-resistant mutant NO.22. (A and B) Growth curve of drug-resistant mutant NO.22 and parental strain NO.22 cultured separately in vitro and in vivo. (C and D) Relative fitness of fosfomycin-resistant mutant NO.22R and parental strain NO.22 in co-culture in vitro and vivo. Relative fitness value less than 1 indicates fitness defect, which incurs fitness cost and a value greater than 1 indicates fitness benefit.
Competitiveness of Fosfomycin-Resistant Strain
To further investigate whether the emergence of drug-resistant strains during treatment affects the growth of normal strains, we adopted a co-cultivation method. Both in vitro and in vivo adaptive data showed that NO.22 had a fitness cost after drug resistance. As depicted in , In vitro studies confirmed that the competition of drug-resistant strains showed the best result at 4 h, with a value of 0.62; then, the adaptability increased and competition declined and tended to level off. The in vivo data indicated that adaptivity was higher (over 0.7), the competition was lower, and the peak value was 0.74 at 8 h in . These data suggest that susceptible strains gradually replace resistant strains during co-culture; therefore, susceptible strains are still the main target of treatment. The in vitro static time-kill assay showed that the single-agent treatment with fosfomycin would be resistant within 4 h, which might be related to the low adaptation in vitro.
Static Time-Kill Assay of the Fosfomycin-Resistant Strain
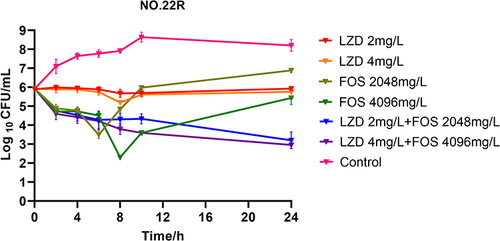
We selected strain NO.22 in this assay, which induced the least virulent and lethal strains of the drug-resistant strains. To observe the bactericidal effect of linezolid combined with fosfomycin on drug-resistant strains, we performed a static time-kill assay in vitro. As shown in , the treatment effect of the linezolid monotherapy group changed, showing a slight or no bacterial killing effect, which showed an increase of 0.03 log10 value compared to the previous value (−0.64 log10). However, the resistance of fosfomycin was controlled and the 4096 mg/L fosfomycin group showed a bactericidal effect, which decreased by 0.49 log10 value at 24 h compared with the previous value (+1.14 log10). The sterilization effect of the combined treatment medication group was still significant, and all of them could decrease by 2 × log10 value, which was −2.72 log10 and −2.98 log10, respectively. The drug combination still showed an excellent synergistic effect in the resistant strains, and the combined medication could effectively inhibit fosfomycin resistance.
Discussion
We confirmed that linezolid combined with fosfomycin has a good synergistic effect on susceptible Enterococcus based on an in vitro checkerboard assay, static time-kill assay, and an in vivo infection model of G. mellonella. The virulence of fosfomycin-resistant strains was studied using the survival curve of G. mellonella and RT-qPCR, and the therapeutic effect of linezolid combined with fosfomycin on drug-resistant mutants was investigated using an in vitro static time-kill assay. Finally, the fitness cost of the strains acquiring drug resistance was analyzed using in vitro and in vivo survival curves and competitive experiments. These studies help assess the resistance risk of resistant strains and thus elucidate the resistance mechanism of Enterococcus, which will provide important guidance for the development of future therapeutic regimens.
First, in vitro assay data showed that linezolid combined with fosfomycin had a highly synergistic effect, of which the linezolid group showed similar bacteriostatic effects, causing a ~1 log10 decrease in the three clinical strains after 24 h compared to the initial concentration at 0 h. However, Yan et al showed a trend of gradual drug resistance when a sub-inhibitory concentration of 1 mg/L linezolid was used in the treatment of VRE.Citation11 According to a survey of 1442 clinically collected Enterococcus isolates in the Czech Republic from 2009 to 2019, linezolid-resistant Enterococcus faecalis (LREF) increased from 0 in 2009 to 36 in 2019.Citation33 Subsequently, a global statistical analysis showed that of 69,291 Enterococcus isolates, 1646 were LREF isolates, indicating a prevalence rate of 2.2%.Citation34 Linezolid-resistant strains emerged gradually in VRE, suggesting a possible association with long-term linezolid exposure.Citation35,Citation36 Although LREF did not appear in this study, the data suggest that linezolid-resistant Enterococcus also began to proliferate.Citation37,Citation38 Therefore, the public should strictly control the dose and time of linezolid in the course of treatment. The single fosfomycin group showed a good sterilization effect in the early stages and regrowth in the later stages. Qi et al further showed that the phenomenon of fosfomycin also appears in VRE,Citation12 indicating that fosfomycin may not be directly applied to the treatment of Enterococcus. Surprisingly, linezolid combined with fosfomycin exhibited a good synergistic effect and exerted a sustained bactericidal effect. Yan et al further suggested that linezolid combined with fosfomycin could prolong the post-antibiotic effect,Citation11 which was beneficial for prolonging the treatment time. Jiang et al speculated that the combination of drugs might be a beneficial result of the synergistic closure of their respective mutation selection windows (MSW).Citation39 Xie et al found that the combination of drugs significantly reduced the thickness of bacterial cell walls,Citation29 while Chai et al found that the combination of drugs reduced biofilm formation,Citation40 which may be the reason for the improved efficacy of the treatment. Combination therapy with fosfomycin drugs has shown effective benefits in clinical treatment. Clinical data have shown that fosfomycin combination therapy can improve the clinical success rate of MRSA lung infections, VRE urinary tract infections, etc.Citation41,Citation42 These data support further investigation of the effects of fosfomycin on infections caused by gram-positive cocci with advanced antimicrobial resistance. Physicians have successfully used linezolid and fosfomycin to rescue patients with severe VRE bacteremia.Citation43 This suggests the clinical utility of linezolid combined with fosfomycin.
In contrast to the in vitro studies, the in vivo data showed a significant improvement in the efficacy of fosfomycin alone, with fosfomycin being more effective than linezolid in improving the survival rate. A similar situation emerged in Li’s study on fosfomycin in the MRSA G. mellonella infection model,Citation16 suggesting that this may be related to the interaction of fosfomycin with the host. Studies have shown that fosfomycin has an immunomodulatory effect,Citation44 which can enhance the killing effect on host cells and reduce the emergence of drug-resistant mutants, thereby significantly shrinking MSW and reducing the emergence of drug resistance.Citation45 For synergism, the survival rate was often used in the previous infection model of G. mellonella; however, this single performance metric may not be sufficient to account for the synergistic treatment effect of the drug. Bacterial load, as the ultimate drug therapy target, is particularly important for the evaluation of efficacy. In a previous study on the synergistic effect of novel β-lactams against Enterococcus, Thieme et al assessed the synergistic effect of the drug by increasing the hemolymph load of the larvae,Citation46 so that the in vivo additive and synergistic effect can be better displayed. Therefore, in this study, the hemolymph load of the larvae was extracted and the combined treatment effect was verified by observing the bacterial load between the treatment groups. The combination group also showed the best effect in terms of bacterial killing, as indicated by the previous survival rate, in which the low-dose combination group (5 mg/kg + 100 mg/kg) could cause a killing effect of −4.98 log10 value compared with the growth group. However, the in vivo bactericidal effect of fosfomycin was surprising, as the bactericidal effect in the high-dose group (200 mg/kg) was similar to that in the low-combination group. When studying the basic efficacy and synergy of the drug, the infection model of G. mellonella became a suitable choice because of its easy operation and breeding. However, owing to the lack of studies on immune metabolism, there may be insufficient studies on host immunity compared with mouse infection models.Citation47,Citation48 More experiments are needed in the future to explore the applicability of G. mellonella.
The broad-spectrum activity of fosfomycin, the efficacy of intravenous and oral preparations, and the safety of tolerability make it a clinically superior option for the treatment of multidrug-resistant bacteria.Citation49,Citation50 However, in vitro and clinical studies have frequently observed resistance to fosfomycin, which is worthy of our attention. Recent studies have shown that when fosfomycin is used in the clinical treatment of Enterococcus infection, resistance at 1024 mg/L or even higher doses will occur,Citation51 which will seriously hinder treatment and increase the pressure of antimicrobial drug selection. This suggests that continuous monitoring and in-depth research on fosfomycin resistance are required. In this study, we found that the in vitro static time-kill assay for all single-dose fosfomycin groups in the treatment process will have a good therapeutic effect in the early stage, but gradually resist over time, which will affect the outcome of treatment. Karsten et al monitored the growth curve of Enterococcus exposed to fosfomycin every 15 min and revealed that low doses of fosfomycin may even promote the growth of the strain itself at 48 h; in contrast, high doses completely and irreversibly inhibit the growth of Enterococcus.Citation52 However, we determined from the hemolymph load counts in the G. mellonella infection model that the in vivo resistance disappears for all fosfomycin dose groups.
Although the cause of this occurrence of Enterococcus is unclear, exposure of the strain to fosfomycin can be induced to expand the enrichment, survival, and promotion of fosfomycin mutants through known selection mechanisms. Scortti et al found that the fosX enzyme can inactivate fosfomycin entering Listeria monocytogenes cells through a non-characteristic transport mechanism, resulting in high levels of bacterial resistance.Citation53 However, through genome research, the combined effect of the virulence genes prfA and hpt on fosfomycin transport in vivo inhibits the effect of fosX on fosfomycin resistance, which causes the strain to be susceptible to fosfomycin in vivo.Citation54 This suggests that fosfomycin-resistant Enterococcus may also regulate the susceptibly in vitro and in vivo in this way. If there is an interaction between virulence and resistance gene regulatory networks in strains, a detailed understanding of these interactions is critical for predicting the development of strain resistance and the impact of antimicrobial formulation on resistant strains.Citation55 Therefore, we need in-depth information on the relationship between fosfomycin resistance and virulence in Enterococcus. Thus, we induced high levels of fosfomycin resistance in these strains. Based on the survival curve of G. mellonella, we found that the virulence of Enterococcus decreased after acquiring resistance, which is similar to that of fosfomycin in E. coli, where the virulence of acquired resistant mutants tended to decrease.Citation56 Wang et al further found that the downregulation of the virulence of fosfomycin was concentration-dependent on the fosfomycin-resistant mutants obtained from the hollow fiber model.Citation24 Subsequently, RT-qPCR revealed that the overall virulence factor of the mutant decreased, with cylA and esp showing a decreasing trend on average, while asaL showed a slightly increasing trend at NO.24. A similar situation was found in previous studies of daptomycin-resistant methicillin-resistant Staphylococcus aureus and artificially induced Streptococcus that acquired daptomycin resistance.Citation57,Citation58 The resistance gene VraSR encoding daptomycin also affects daptomycin adhesion of daptomycin-resistant strains to epithelial cells, which affects the colonization and survival of daptomycin-resistant strains in the host. Therefore, the impact of decreased virulence factors on the host is complicated, and asaL has been associated with mortality in clinical case studies;Citation59 thus, the acquisition of resistance may lead to a decrease in the killing rate of the strain. However, if the mutant can spread and infect more easily and adversely affect the choice of therapeutic agents, it could pose a deeper challenge to treatment.
There is an urgent need to understand the underlying causes of drug resistance because of its frequent emergence. Among the different factors contributing to the rise in antibiotic resistance, the fitness cost is considered to be the most prominent, which is a close indicator of the relationship between the frequency of antimicrobial use and the prevalence of drug-resistant bacteria in the future.Citation60,Citation61 The fitness cost theory and studies have shown that in an environment absent of or low in antibiotics, drug-resistant strains will be disadvantaged in a competitive environment with susceptible strains, thereby maintaining or even prolonging the effective time of the corresponding antibiotics.Citation62 Therefore, it is particularly critical to evaluate the fosfomycin-resistant Enterococcus; the adaptability of strains will change with the change in environment, which leads to the differences between in vitro and in vivo competitive experiments.Citation63 Thus, we studied the adaptability of drug-resistant strains based on in vitro and in vivo growth curves and competition experiments. The data show that although the growth rate of the strain has a slight tendency to slow down after acquiring resistance, there will be an fitness cost in the process of acquiring fosfomycin resistance. The adaptability of the strain in vitro for 4 h was extremely low, and the body maintained a high degree of adaptability and weak competitiveness in vivo. This may explain why drug-resistant strains develop resistance at an early stage of in vitro therapy. However, in recent years, there have been no or high-adaptive-cost mutations in strains and compensatory evolution to promote the survival of drug-resistant strains in the environment. In a previous study of the rifampicin resistance mutation site rpoB H526Y, this binding site was found to increase the transcriptional gene, thereby increasing the adaptive cost of resistance. This also suggests that the use of drugs that can inhibit rpoB H526Y in combination with rifampicin reduces drug resistance.Citation64 In this study, we speculated that linezolid may inhibit drug resistance by inhibiting the fosfomycin mutation site. In the future, it will reveal the potential molecular mechanism of fitness cost through genomic research to better understand the resistance of the strain and speed up the discovery of new combined therapies. In general, fitness cost, drug resistance, and virulence may have complex links or even overlaps in genetics, which has significant research potential.
Whether the emergence of drug-resistant mutants will affect the efficacy of previous antimicrobial agents is also of concern and is related to clinical drug selection. Therefore, we studied the efficacy of linezolid, fosfomycin, and their combination against fosfomycin-resistant Enterococcus through in vitro static time-kill assays. These data suggested that linezolid does not have a bactericidal effect on resistant strains as it does on susceptible strains. Xie also found similar results,Citation29 suggesting that linezolid may no longer be the dominant option in the clinical treatment of fosfomycin-resistant mutants. Therefore, increasing the drug dose may be an appropriate choice. Continuous infusion of linezolid is one of the choices for clinical response, as it can avoid the toxic side effects caused by an increased dosage. It may also help maintain the serum level required for treatment and limit plasma concentration fluctuations.Citation65 However, the advantages of continuous infusion may only occur in special clinical environments and special populations, and side effects are frequent.Citation66,Citation67 Continuous infusion greatly increases the time of patients in the hospital and the possibility of cross infection. Also, there may be an increased risk of drug-resistant strains because the continuous infusion of linezolid will continue to maintain drug concentrations around the MIC.Citation68
Fosfomycin also induces drug resistance. The advantages of linezolid combined with fosfomycin are fully demonstrated, which can directly inhibit the occurrence of fosfomycin resistance. Previously, fosfomycin combined with chloramphenicol showed a good synergistic bactericidal effect in vitro and in the G.mellonella infection model,Citation69 and fosfomycin combined with daptomycin for the treatment of linezolid-resistant VRE also showed an excellent bacteriostatic effect;Citation70 therefore, fosfomycin has great potential as a combination therapy. The high resistance to fosfomycin suggests that the dose of the drug needs to be strictly controlled clinically.Citation32 Recent studies on vancomycin in the treatment of enterococcal infection revealed that parameters such as AUC/MIC and valley concentration can guide the concentration control of vancomycin, thereby improving treatment results and reducing side effects such as nephrotoxicity.Citation71,Citation72 This suggests that we need to conduct in-depth studies of relevant PK/PD models to identify drug-related therapies and resistance targets to optimize the clinical choice of individualized doses.
However, there were some limitations to our study. Although artificially induced strains are the appropriate means to study the fitness cost of strains, there are still differences between them and drug-resistant strains in clinical practice. In the future, further exploration can be carried out using highly drug-resistant and sensitive strains screened in clinical practice. Simultaneously, this experiment only studied drug resistance from the perspective of fitness cost, which belongs to the classification of heteroresistance. In reality, there are very complex classifications for the resistance mechanism of fosfomycin, including mutations in murA genes conferring intrinsic resistance to fosfomycin, resistance by changes in fosfomycin transport caused by mutations in structural genes encoding GlpT and UhpT membrane transporters, and acquisition of plasmid-encoded genes fosA, fosX, etc. that inactivate antibiotics, also resulting in fosfomycin resistance.Citation73 This suggests a need for future studies of relevant resistance mechanisms from a genetic perspective. Some genes encoding drug resistance have the same recognition site as the virulence gene encoding Enterococcus, which indicates that there is an association between drug-resistance genes and the upstream control of virulence genes.Citation54 At present, the association between Enterococcus fosfomycin-resistance genes and virulence genes is unclear, and further research is needed. Further animal and clinical trials are needed to verify the utility of this drug combination.
Conclusion
The combination of linezolid and fosfomycin has a significant synergistic effect in the treatment of fosfomycin-susceptible and -resistant Enterococcus, and linezolid alone may negatively affect fosfomycin-resistant Enterococcus. The treatment of Enterococcus infections with fosfomycin showed that increasing the dose of the drug does not address bacterial resistance. When the drug concentration changes, bacteria can develop resistance in various ways, which has a complex impact on virulence. Therefore, clinicians require targeted drug selection in the face of drug-resistant strains. In addition, although the fosfomycin-resistant Enterococcus mutants were not as competitive as the susceptible strains in this study, the generation of adaptive costs was an important factor to consider in treatment planning, requiring continuous monitoring and research. Therefore, further in vivo and in vitro studies will be conducted to investigate the relationship between the existence of adaptive costs and the resistance of different fosfomycin genes. Moreover, there is a complex relationship between Enterococcus drug resistance and virulence gene networks; thus, it is necessary to further study the control of fosfomycin-resistance genes through genomics and the potential association between adaptive cost and Enterococcus virulence and other genes.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81173133), the Fund of Excellent Talents in Colleges and Universities of Anhui Province, China (gxbjZD06), and the Fund of Academic Leaders of Anhui Province, China (2015D068).
References
- Starling S. Bacterial evolution: the origins of pathogenic enterococci. Nat Rev Microbiol. 2017;15(7):382–383. doi:10.1038/nrmicro.2017.65
- Ch’ng JH, Chong KKL, Lam LN, et al. Biofilm-associated infection by enterococci. Nat Rev Microbiol. 2019;17(2):82–94. doi:10.1038/s41579-018-0107-z
- Dahl A, Iversen K, Fosbol E, et al. Reply: Enterococcus faecalis infective endocarditis. J Am Coll Cardiol. 2019;74(19):2435–2436. doi:10.1016/j.jacc.2019.09.011
- García-Solache M, Rice LB. The Enterococcus: a model of adaptability to its environment. Clin Microbiol Rev. 2019;32(2):e00058–18. doi:10.1128/CMR.00058-18
- Gouliouris T, Coll F, Ludden C, et al. Quantifying acquisition and transmission of Enterococcus faecium using genomic surveillance. Nat Microbiol. 2021;6(1):103–111. doi:10.1038/s41564-020-00806-7
- Arredondo-Alonso S, Top J, McNally A, et al. Plasmids shaped the recent emergence of the major nosocomial pathogen Enterococcus faecium. mBio. 2020;11(1):e03284–19. doi:10.1128/mBio.03284-19
- Abbott IJ, Meletiadis J, Belghanch I, et al. Fosfomycin efficacy and emergence of resistance among Enterobacteriaceae in an in vitro dynamic bladder infection model. J Antimicrob Chemother. 2018;73(3):709–719. doi:10.1093/jac/dkx441
- Van Tyne D, Gilmore MS. Raising the alarmone: within-host evolution of antibiotic-tolerant. Enterococcus Faecium mBio. 2017;8(1):e00066–17.
- Chuang YC, Lin HY, Chen PY, et al. Survival of patients with vancomycin-resistant Enterococcus faecium bacteremia treated with conventional or high doses of daptomycin or linezolid is associated with the rate of bacterial clearance. Crit Care Med. 2018;46(10):1634–1642. doi:10.1097/CCM.0000000000003264
- Schwartz MD, Shive DK, Shaikh ZH. Delayed discovery of linezolid-resistant, vancomycin-resistant Enterococcus faecium: lessons learned. Clin Infect Dis. 2004;38(1):155–156. doi:10.1086/380465
- Yan Y, Yang G, Li Y, et al. Factorial design and post-antibiotic sub-MIC effects of linezolid combined with fosfomycin against vancomycin-resistant enterococci. Ann Transl Med. 2022;10(3):148. doi:10.21037/atm-21-4595
- Qi C, Xu S, Wu M, et al. Pharmacodynamics of linezolid-plus-fosfomycin against vancomycin-susceptible and -resistant enterococci in vitro and in vivo of A Galleria mellonella larval infection model. Infect Drug Resist. 2019;12:3497–3505. doi:10.2147/IDR.S219117
- Tao Y, Duma L, Rossez Y. Galleria mellonella as a good model to study Acinetobacter baumannii pathogenesis. Pathogens. 2021;10(11):1483. doi:10.3390/pathogens10111483
- Piatek M, Sheehan G, Kavanagh K. Galleria mellonella: the versatile host for drug discovery, in vivo toxicity testing and characterising host-pathogen interactions. Antibiotics. 2021;10(12):1545. doi:10.3390/antibiotics10121545
- Ménard G, Rouillon A, Cattoir V, et al. Galleria mellonella as a suitable model of bacterial infection: past, present and future. Front Cell Infect Microbiol. 2021;11:782733. doi:10.3389/fcimb.2021.782733
- Li L, Chen H, Liu Y, et al. Synergistic effect of linezolid with fosfomycin against Staphylococcus aureus in vitro and in an experimental Galleria mellonella model. J Microbiol Immunol Infect. 2020;53(5):731–738. doi:10.1016/j.jmii.2018.12.007
- Ochoa S, Fernández F, Devotto L, et al. Virulence assessment of enterohepatic Helicobacter species carried by dogs using the wax moth larvae Galleria mellonella as infection model. Helicobacter. 2021;26(4):e12808. doi:10.1111/hel.12808
- Yang HF, Pan AJ, Hu LF, et al. Galleria mellonella as an in vivo model for assessing the efficacy of antimicrobial agents against Enterobacter cloacae infection. J Microbiol Immunol Infect. 2017;50(1):55–61. doi:10.1016/j.jmii.2014.11.011
- Mao J, Li T, Zhang N, et al. Dose optimization of combined linezolid and fosfomycin against Enterococcus by using an in vitro pharmacokinetic/pharmacodynamic model. Microbiol Spectr. 2021;9(3):e0087121. doi:10.1128/Spectrum.00871-21
- Melnyk AH, Wong A, Kassen R. The fitness costs of antibiotic resistance mutations. Evol Appl. 2015;8(3):273–283. doi:10.1111/eva.12196
- Alame Emane AK, Guo X, Takiff HE, et al. Drug resistance, fitness and compensatory mutations in Mycobacterium tuberculosis. Tuberculosis. 2021;129:102091. doi:10.1016/j.tube.2021.102091
- Kempf I, Zeitouni S. Coût biologique de la résistance aux antibiotiques: analyse et conséquences [The cost of antibiotic resistance: analysis and consequences]. Pathol Biol. 2012;60(2):e9–e14. French. doi:10.1016/j.patbio.2009.10.013
- Li W, Liu Z, Yin W, et al. MCR expression conferring varied fitness costs on host bacteria and affecting bacteria virulence. Antibiotics. 2021;10(7):872. doi:10.3390/antibiotics10070872
- Wang S, Liu H, Mao J, et al. Pharmacodynamics of linezolid plus fosfomycin against vancomycin-resistant Enterococcus faecium in a hollow fiber infection model. Front Microbiol. 2021;12:779885. doi:10.3389/fmicb.2021.779885
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; 30th Informational supplement.CLSI M100-S30. Wayne, PA: Clinical and Laboratory Standards Institute; 2020.
- Davis H, Brown R, Ashcraft D, et al. In vitro synergy with fosfomycin plus doxycyclin against linezolid and vancomycin-resistant Enterococcus faecium. J Glob Antimicrob Resist. 2020;22:78–83. doi:10.1016/j.jgar.2020.01.014
- Yang W, Liu J, Blažeković B, et al. In vitro antibacterial effects of Tanreqing injection combined with vancomycin or linezolid against methicillin-resistant Staphylococcus aureus. BMC Complement Altern Med. 2018;18(1):169. doi:10.1186/s12906-018-2231-8
- Jemel S, Guillot J, Kallel K, et al. In vivo efficacy of voriconazole in a Galleria mellonella model of invasive infection due to azole-susceptible or resistant Aspergillus fumigatus isolates. J Fungi. 2021;7(12):1012. doi:10.3390/jof7121012
- Xie N, Jiang L, Chen M, et al. In vitro and in vivo antibacterial activity of linezolid plus fosfomycin against Staphylococcus aureus with resistance to one drug. Infect Drug Resist. 2021;14:639–649. doi:10.2147/IDR.S290332
- Billal DS, Fedorko DP, Yan SS, et al. In vitro induction and selection of fluoroquinolone-resistant mutants of Streptococcus pyogenes strains with multiple emm types. J Antimicrob Chemother. 2007;59(1):28–34. doi:10.1093/jac/dkl428
- Jiang L, Cai W, Tang F, et al. Characterization of fitness cost caused by tigecycline-resistance gene tet (X6) in different host bacteria. Antibiotics. 2021;10(10):1172. doi:10.3390/antibiotics10101172
- Xia X, Yang L, Ling Y, et al. Emergence and mechanism of resistance of tulathromycin against Mycoplasma hyopneumoniae in a PK/PD model and the fitness costs of 23S rRNA mutants. Front Vet Sci. 2022;9:801800. doi:10.3389/fvets.2022.801800
- Mališová L, Jakubů V, Pomorská K, et al. Spread of linezolid-resistant Enterococcus spp. in human clinical isolates in the Czech Republic. Antibiotics. 2021;10(2):219. doi:10.3390/antibiotics10020219
- Dadashi M, Sharifian P, Bostanshirin N, et al. The global prevalence of daptomycin, tigecycline, and linezolid-resistant Enterococcus faecalis and Enterococcus faecium strains from human clinical samples: a systematic review and meta-analysis. Front Med. 2021;8:720647. doi:10.3389/fmed.2021.720647
- Olearo F, Both A, Belmar Campos C, et al. Emergence of linezolid-resistance in vancomycin-resistant Enterococcus faecium ST117 associated with increased linezolid-consumption. Int J Med Microbiol. 2021;311(2):151477. doi:10.1016/j.ijmm.2021.151477
- Smith TT, Tamma PD, Do TB, et al. Prolonged linezolid use is associated with the development of linezolid-resistant Enterococcus faecium. Diagn Microbiol Infect Dis. 2018;91(2):161–163. doi:10.1016/j.diagmicrobio.2018.01.027
- Egan SA, Shore AC, O’Connell B, et al. Linezolid resistance in Enterococcus faecium and Enterococcus faecalis from hospitalized patients in Ireland: high prevalence of the MDR genes optrA and poxtA in isolates with diverse genetic backgrounds. J Antimicrob Chemother. 2020;75(7):1704–1711. doi:10.1093/jac/dkaa075
- Zhang Y, Dong G, Li J, et al. A high incidence and coexistence of multiresistance genes cfr and optrA among linezolid-resistant enterococci isolated from a teaching hospital in Wenzhou, China. Eur J Clin Microbiol Infect Dis. 2018;37(8):1441–1448. doi:10.1007/s10096-018-3269-8
- Jiang L, Xie N, Chen M, et al. Synergistic combination of Linezolid and fosfomycin closing each other’s mutant selection window to prevent enterococcal resistance. Front Microbiol. 2021;11:605962. doi:10.3389/fmicb.2020.605962
- Chai D, Liu X, Wang R, et al. Efficacy of Linezolid and fosfomycin in catheter-related biofilm infection caused by methicillin-resistant Staphylococcus aureus. Biomed Res Int. 2016;2016:6413982. doi:10.1155/2016/6413982
- Falagas ME, Roussos N, Gkegkes ID, et al. Fosfomycin for the treatment of infections caused by Gram-positive cocci with advanced antimicrobial drug resistance: a review of microbiological, animal and clinical studies. Expert Opin Investig Drugs. 2009;18(7):921–944. doi:10.1517/13543780902967624
- Cai T, Tamanini I, Mattevi D, et al. Fosfomycin trometamol and N-acetyl-L-cysteine as combined oral therapy of difficult-to-treat chronic bacterial prostatitis: results of a pilot study. Int J Antimicrob Agents. 2020;56(1):105935. doi:10.1016/j.ijantimicag.2020.105935
- Hemapanpairoa J, Changpradub D, Thunyaharn S, et al. Vancomycin-resistant enterococcal infection in a Thai university hospital: clinical characteristics, treatment outcomes, and synergistic effect. Infect Drug Resist. 2019;12:2049–2057. doi:10.2147/IDR.S208298
- Pan AJ, Mei Q, Ye Y, et al. Validation of the mutant selection window hypothesis with fosfomycin against Escherichia coli and Pseudomonas aeruginosa: an in vitro and in vivo comparative study. J Antibiot. 2017;70(2):166–173. doi:10.1038/ja.2016.124
- Shen F, Tang X, Cheng W, et al. Fosfomycin enhances phagocyte-mediated killing of Staphylococcus aureus by extracellular traps and reactive oxygen species. Sci Rep. 2016;6:19262. doi:10.1038/srep19262
- Thieme L, Hartung A, Makarewicz O, et al. In vivo synergism of ampicillin, gentamicin, ceftaroline and ceftriaxone against Enterococcus faecalis assessed in the Galleria mellonella infection model. J Antimicrob Chemother. 2020;75(8):2173–2181. doi:10.1093/jac/dkaa129
- Killiny N. Generous hosts: why the larvae of greater wax moth, Galleria mellonella is a perfect infectious host model? Virulence. 2018;9(1):860–865. doi:10.1080/21505594.2018.1454172
- Stanojević S, Blagojević V, Ćuruvija I, et al. Lactobacillus rhamnosus affects rat peritoneal cavity cell response to stimulation with gut microbiota: focus on the host innate immunity. Inflammation. 2021;44(6):2429–2447. doi:10.1007/s10753-021-01513-z
- Trinh TD, Smith JR, Rybak MJ. Parenteral Fosfomycin for the treatment of multidrug resistant bacterial infections: the rise of the epoxide. Pharmacotherapy. 2019;39(11):1077–1094. doi:10.1002/phar.2326
- Tsegka KG, Voulgaris GL, Kyriakidou M, et al. Intravenous fosfomycin for the treatment of patients with bone and joint infections: a review. Expert Rev Anti Infect Ther. 2022;20(1):33–43. doi:10.1080/14787210.2021.1932463
- Guo Y, Tomich AD, McElheny CL, et al. High-level Fosfomycin resistance in vancomycin-resistant Enterococcus faecium. Emerg Infect Dis. 2017;23(11):1902–1904. doi:10.3201/eid2311.171130
- Theophel K, Schacht VJ, Schlüter M, et al. The importance of growth kinetic analysis in determining bacterial susceptibility against antibiotics and silver nanoparticles. Front Microbiol. 2014;5:544. doi:10.3389/fmicb.2014.00544
- Scortti M, Lacharme-Lora L, Wagner M, et al. Coexpression of virulence and fosfomycin susceptibility in Listeria: molecular basis of an antimicrobial in vitro-in vivo paradox. Nat Med. 2006;12(5):515–517. doi:10.1038/nm1396
- Scortti M, Han L, Alvarez S, et al. Epistatic control of intrinsic resistance by virulence genes in Listeria. PLoS Genet. 2018;14(9):e1007525. doi:10.1371/journal.pgen.1007525
- Deng Y, Xu H, Su Y, et al. Horizontal gene transfer contributes to virulence and antibiotic resistance of Vibrio harveyi 345 based on complete genome sequence analysis. BMC Genomics. 2019;20(1):761. doi:10.1186/s12864-019-6137-8
- Pourbaix A, Guérin F, Lastours V, et al. Biological cost of fosfomycin resistance in Escherichia coli in a murine model of urinary tract infection. Int J Med Microbiol. 2017;307(8):452–459. doi:10.1016/j.ijmm.2017.09.019
- Taglialegna A, Varela MC, Rosato RR, et al. VraSR and virulence trait modulation during daptomycin resistance in methicillin-resistant Staphylococcus aureus infection. mSphere. 2019;4(1):e00557–18. doi:10.1128/mSphere.00557-18
- Parrett A, Reed JM, Gardner SG, et al. Metabolic changes associated with adaptive resistance to daptomycin in Streptococcus mitis-oralis. BMC Microbiol. 2020;20(1):162. doi:10.1186/s12866-020-01849-w
- Marchi AP, Perdigão Neto LV, Martins RCR, et al. Vancomycin-resistant enterococci isolates colonizing and infecting haematology patients: clonality, and virulence and resistance profile. J Hosp Infect. 2018;99(3):346–355. doi:10.1016/j.jhin.2017.10.010
- Nielsen KL, Pedersen TM, Udekwu KI, et al. Fitness cost: a bacteriological explanation for the demise of the first international methicillin-resistant Staphylococcus aureus epidemic. J Antimicrob Chemother. 2012;67(6):1325–1332. doi:10.1093/jac/dks051
- Yokoyama M, Stevens E, Laabei M, et al. Epistasis analysis uncovers hidden antibiotic resistance-associated fitness costs hampering the evolution of MRSA. Genome Biol. 2018;19(1):94. doi:10.1186/s13059-018-1469-2
- Knight GM, Colijn C, Shrestha S, et al. The distribution of fitness costs of resistance-conferring mutations is a key determinant for the future burden of drug-resistant tuberculosis: a model-based analysis. Clin Infect Dis. 2015;61(Suppl 3):S147–S154. doi:10.1093/cid/civ579
- Hubbard ATM, Jafari NV, Feasey N, et al. Effect of environment on the evolutionary trajectories and growth characteristics of antibiotic-resistant Escherichia coli mutants. Front Microbiol. 2019;10:2001. doi:10.3389/fmicb.2019.02001
- Rasouly A, Shamovsky Y, Epshtein V, et al. Analysing the fitness cost of antibiotic resistance to identify targets for combination antimicrobials. Nat Microbiol. 2021;6(11):1410–1423. doi:10.1038/s41564-021-00973-1
- El-Gaml RM, El-Khodary NM, Abozahra RR, et al. Applying pharmacokinetic/pharmacodynamic measurements for linezolid in critically ill patients: optimizing efficacy and reducing resistance occurrence. Eur J Clin Pharmacol. 2022;78(8):1301–1310. doi:10.1007/s00228-022-03340-z
- La H, Bodolea C, Vlase L, et al. Linezolid administration to critically Ill patients: intermittent or continuous infusion? A systematic literature search and review. Antibiotics. 2022;11(4):436. doi:10.3390/antibiotics11040436
- Zhu LL, Zhou Q. Optimal infusion rate in antimicrobial therapy explosion of evidence in the last five years. Infect Drug Resist. 2018;11:1105–1117. doi:10.2147/IDR.S167616
- Boak LM, Li J, Rayner CR, et al. Pharmacokinetic/pharmacodynamic factors influencing emergence of resistance to linezolid in an in vitro model. Antimicrob Agents Chemother. 2007;51(4):1287–1292. doi:10.1128/AAC.01194-06
- Lagatolla C, Milic J, Imperi F, et al. Synergistic activity of fosfomycin and chloramphenicol against vancomycin-resistant Enterococcus faecium (VREfm) isolates from bloodstream infections. Diagn Microbiol Infect Dis. 2021;99(2):115241. doi:10.1016/j.diagmicrobio.2020.115241
- Liu BG, Yuan XL, He DD, et al. Research progress on the oxazolidinone drug linezolid resistance. Eur Rev Med Pharmacol Sci. 2020;24(18):9274–9281. doi:10.26355/eurrev_202009_23009
- Katip W, Okonogi S, Oberdorfer P. The thirty-day mortality rate and nephrotoxicity associated with trough serum vancomycin concentrations during treatment of enterococcal infections: a propensity score matching analysis. Front Pharmacol. 2022;12:773994. doi:10.3389/fphar.2021.773994
- Katip W, Oberdorfer P, Monocentric Retrospective A. Study of AUC/MIC ratio of vancomycin associated with clinical outcomes and nephrotoxicity in patients with enterococcal infections. Pharmaceutics. 2021;13(9):1378. doi:10.3390/pharmaceutics13091378
- Falagas ME, Athanasaki F, Voulgaris GL, et al. Resistance to fosfomycin: mechanisms, frequency and clinical consequences. Int J Antimicrob Agents. 2019;53(1):22–28. doi:10.1016/j.ijantimicag.2018.09.013