Abstract
The incidence of fungal infections is increasing at an alarming rate and has posed a great challenge for science in recent years. The rise in these infections has been related to the increase in immunocompromised patients and the resistance of different species to antifungal drugs. Infections caused by the different Candida species, especially Candida albicans, are one of the most common mycoses in humans, and the etiological agents are considered opportunistic pathogens associated with high mortality rates when disseminated infections occur. Candida lusitaniae is considered an emerging opportunistic pathogen that most frequently affects immunocompromised patients with some comorbidity. Although it is a low-frequency pathogen, and the mortality rate of C. lusitaniae-caused candidemia does not exceed 5%, some isolates are known to be resistant to antifungals such as amphotericin B, 5-fluorocytosine, and fluconazole. In this paper, a detailed review of the current literature on this organism and its different aspects, such as its biology, possible virulence factors, pathogen-host interaction, diagnosis, and treatment of infection, is provided. Of particular interest, through Blastp analysis we predicted possible virulence factors in this species.
Introduction
Candida spp. are often part of the normal microbiota that resides in non-sterile human tissues and as a such, are often found as part of the respiratory, gastrointestinal, urinary, and genital tracts, in the skin, fingernails, and oral cavity.Citation1,Citation2 Candidiasis is the name given to the infections caused by members of the Candida genus, and traditionally, the most frequently isolated species from clinical specimens is Candida albicans, which is associated with high morbidity and mortality rates.Citation3–7 However, C. albicans is not the sole species of this fungal genus associated with human diseases.Citation8,Citation9 The emergent pathogen Candida lusitaniae is an opportunistic haploid yeast that has been reported as the etiological cause of infection in humans, most frequently in immunocompromised patients who often have comorbidities.Citation10 Even though it is considered a low-frequency emerging nosocomial pathogen and susceptible to conventional antifungal therapies, C. lusitaniae has attracted attention because some isolates are resistant to amphotericin B, 5-fluorocytosine, or fluconazole.Citation7,Citation11–13 From the infections caused by Candida spp., C. lusitaniae is responsible for approximately 19.3% of fungemia cases in cancer patients,Citation14 and approximately 1.7% of all cases of genitourinary candidiasis in ambulatory patients.Citation15
Before the fluconazole era, C. lusitaniae infections were associated with high mortality rates; however, nowadays this is uncommon and mortality usually does not exceed 5%.Citation10 Additionally to fungemia, C. lusitaniae has also been associated with peritonitis, meningitis, and urinary tract infections.Citation7,Citation10 Thus far, the basic aspects of this organism have been poorly studied, and there is an increasing need to develop new alternatives to diagnose and treat this species and others belonging to the Candida genus. With no doubt, these facts make this fungal species of interest for both applied and basic science. Here, we offer a critical revision of the most relevant information on both C. lusitaniae clinical and basic aspects and the caused infection.
Basic Biological Attributes of Candida lusitaniae
C. lusitaniae is a dimorphic organism that produces ovoid, ellipsoidal, or elongated yeast cells of a size of 2–6×2–10 µm, similar to other Candida species, such as Candida tropicalis,Citation16 and colonies are creamy in color and appearance, soft and smooth.Citation10 At the difference of C. albicans, the most studied species of this genus, C. lusitaniae is not capable of developing true hyphae, only pseudohyphae, which is a blastoconidium with a constricted budding neck between conidium and the first compartment of the emerging germ tube.Citation17,Citation18 It is worthy to mention that the dimorphism in this organism has been related to the fungal resistance to amphotericin B.Citation18–20 In addition, this morphological plasticity offers a possibility for a daughter cell to survive the host immune defenses.Citation20 In CHROMagar, the colonies generate a pinkish to purple color, allowing their differentiation from C. tropicalis, since both species are morphologically similar.Citation19
The Candida genus once thought to gather asexual species, has now revealed some species with other reproductive cycles. C. albicans may go through parasexual and asexual cycles, while C. lusitaniae shows asexual and sexual cycles, being its teleomorph Clavispora lusitaniae and assuring meiosis during spore formation.Citation21,Citation22 The C. lusitaniae mating-type (MAT) locus has been reported to be like the one found in C. albicans, and the sexual cycle is regulated by the biallelic locus MATa and MATα.Citation23 The strain MATα has four out of five genes homologs to C. albicans MLTα, whereas the MATa locus has a translocation that differentiates it from the C. albicans MATa.Citation21 C. lusitaniae genome contains the genes MATα1 and MATa2, encoding for transcription factors required for cell mating and identity, and MATa1 required for sporulation.Citation21 The mating between α and a cells is performed when they are co-cultured and during stress by starvation.Citation21 Then, the pheromones induce the conjugative tube, followed by cell and nuclear fusions, and finally, the formation of the asci that contains two spores.Citation21
The recombination during the sexual cycle is SPO11 dependent. This gene encodes for a meiosis-specific topoisomerase and is a homolog of SPO11 found in other eukaryotes.Citation21,Citation23 In addition, C. lusitaniae CLS12, a dispensable gene for filamentation, is involved in mating and is a homolog of the Saccharomyces cerevisiae STE12, a mating, and filamentation regulator.Citation24
Although the internal and external C. lusitaniae structures and organelles are not very well characterized, it is known that like other Candida species it possesses a cell wall, cell membrane, endoplasmic reticulum, ribosomes, and Golgi apparatus.Citation25 The C. lusitaniae cell wall has not been analyzed by transmission electron microscopy, but in nearby species such as Candida krusei, Candida parapsilosis, C. tropicalis, and C. albicans, it is known that the wall is uniform and with well-defined layers with different compositions.Citation26–35 The outermost layer is observed as an electron-dense material of approximately 20 nm thick, corresponding to mannosylated glycoproteins, then, an electron-transparent layer in the middle, which has an appearance of being composed of spongy material and dispersion granules, and an inner layer of around 100 nm formed by a transparent matrix that contains filamentous structures.Citation27,Citation30,Citation36–38 This innermost layer is generally composed of chitin and β-glucans in Candida spp;Citation29,Citation31–34,Citation39–44 while the outermost layer contains proteins modified with both N-linked and O-linked mannans.Citation30,Citation38,Citation45–49 The C. lusitaniae mannan structure is significantly different from other Candida species and closer to that described in C. albicans, showing β-1,2-mannose residues as part of the N-linked mannan side chains.Citation50 In C. lusitaniae, the structural polysaccharides β-1,3-glucan and chitin, as in other Candida species, are located underneath the cell wall proteins, most of which are covalently linked to β-1,6-glucan by glycosylphosphatidylinositol anchors.Citation51 In terms of immunological recognition by the host, the cell wall is the one that fulfills the most important function, since displays molecules that have a positive role in this immune sensing but also can disguise the interaction with immune effectors.Citation41,Citation51–53
So far, the C. lusitaniae metabolism has been poorly studied and scarce information is currently available. This organism is known to metabolize glucose, cellobiose, and cellotriose but fails to degrade cellotetrose, a phenotypical trait that differentiates this species from Candida guilliermondii.Citation54 Moreover, C. lusitaniae can also use galactose, sucrose, maltose, lactate, and trehalose as carbon sources.Citation55 Similar to other yeast-like species, it possesses a fermentative metabolism capable of producing ethanol from D-xylose under anaerobic conditions.Citation55 This ability to adapt its metabolism to assimilate different carbon sources has been linked to the resistance to some drugs, such as amphotericin B; cells growing in lactate are about 10 times more resistant to the drug than those growing in presence of glucose as a carbon source.Citation56 Based on these metabolic characteristics, this Candida species can be identified by the assimilation of sorbose, rhamnose, and 2-keto-D-gluconate.Citation55
The C. lusitaniae genome is distributed in eight chromosomes and belongs to the Candida CTG clade.Citation57 This clade is composed of C. albicans, C. lusitaniae, Candida dubliniensis, C. tropicalis, C. guilliermondii, and C. parapsilosis, and they have in common the nonconventional use of the CUG codon to encode for serine instead of leucine.Citation57 Since C. lusitaniae has a sexual stage, it can go from haploid to diploid; being asexual cells often found as haploid organisms.Citation58
The C. lusitaniae (ATCC 42720) nuclear genome sequence contains 12.11 Mbp, with a GC content of 44.5%, a total of 6153 protein-encoding genes, and five pseudogenes.Citation59 A recent study reported the sequencing of five C. lusitaniae strains.Citation60 The strain DSY4606 (P1) contains 12.08 Mbp, the GC content is 44.53%, 5676 protein-encoding genes from a total of 5882 genes that have been predicted, along with nine rRNA genes and 197 tRNA genes.Citation60 The other strains analyzed in this study were P2 to P5 and the number of the genes varied from 5869 to 5892, suggesting small variations in the gene numbers and that the phenotypic plasticity could be due to recombination events during sexual reproduction.Citation60
The Virulence Factors Repertoire
Cell adhesion is essential in various biological processes and many fungi such as Candida spp. contain a family of cell wall glycoproteins named adhesins, which are responsible for offering unique adhesion properties.Citation61 Adhesins are indispensable for fungal cell–cell interactions and to mediate the host-fungus interplay.Citation62 The Candida spp. adhesion to host cells, in particular epithelial cells, is the first step in the infective process. C. lusitaniae also colonizes the host’s epithelial cells as part of the first events of the infection but does not cause damage like other Candida species. It was reported that C. albicans is significantly more adherent (61.6%) to buccal epithelial cells than C. lusitaniae (2%),Citation63 and a similar trend was observed when the fungal ability to bind mucin was tested.Citation64 These low adhesion properties correlate with the low virulence reported for C. lusitaniae. Like other Candida species, C. lusitaniae can also adhere to plastic surfaces, such as indwelling catheters, cannulas, and drains.Citation65 The ALS gene family members, EAP1, ECM33, HWP1, IFF4, INT1, and MP65, are encoding for the major C. albicans adhesins.Citation66 Even though none of the putative orthologs of these genes have been characterized in C. lusitaniae, these can be found within its genome (), making it likely that adhesion occurs via these cell surface adhesins.
Table 1 Prediction of the Most Important Virulence Factors in Candida lusitaniae
Cell surface hydrophobicity and biofilm formation are known as relevant virulence factors in Candida spp. pathogenesis. The wall hydrophobicity is provided by the presence of hydrophobic proteins that are embedded within the Candida cell wall,Citation67 and several studies have linked the cell wall hydrophobicity with Candida adhesion to epithelial cells.Citation68,Citation69 A study involving 15 C. lusitaniae isolates showed that these had a higher wall hydrophobicity (37.52%), compared to C. albicans cells (8.48%).Citation68 Increased hydrophobicity has been related to cell adhesion; however, this is not the case for C. lusitaniae, where this species has been reported to be more hydrophobic but less adherent.Citation68 This result could be explained by the fact that there are other factors apart from hydrophobicity that are related to adherence, among these, fungal cells can switch between hydrophobic and hydrophilic phenotypes, due to changes in the environmental conditions such as the temperature, nutrient composition, growth phases, and culture medium used for cell propagation.Citation70,Citation71 This phenomenon has been reported in other Candida species, such as in C. dubliniensis.Citation68,Citation72
Biofilm formation is an important factor that confers protection to the fungal cells, making them resistant to chemical or physical damage.Citation73 A study of mixed biofilms between C. albicans and C. lusitaniae showed this was not viable, but between C. tropicalis and C. dubliniensis was successfully established, suggesting that the hypha production by the two species was required for biofilm formation.Citation74 In another study, it was demonstrated that the ability of C. lusitaniae to form biofilms is influenced by the culturing media, being capable of doing so in YNB, but not in RMPI broth.Citation57 In C. albicans, biofilm formation is regulated by seven principal genes BCR1, BRG1, EFG1, HSP90, NDT80, ROB1, and ZAP1, which are likely to be within the C. lusitaniae genome (). However, research is needed to assess the contribution of these genes during C. lusitaniae biofilm formation.
During the pathogenic process, hydrolytic enzymes are paramount for success, and among them are the secreted aspartyl proteinase (SAP), phospholipase, and lipases. In C. albicans, lipases are described in some infection models but their function is not clear yet.Citation75 The putative orthologs of C. albicans genes encoding for lipases and phospholipases found within the C. lusitaniae genome are shown in . The SAPs have been described in C. albicans and some studies have focused on finding homologs in the other medically relevant Candida species. Here, shows the putative SAPs orthologs found in C. lusitaniae, although the function is still unknown. SAPs help the pathogen penetrate the host and to evade the immune response, this way being an important element in pathogens’ virulence.Citation76 When comparing the proteolytic activity of four different Candida species, C. lusitaniae showed the highest hydrolytic activity but at the same time low enzyme secretion.Citation76 This observation may help to understand the low virulence of this species and its poor ability to kill laboratory animals.
As mentioned, C. lusitaniae belongs to the genus members that are not capable of forming true hyphae, only pseudohyphae. Dimorphism in fungi, such as C. albicans is related to the expression of some virulence factors that are morphology specific.Citation27 Among the main dimorphism regulators found in C. albicans are Cph1, a transcriptional regulator that controls filamentous growth; Hgc1, an essential protein for hyphal morphogenesis; and Nrg1/Tup1, transcriptional repressors that contribute to filamentation.Citation77–79 According to our analysis, the C. lusitaniae genome contains putative orthologs of these genes (), suggesting that the process that controls dimorphism is differently regulated in C. albicans and C. lusitaniae.
The phenotypic switching is strongly related to C. albicans virulence, allowing the fungus to adjust to different environmental conditions through the expression of different and selective genes.Citation80 C. lusitaniae undergoes phenotypic changes when cultured on YPD-CuSO4 agar, generating white and light brown colonies containing exclusively yeast cells, and dark brown colonies containing pseudohyphae.Citation80 Light brown colonies showed a minimum inhibitory concentration (MIC) of 2–4 µg/mL for amphotericin B, the dark brown colonies of 8 µg/mL, and the white colonies of 256 µg/mL, underlining that this phenotypic switching is related to drug resistance.Citation80
Other virulence factors that play important roles in fungi are thermotolerance and immune evasion. Thermotolerance is responsible for facilitating the growth and colonization of the fungal cell once entering the host tissues. The host temperature is usually higher than the optimal for fungal growth, thus adaptation to this stressing milieu is essential for cell fitness and the ability to damage the host cells and tissues. Our bioinformatic analysis suggests that the C. lusitaniae genome contains putative orthologs of the genes for thermotolerance HSP60 and HSP104, which encode heat shock proteins (). Hsp60 acts as an immunogenic trigger in the orchestration of diseases when there is thermal stress and Hsp104 is a survival mediator, in response to increased temperature.Citation81,Citation82 Immune evasion is a mechanism that involves many other processes, such as biofilm and protease production, morphological changes, and protein synthesis to overcome oxidative stress.Citation41 Some of these processes have already been explained earlier in this section. For immune evasion, two possible orthologs of C. albicans Hgt1 and Msb2 were found in C. lusitaniae. However, no ortholog of the Pra1 gene was found (). However, the mechanisms of thermotolerance and immune evasion are probably similar in both fungi, due to the results obtained in our bioinformatics analysis ().
The Candida lusitaniae-Immune System Interaction
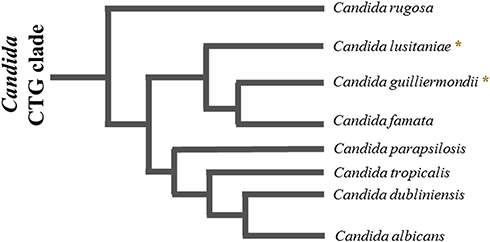
Currently, the most studied fungus–host interaction is that of C. albicans. However, although there are species-dependent variations in the way the host immune system recognizes Candida spp., the core processes may be similar and involve the recognition of the microorganism through pattern recognition receptors (PRRs). To the best of our knowledge, no study has explored the C. lusitaniae-host interaction; however, for Candida guilliermondii, the phylogenetically closest species within the Candida genus (), the characteristics of this interaction have been more studied.Citation33 Therefore, it is possible to speculate that the current information we have regarding C. guilliermondii could be extrapolated to C. lusitaniae.Citation83
Figure 1 Schematic representation of the phylogenetic relationship between species of the Candida CTG clade. The species C. rugosa, C lusitaniae, C guilliermondii, C famata, C parapsilosis, C tropicalis, C dubliniensis and C. albicans are part of the CTG clade of Candida. (٭) represents the species of our interest, C lusitaniae, and C. guilliermondii, which are phylogenetically closer to each other. The lengths of the branches are arbitrary.
Immune detection of fungal species is an important process in establishing a protective antifungal immune response. For this interaction to take place, the components of the cell wall must be recognized.Citation30,Citation33,Citation84 The fungal cell wall is a highly dynamic structure that provides protection, controls communication with the extracellular environment, maintains cell integrity, and functions as a molecular scaffold to display virulence factors.Citation33,Citation38,Citation44,Citation52 This structure has pathogen-associated molecular patterns (PAMPs), which are recognized by the immune system through PRRs, most of them located on the cell surface of immune cells.Citation85–87 The main PAMPs found in the different Candida species, such as C. guilliermondii, are chitin, β-1,3- and β-1,6-glucans, and N-linked and O-linked mannans.Citation51
It has been shown that during the immune response against Candida species, such as C. guilliermondii, murine neutrophils, and phagocytic cells can discriminate among species.Citation88,Citation89 Murine phagocytic cells, bone marrow cells, and spleen cells have a greater ability to kill C. guilliermondii when compared to the phagocytic rate of C. albicans cells.Citation88 Human monocytes differentially recognize some species of Candida such as C. tropicalis and C. krusei, but not C. guilliermondii, which is involved in increased stimulation of the complement components C3 and colony-stimulating factor of granulocytes and macrophages.Citation89 C. guilliermondii shows a limited ability to stimulate tumor necrosis factor α (TNFα) when coincubated with peritoneal macrophages.Citation90
Human peripheral blood mononuclear cells (PBMCs) are often used to evaluate pathogen–host interaction in different fungal species since they can produce different types of cytokines when the PRRs are activated by PAMPs. Although the different species of Candida show a similar cell wall composition, some differences could affect the interaction with components of innate immunity.Citation33,Citation44 In C. guilliermondii it has been observed that low levels of β-1,3-glucan induce lower cytokine levels when this polysaccharide is exposed to the cell surface.Citation33
C. guilliermondii cells stimulate higher levels of the cytokines TNFα, IL-6, IL-1β, and IL-10, compared to C. albicans where stimulation is very low.Citation33 When cells are heat-inactivated (HK), higher levels of cytokines are stimulated than C. guilliermondii live cells; however, differences are observed when cytokine profiles are compared with those stimulated by C. albicans.Citation39,Citation73 C. guilliermondii stimulates a lower production of TNFα, IL-1β, and IL-6 than C. albicans when the HK and β-elimination treatments are used, but the anti-inflammatory cytokine IL-10 is highly produced in presence of C. guilliermondii cells.Citation33 It is tempting to suggest that a similar cytokine profile may be stimulated by C. lusitaniae cells.
Blockade of receptors such as Dectin-1 with laminarin does not affect the ability of C. albicans to stimulate cytokine production; however, a significant reduction in cytokine levels is observed when HK cells are used.Citation31–34,Citation39,Citation40,Citation42,Citation43,Citation47 For C. guilliermondii though the presence of laminarin affects the cytokine stimulation by live or β-eliminated cells, indicating that a difference with C. albicans, O-linked mannans along with β-1,3-glucan sensing are key interactions for a strong cytokine stimulation.Citation33
Assays with macrophages revealed that most Candida species are uptake and internalized in acid phagolysosomes; however, the species that experienced this process in greater proportion are C. tropicalis, C. guilliermondii, and C. krusei at the difference of C. albicans and C. auris.Citation33
In addition to the responses mentioned above, complement proteins play an important role in the defense of the host against the pathogen, especially against members of the Candida species, since they promote phagocytosis and activate inflammatory responses.Citation91,Citation92 This system is a link between innate and adaptive immunity in such a way that a complete immune response against the pathogen is created.Citation93 In addition to C. albicans, species such as C. lusitaniae, C. glabrata, C. parapsilosis, and C. tropicalis have also been reported to bind to complement proteins.Citation94
Once again, due to close phylogenetic relationship between C. lusitaniae and C. guilliermondii, we suggest that the immune response in both species is similar; however, this must be experimentally verified.
Candida lusitaniae-Caused Candidiasis
C. albicans is the main species causing candidiasis in humans. However, other species of this genus have raised particular concern by exhibiting resistance to broad-spectrum antifungals commonly used to treat candidiasis.Citation95 This is the case of C. lusitaniae which, despite being a rare pathogen, has aroused special interest as a nosocomial pathogen due to its increased prevalence in recent years, being characterized by infecting immunocompromised patients, patients receiving prolonged antibiotic therapy, hospitalized, patients with underlying malignancies, and undergoing chemotherapies or bone marrow transplants.Citation7,Citation10,Citation12,Citation96 Another risk factor for candidiasis is the use of catheters, as they are major yeast reservoirs that promote fungemia, and C. lusitaniae is no exception.Citation97,Citation98 Case reports in the literature have demonstrated the ability of C. lusitaniae to form biofilms, causing endogenous infections,Citation99,Citation100 and like other causative agents of candidiasis, the possibility of acquiring this species through person-to-person contact has been reported, at least in the intensive care unit.Citation101
In a study conducted on patients with candidemia, it was shown that 43.5% of patients were infected by non-albicans species. Of this percentage, most patients were found to have a neutrophil count of fewer than 500 cells/μL.Citation95 Another study, conducted at a Texas cancer center between 1988 and 1999, reported that 75% of patients were neutropenic at the time of C. lusitaniae infection, with a mortality rate in these cases of 25%, which could be related to its high resistance to amphotericin B.Citation7 In fact, most of the severe C. lusitaniae infections reported resistance to this antifungal drug.Citation16,Citation102–104
C. lusitaniae has been isolated most frequently from the respiratory tract, followed by urine and blood samples. It has also been isolated from the peritoneum, vagina, and skin.Citation11,Citation12,Citation103,Citation105 In mouse models, kidney colonization by C. lusitaniae was found to be indifferent to the animal’s immunocompetence.Citation18 Unusually, cases of keratitis have been reported where C. lusitaniae was one of the etiological agents.Citation106,Citation107 The unusualness of these clinical cases was verified when a study published in 2012 reported that only 3 of 18 mouse models developed keratitis after being challenged with wild-type C. lusitaniae yeast cells.Citation18
Clinical data on invasive infections caused by C. lusitaniae are scarce.Citation108 However, as mentioned, previous reports have shown that infections caused by C. lusitaniae usually appear in patients with hematological malignancies.Citation108,Citation109 A patient with acute lymphoblastic leukemia who underwent hematopoietic cell transplantation developed catheter-associated C. lusitaniae candidemia while undergoing amphotericin B therapy.Citation108 Similar to this case, there are several patients with malignant neoplasms, mainly leukemia, which are affected by this pathogen. Most patients have been reported to have both neutropenia and stem cell transplantation, known independent factors to develop this systemic infection.Citation12,Citation109,Citation110 In 2003, 55 cases of C. lusitaniae-caused candidiasis were reported, predominantly bloodstream infections.Citation10 Three-quarters of the studied population had underlying medical conditions, which led to a mortality rate of 5%.Citation10
Another study conducted by the International Pediatric Fungal Network, between 2007 and 2011, showed that C. lusitaniae was found in 8 out of 201 isolates collected from 196 non-neonatal pediatric patients.Citation111 The University Children’s Hospital Münster obtained data on infections caused by different Candida species between 1998 and 2006. Among these, C. lusitaniae was found to be the causal agent of 7.1% of candidemias in patients under 20 years old.Citation108,Citation112 Most of these patients were immunocompromised, had an indwelling venous catheter, and were receiving broad-spectrum antibiotic treatment.Citation112 These three conditions are shared with another clinical study, where 12 patients showed fungemia due to C. lusitaniae.Citation7 Ten of these patients had received cytotoxic drugs and nine patients were neutropenic.Citation7 A case study reported the presence of C. lusitaniae in an immunocompetent patient with intraperitoneal infection after undergoing laparoscopic hydrosalpinx surgery.Citation113 Intra-abdominal infections are a morbidity cause in patients undergoing abdominal surgery and are commonly caused by Candida species.Citation113
Diagnosis of Candida lusitaniae in Clinical Samples
The effective Candida strains identification at the species level in the clinical area has become very important due to the high incidence of candidiasis in recent years. Different strategies have been used over time to differentiate and identify the different Candida species. Chromogenic agars, such as Candida ID agar and CHROMagar Candida agar,Citation114–116 have been used for the detection and presumptive identification of Candida spp., especially C. albicans. Candida ID agar is based on a chromogenic substrate of indolyl glucosaminide that is hydrolyzed by the different Candida species and generates different colors in the colonies. In the case of C. lusitaniae, the colonies appear pink in this agar; however, although the color could be informative for identification, other species such as C. tropicalis and C. guilliermondii develop colonies of this same color.Citation116 C. lusitaniae identification on CHROMagar Candida, a medium that also uses a chromogenic substrate of β-glucosaminidase, shows purple and white colonies, however, these colonies have the same colors as C. krusei and C. parapsilosis, which could not ensure the correct C. lusitaniae identification.Citation116 In corn meal agar, C. lusitaniae shows ovoid yeast cells, which are arranged in pairs and chains, also, abundantly branched and curved pseudohyphae can be seen. Some strains of this species have rudimentary or null pseudohyphae.Citation117
In recent years, methods have been developed to allow early identification of the different Candida species, trying to reduce morbidity and mortality of infected patients.Citation118 Candida spp. identification by traditional methods such as morphology analysis can take 3–5 days or even longer for unusual species.Citation119 The design of Candida species-specific probes has helped identify more than 18 species of this genus, including C. lusitaniae.Citation119 For this, universal fungal primers, multicopy genetic targets, and species-specific probes are used, which are directed to the ITS2 region of the gene that encodes rRNA.Citation120 The API 20C carbohydrate assimilation system is also a gold standard for phenotypic characterization of non-albicans species.Citation116,Citation119
For C. lusitaniae identification, the ability of the API Candida system (bioMérieux, France) to identify isolates of this species has been evaluated.Citation121 Of 52 clinical isolates that had been previously identified based on their morphology, 48 of these were identified as C. lusitaniae at 48 hours. Subsequently, 44 of the isolates were identified as C. lusitaniae at 24 hours, and the other four were discarded because they assimilated cellobiose more slowly.Citation121 The morphological identification determined that the strains corresponded to the species C. lusitaniae; however, this identification was verified using the ID 32C system, which was chosen for its extensive database.Citation121 By this system, all the strains were identified as C. lusitaniae, two of these by applying complementary tests and reincubating for another 24 hours.Citation121 Using the API Candida system, only 12 strains were identified as C. lusitaniae at 24 hours. In other words, the API system is not effective for the identification of this species and it is proposed that it is necessary to include morphological characteristics to avoid misidentification of C. lusitaniae as Candida famata.Citation122 Although API Candida is considered a promising system for the identification of Candida species, it is not the most adequate to identify C. lusitaniae.Citation121
Molecular taxonomic methods have also been used for C. lusitaniae identification.Citation110 Using these methodologies, it was determined that the DNA bases of two clinical isolates were 45.1% guanine plus cytosine molecules from one strain, compared to 44.7% guanine plus cytosine molecules from the second strain. DNA/DNA reassociation experiments showed that there was a complementarity greater than 95% between the DNA of the two C. lusitaniae clinical isolates.Citation110
Therapeutic Options to Treat Infections Caused by Candida lusitaniae
Four classes of antifungal drugs are currently used to treat systemic candidiasis: azoles, such as fluconazole, itraconazole, posaconazole, and voriconazole; polyenes, like conventional amphotericin B and its lipid formulations; the echinocandins caspofungin and micafungin; and the pyrimidine analog flucytosine.Citation123 Several non-albicans Candida species are inherently resistant or less susceptible to various classes of antifungals, and the introduction of new azoles such as fluconazole has increased the frequency of multidrug-resistant strains.Citation123
C. lusitaniae is known to develop resistance to amphotericin B; however, this species is considered susceptible to flucytosine and azoles.Citation124 Although in most cases this pattern is consistent, several studies have shown that this species can develop resistance to flucytosine and azoles, classifying it as a species difficult to manage due to the variation in antifungal susceptibilities.Citation7,Citation124–127 Echinocandins are the most widely used antifungal drugs for the treatment of C. lusitaniae-caused candidemia, which targets the β-1,3-D-glucan synthase encoded by the FKS genes,Citation128 but their use has resulted in reported emerging resistance in several strains.Citation129 Mutations in the FKS genes specifically FKS1 and FKS2 are responsible for the increased MIC in some species. In C. lusitaniae, it is reported that a nonsense mutation occurs in the FKS1 hot spot 1 at position 645 (S645F), which leads to an increase in the MIC for several echinocandins.Citation129 Caspofungin resistance correlates with three new FKS1 mutations (S638Y, S638P, and S631Y); which correspond to positions Ser645 and Ser643 of C. albicans Fks1, and have been related to echinocandins resistance.Citation127,Citation129 Furthermore, resistance to fluconazole in this species is thought to be associated with the overexpression of a major facilitator gene (MFS7), and mutations in the transcriptional activator MRR1 in C. albicans.Citation130
Clinical cases of neonates with kidney infections caused by C. lusitaniae have shown that changes in colony morphology are associated with resistance to amphotericin B and azoles.Citation126 Previous reports have indicated that initial therapy is based on the use of amphotericin B and that it is used mainly as monotherapy.Citation131 However, due to increased resistance to this antifungal, therapy began to be replaced by fluconazole or combined therapy. This therapy seemed to work in 85% of patients, who were cured, and the mortality rate decreased, presenting only 12% mortality in patients treated under this scheme.Citation7 The response appears to be different for clinical cases, and largely depends on the patient’s immunity. In a study of 46 patients, one-third were cured with amphotericin B, one-third with fluconazole, and one-third with flucytosine.Citation126 Although exclusive therapy with amphotericin B is not ruled out, an initial combination with flucytosine is recommended.Citation126 Fluconazole therapy is effective in many cases and is recommended for treating disseminated candidiasis caused by C. lusitaniae.Citation7,Citation132 However, it is necessary to carry out in vitro tests as soon as the presence of this species is identified, to determine the most appropriate treatment for each patient.Citation126
Clinical reports have described that C. lusitaniae can generate multiresistant isolates when fluconazole antifungals are combined with amphotericin B.Citation129 This type of combined treatment can be counterproductive and it is suggested that should be avoided, especially when C. lusitaniae is involved in deep-seated infections in immunocompromised patients.Citation126,Citation129
Something that has attracted attention in this species is the phenotypic change that it can develop in the culture medium. Two colony color variations have been demonstrated in CHROMagar Candida, causing the phenotype of full-size colonies and small colonies, both of which are included in the MATa genotype.Citation20,Citation126,Citation133,Citation134 These phenotypical switching affected susceptibility to amphotericin B.Citation20,Citation126 Moreover, changes in phenotype are observed when cross-resistance to fluconazole and itraconazole develops. This phenomenon had been already reported in different clinical cases that had documented acquired resistance to amphotericin B.Citation135 It seems that this change is influenced by the adaptation of the organism to environmental changes.Citation126 Similar findings have been reported also in Candida glabrata.Citation133 These events of resistance to antifungals such as amphotericin B appear to be correlated with decreased ergosterol levels as a result of a defect in sterol isomerase.Citation136 Although it is not ruled out that the resistance may be mediated by other mechanisms, such as the alteration of other steps in the biosynthesis of sterols, changes in the plasmatic membrane phospholipids, modifications in the cell wall structure, and the increase in the catalase activity.Citation137
Finally, the correct treatment of infections caused by C. lusitaniae requires early control of susceptibility to antifungal drugs and an exhaustive examination of cultures to evaluate the possible morphological changes above mentioned.
Concluding Remarks
In recent years, research on candidiasis has been increasing but most of the work has been focused on understanding the biological, epidemiological, clinical, and biological aspects of species such as C. albicans and to a lesser extent on other species such as C. parapsilosis, C. glabrata, C. tropicalis, and C. krusei.Citation27 C. lusitaniae is perhaps a forgotten pathogen because of the low frequency of isolation in healthcare centers. However, the infection caused by this species can be fatal in immunocompromised patients and the microorganism can develop resistance to antifungal drugs such as amphotericin B, and azoles, making it a difficult species to treat.
Bioinformatic tools have been key to understanding basic aspects of neglected species like this one since they allow us to generate gene predictions, which help to detect differences and similarities in terms of virulence, drug resistance, and relevant biological information when compared to thoroughly studied species like C. albicans and C. tropicalis. This information could be useful to develop new techniques for diagnosis, and treatment and to find other therapeutic targets against C. lusitaniae.
The information collected in this work highlights that there is still a lack of information about this species, which could be an opportunity area to develop more exhaustive studies that allow the scientific community to elucidate important aspects of this species’ biology. It would be interesting to develop new methodologies that allow the rapid and efficient identification of C. lusitaniae strains since the methodologies currently used are not very specific and could give false negatives or false positives, as is the case of the morphological evaluation in Candida ID agar and CHROMagar Candida. Finally, the study of the interaction with humoral and cellular components of the host immunity results paramount to understanding the C. lusitaniae-host interaction and proposing immunomodulatory options to treat the caused infections by this Candida species.
Author Contributions
All authors made a significant contribution to the work reported, in the conception, design, execution, acquisition of data, analysis, and interpretation, critically reviewed the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
This work was supported by Consejo Nacional de Ciencia y Tecnología (ref. FC 2015-02-834 and CF-2019-6380), and Red Temática Glicociencia en Salud (CONACYT-México).
References
- Aguiar Cordeiro R, de Jesus Evangelista AJ, Serpa R, et al. β-lactam antibiotics & vancomycin increase the growth & virulence of Candida spp. Future Microbiol. 2018;13(8):869–875. doi:10.2217/fmb-2018-0019
- Goncalves B, Ferreira C, Alves CT, Henriques M, Azeredo J, Silva S. Vulvovaginal candidiasis: epidemiology, microbiology and risk factors. Crit Rev Microbiol. 2016;42(6):905–927. doi:10.3109/1040841X.2015.1091805
- Pfaller MA, Diekema DJ, Mendez M, et al. Candida guilliermondii, an opportunistic fungal pathogen with decreased susceptibility to fluconazole: geographic and temporal trends from the ARTEMIS DISK antifungal surveillance program. J Clin Microbiol. 2006;44(10):3551–3556. doi:10.1128/JCM.00865-06
- Pfaller MA, Jones RN, Messer SA, Edmond MB, Wenzel RP. National surveillance of nosocomial blood stream infection due to species of Candida other than Candida albicans: frequency of occurrence and antifungal susceptibility in the SCOPE Program. Diagn Microbiol Infect Dis. 1998;30(2):121–129. doi:10.1016/S0732-8893(97)00192-2
- Pfaller M, Neofytos D, Diekema D, et al. Epidemiology and outcomes of candidemia in 3648 patients: data from the Prospective Antifungal Therapy (PATH Alliance®) registry, 2004–2008. Diagn Microbiol Infect Dis. 2012;74(4):323–331. doi:10.1016/j.diagmicrobio.2012.10.003
- Andes DR, Safdar N, Baddley JW, et al. The epidemiology and outcomes of invasive Candida infections among organ transplant recipients in the United States: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Transpl Infect Dis. 2016;18(6):921–931. doi:10.1111/tid.12613
- Minari A, Hachem R, Raad I. Candida lusitaniae: a cause of breakthrough fungemia in cancer patients. Clin Infect Dis. 2001;32(2):186–190. doi:10.1086/318473
- Fakhim H, Vaezi A, Dannaoui E, et al. Comparative virulence of Candida auris with Candida haemulonii, Candida glabrata and Candida albicans in a murine model. Mycoses. 2018;61(6):377–382. doi:10.1111/myc.12754
- Fakhim H, Vaezi A, Javidnia J, et al. Candida africana vulvovaginitis: prevalence and geographical distribution. J Mycol Med. 2020;30(3):100966. doi:10.1016/j.mycmed.2020.100966
- Hawkins JL, Baddour LM. Candida lusitaniae infections in the era of fluconazole availability. Clin Infect Dis. 2003;36(2):e14–18. doi:10.1086/344651
- Baker JG, Nadler HL, Forgacs P, Kurtz SR. Candida lusitaniae: a new opportunistic pathogen of the urinary tract. Diagn Microbiol Infect Dis. 1984;2(2):145–149. doi:10.1016/0732-8893(84)90010-5
- Sanchez V, Vazquez JA, Barth-Jones D, Dembry L, Sobel JD, Zervos MJ. Epidemiology of nosocomial acquisition of Candida lusitaniae. J Clin Microbiol. 1992;30(11):3005–3008. doi:10.1128/jcm.30.11.3005-3008.1992
- Zhang H, Ran Y, Li D, et al. Clavispora lusitaniae and Chaetomium atrobrunneum as rare agents of cutaneous infection. Mycopathologia. 2010;169(5):373–380. doi:10.1007/s11046-009-9266-9
- Jung DS, Farmakiotis D, Jiang Y, Tarrand JJ, Kontoyiannis DP. Uncommon Candida species fungemia among cancer patients, Houston, Texas, USA. Emerg Infect Dis. 2015;21(11):1942–1950. doi:10.3201/eid2111.150404
- Obisesan OJ, Olowe OA, Taiwo SS. Phenotypic detection of genitourinary candidiasis among sexually transmitted disease clinic attendees in Ladoke Akintola University Teaching Hospital, Osogbo, Nigeria. J Environ Public Health. 2015;2015:401340. doi:10.1155/2015/401340
- Hadfield TL, Smith MB, Winn RE, Rinaldi MG, Guerra C. Mycoses caused by Candida lusitaniae. Rev Infect Dis. 1987;9(5):1006–1012. doi:10.1093/clinids/9.5.1006
- Boisnard S, Ruprich-Robert G, Florent M, Da Silva B, Chapeland-Leclerc F, Papon N. Role of Sho1p adaptor in the pseudohyphal development, drugs sensitivity, osmotolerance and oxidant stress adaptation in the opportunistic yeast Candida lusitaniae. Yeast. 2008;25(11):849–859. doi:10.1002/yea.1636
- Zhang J, Silao FG, Bigol UG, et al. Calcineurin is required for pseudohyphal growth, virulence, and drug resistance in Candida lusitaniae. PLoS One. 2012;7(8):e44192. doi:10.1371/journal.pone.0044192
- McClenny NB, Fei H, Baron EJ, et al. Change in colony morphology of Candida lusitaniae in association with development of amphotericin B resistance. Antimicrob Agents Chemother. 2002;46(5):1325–1328. doi:10.1128/AAC.46.5.1325-1328.2002
- Yoon SA, Vazquez JA, Steffan PE, Sobel JD, Akins RA. High-frequency, in vitro reversible switching of Candida lusitaniae clinical isolates from amphotericin B susceptibility to resistance. Antimicrob Agents Chemother. 1999;43(4):836–845. doi:10.1128/AAC.43.4.836
- Reedy JL, Floyd AM, Heitman J. Mechanistic plasticity of sexual reproduction and meiosis in the Candida pathogenic species complex. Curr Biol. 2009;19(11):891–899. doi:10.1016/j.cub.2009.04.058
- Gargeya IB, Pruitt WR, Simmons RB, Meyer SA, Ahearn DG. Occurrence of Clavispora lusitaniae, the teleomorph of Candida lusitaniae, among clinical isolates. J Clin Microbiol. 1990;28(10):2224–2227. doi:10.1128/jcm.28.10.2224-2227.1990
- Francois F, Noel T, Pepin R, et al. Alternative identification test relying upon sexual reproductive abilities of Candida lusitaniae strains isolated from hospitalized patients. J Clin Microbiol. 2001;39(11):3906–3914. doi:10.1128/JCM.39.11.3906-3914.2001
- Young LY, Lorenz MC, Heitman J. A STE12 homolog is required for mating but dispensable for filamentation in Candida lusitaniae. Gene. 2000;155(1):17–29.
- Leite de Andrade MC, Soares de Oliveira MA, Santos F, et al. A new approach by optical coherence tomography for elucidating biofilm formation by emergent Candida species. PLoS One. 2017;12(11):e0188020. doi:10.1371/journal.pone.0188020
- Diaz-Jimenez DF, Mora-Montes HM, Hernandez-Cervantes A, Luna-Arias JP, Gow NA, Flores-Carreon A. Biochemical characterization of recombinant Candida albicans mannosyltransferases Mnt1, Mnt2 and Mnt5 reveals new functions in O- and N-mannan biosynthesis. Biochem Biophys Res Commun. 2012;419(1):77–82. doi:10.1016/j.bbrc.2012.01.131
- Gomez-Gaviria M, Mora-Montes HM. Current aspects in the biology, pathogeny, and treatment of Candida krusei, a neglected fungal pathogen. Infect Drug Resist. 2020;13:1673–1689. doi:10.2147/IDR.S247944
- Mora-Montes HM, Ponce-Noyola P, Villagomez-Castro JC, Gow NA, Flores-Carreon A, Lopez-Romero E. Protein glycosylation in Candida. Future Microbiol. 2009;4(9):1167–1183. doi:10.2217/fmb.09.88
- Estrada-Mata E, Navarro-Arias MJ, Perez-Garcia LA, et al. Members of the Candida parapsilosis complex and Candida albicans are differentially recognized by human peripheral blood mononuclear cells. Front Microbiol. 2015;6:1527. doi:10.3389/fmicb.2015.01527
- Gómez-Gaviria M, García-Carnero LC, Tamez-Castrellón AK, Mora-Montes HM. The cell wall of medically relevant yeasts and molds. In: Zaragoza Ó, Casadevall A, editors. Encyclopedia of Mycology. Oxford: Elsevier; 2021:12–22.
- Hernandez-Chavez MJ, Clavijo-Giraldo DM, Novak A, et al. Role of protein mannosylation in the Candida tropicalis-host interaction. Front Microbiol. 2019;10:2743. doi:10.3389/fmicb.2019.02743
- Hernandez-Chavez MJ, Franco B, Clavijo-Giraldo DM, Hernandez NV, Estrada-Mata E, Mora-Montes HM. Role of protein phosphomannosylation in the Candida tropicalis-macrophage interaction. FEMS Yeast Res. 2018;18(5). doi:10.1093/femsyr/foy053
- Navarro-Arias MJ, Hernandez-Chavez MJ, Garcia-Carnero LC, et al. Differential recognition of Candida tropicalis, Candida guilliermondii, Candida krusei, and Candida auris by human innate immune cells. Infect Drug Resist. 2019;12:783–794. doi:10.2147/IDR.S197531
- Perez-Garcia LA, Csonka K, Flores-Carreon A, et al. Role of protein glycosylation in Candida parapsilosis cell wall integrity and host interaction. Front Microbiol. 2016;7:306. doi:10.3389/fmicb.2016.00306
- Toth R, Nosek J, Mora-Montes HM, et al. Candida parapsilosis: from genes to the bedside. Clin Microbiol Rev. 2019;32(2). doi:10.1128/CMR.00111-18
- Joshi KR, Wheeler EE, Gavin JB. The ultrastructure of Candida krusei. Mycopathologia. 1975;56(1):5–8. doi:10.1007/BF00493575
- Grigor’eva A, Bardasheva A, Tupitsyna A, et al. Changes in the ultrastructure of Candida albicans treated with cationic peptides. Microorganisms. 2020;8(4):582.
- Gómez-Gaviria M, Vargas-Macías AP, García-Carnero LC, Martínez-Duncker I, Mora-Montes HM. Role of protein glycosylation in interactions of medically relevant fungi with the host. J Fungi. 2021;7(10):875. doi:10.3390/jof7100875
- Navarro-Arias MJ, Defosse TA, Dementhon K, et al. Disruption of protein mannosylation affects Candida guilliermondii cell wall, immune sensing, and virulence. Front Microbiol. 2016;7:1951. doi:10.3389/fmicb.2016.01951
- Navarro-Arias MJ, Dementhon K, Defosse TA, et al. Group X hybrid histidine kinase Chk1 is dispensable for stress adaptation, host-pathogen interactions and virulence in the opportunistic yeast Candida guilliermondii. Res Microbiol. 2017;168(7):644–654. doi:10.1016/j.resmic.2017.04.009
- Hernandez-Chavez MJ, Perez-Garcia LA, Nino-Vega GA, Mora-Montes HM. Fungal strategies to evade the host immune recognition. J Fungi. 2017;3(4):51. doi:10.3390/jof3040051
- Gow NA, Netea MG, Munro CA, et al. Immune recognition of Candida albicans beta-glucan by dectin-1. J Infect Dis. 2007;196(10):1565–1571. doi:10.1086/523110
- Mora-Montes HM, Netea MG, Ferwerda G, et al. Recognition and blocking of innate immunity cells by Candida albicans chitin. Infect Immun. 2011;79(5):1961–1970. doi:10.1128/IAI.01282-10
- Walker LA, Munro CA. Caspofungin induced cell wall changes of Candida species influences macrophage interactions. Front Cell Infect Microbiol. 2020;10:164. doi:10.3389/fcimb.2020.00164
- Shibata N, Kobayashi H, Suzuki S. Immunochemistry of pathogenic yeast, Candida species, focusing on mannan. Proc Jpn Acad Ser B Phys Biol Sci. 2012;88(6):250–265. doi:10.2183/pjab.88.250
- Klis FM, de Groot P, Hellingwerf K. Molecular organization of the cell wall of Candida albicans. Med Mycol. 2001;39(Suppl 1):1–8. doi:10.1080/mmy.39.1.1.8-0
- Mora-Montes HM, Bates S, Netea MG, et al. A multifunctional mannosyltransferase family in Candida albicans determines cell wall mannan structure and host-fungus interactions. J Biol Chem. 2010;285(16):12087–12095. doi:10.1074/jbc.M109.081513
- Mukaremera L, Lee KK, Mora-Montes HM, Gow NAR. Candida albicans yeast, pseudohyphal, and hyphal morphogenesis differentially affects immune recognition. Front Immunol. 2017;8:629. doi:10.3389/fimmu.2017.00629
- Munro CA, Bates S, Buurman ET, et al. Mnt1p and Mnt2p of Candida albicans are partially redundant alpha-1,2-mannosyltransferases that participate in O-linked mannosylation and are required for adhesion and virulence. J Biol Chem. 2005;280(2):1051–1060. doi:10.1074/jbc.M411413200
- Shibata N, Kobayashi H, Okawa Y, Suzuki S. Existence of novel beta-1,2 linkage-containing side chain in the mannan of Candida lusitaniae, antigenically related to Candida albicans serotype A. Eur J Biochem. 2003;270(12):2565–2575. doi:10.1046/j.1432-1033.2003.03622.x
- Gow NAR, Latge JP, Munro CA. The fungal cell wall: structure, biosynthesis, and function. Microbiol spect. 2017;5(3). doi:10.1128/microbiolspec.FUNK-0035-2016
- Díaz-Jiménez DF, Pérez-García LA, Martínez-álvarez JA, Mora-Montes HM. Role of the fungal cell wall in pathogenesis and antifungal resistance. Curr Fungal Infect Rep. 2012;6(4):275–282. doi:10.1007/s12281-012-0109-7
- Gow NA, van de Veerdonk FL, Brown AJ, Netea MG. Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat Rev Microbiol. 2012;10(2):112–122. doi:10.1038/nrmicro2711
- Freer SN. Fermentation and aerobic metabolism of cellodextrins by yeasts. Appl Environ Microbiol. 1991;57(3):655–659. doi:10.1128/aem.57.3.655-659.1991
- Lachance M-A. Chapter 21 - Clavispora Rodrigues de Miranda (1979). In: Kurtzman CP, Fell JW, Boekhout T, editors. The Yeasts. 5th ed. London: Elsevier; 2011:349–353.
- Ene IV, Adya AK, Wehmeier S, et al. Host carbon sources modulate cell wall architecture, drug resistance and virulence in a fungal pathogen. Cell Microbiol. 2012;14(9):1319–1335. doi:10.1111/j.1462-5822.2012.01813.x
- Priest SJ, Lorenz MC. Characterization of virulence-related phenotypes in Candida species of the CUG clade. Eukaryot Cell. 2015;14(9):931–940. doi:10.1128/EC.00062-15
- Sherwood RK, Scaduto CM, Torres SE, Bennett RJ. Convergent evolution of a fused sexual cycle promotes the haploid lifestyle. Nature. 2014;506(7488):387–390. doi:10.1038/nature12891
- Butler G, Rasmussen MD, Lin MF, et al. Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature. 2009;459(7247):657–662. doi:10.1038/nature08064
- Kannan A, Asner SA, Trachsel E, Kelly S, Parker J, Sanglard D. Comparative genomics for the elucidation of multidrug resistance in Candida lusitaniae. mBio. 2019;10(6):e02512–02519. doi:10.1128/mBio.02512-19
- de Groot PWJ, Bader O, de Boer AD, Weig M, Chauhan N. Adhesins in human fungal pathogens: glue with plenty of stick. Eukaryot Cell. 2013;12(4):470–481. doi:10.1128/EC.00364-12
- Willaert RG. Adhesins of yeasts: protein structure and interactions. J Fungi. 2018;4(4):119. doi:10.3390/jof4040119
- Biasoli MS, Tosello ME, Magaró HM. Adherence of Candida strains isolated from the human gastrointestinal tract. Mycoses. 2002;45(11–12):465–469. doi:10.1046/j.1439-0507.2002.00793.x
- de Repentigny L, Aumont F, Bernard K, Belhumeur P. Characterization of binding of Candida albicans to small intestinal mucin and its role in adherence to mucosal epithelial cells. Infect Immun. 2000;68(6):3172–3179. doi:10.1128/IAI.68.6.3172-3179.2000
- Dorko E, Kmet’ová M, Marossy A, Dorko F, Molokácová M. Non-albicans Candida species isolated from plastic devices. Mycopathologia. 1999;148(3):117–122. doi:10.1023/A:1007178806720
- Staniszewska M. Virulence factors in Candida species. Curr Protein Pept Sci. 2020;21(3):313–323. doi:10.2174/1389203720666190722152415
- Nordin MA, Wan Harun WH, Abdul Razak F. An in vitro study on the anti-adherence effect of Brucea javanica and Piper betle extracts towards oral Candida. Arch Oral Biol. 2013;58(10):1335–1342. doi:10.1016/j.archoralbio.2013.07.001
- Muadcheingka T, Tantivitayakul P. Distribution of Candida albicans and non-albicans Candida species in oral candidiasis patients: correlation between cell surface hydrophobicity and biofilm forming activities. Arch Oral Biol. 2015;60(6):894–901. doi:10.1016/j.archoralbio.2015.03.002
- Panagoda GJ, Ellepola AN, Samaranayake LP. Adhesion to denture acrylic surfaces and relative cell-surface hydrophobicity of Candida parapsilosis and Candida albicans. APMIS. 1998;106(7):736–742. doi:10.1111/j.1699-0463.1998.tb00220.x
- Bujdáková H, Didiášová M, Drahovská H, Černáková L. Role of cell surface hydrophobicity in Candida albicans biofilm. Open Life Sci. 2013;8(3):259–262. doi:10.2478/s11535-013-0136-y
- Krasowska A, Sigler K. How microorganisms use hydrophobicity and what does this mean for human needs? Front Cell Infect Microbiol. 2014;4:112. doi:10.3389/fcimb.2014.00112
- Blanco MT, Morales JJ, Lucio L, Pérez-Giraldo C, Hurtado C, Gómez-García AC. Modification of adherence to plastic and to human buccal cells of Candida albicans and Candida dubliniensis by a subinhibitory concentration of itraconazole. Oral Microbiol Immunol. 2006;21(1):69–72. doi:10.1111/j.1399-302X.2005.00260.x
- Gulati M, Nobile CJ. Candida albicans biofilms: development, regulation, and molecular mechanisms. Microbes Infect. 2016;18(5):310–321. doi:10.1016/j.micinf.2016.01.002
- Pathirana RU, McCall AD, Norris HL, Edgerton M. Filamentous non-albicans Candida species adhere to Candida albicans and benefit from dual biofilm growth. Front Microbiol. 2019;10:1188. doi:10.3389/fmicb.2019.01188
- Schaller M, Borelli C, Korting HC, Hube B. Hydrolytic enzymes as virulence factors of Candida albicans. Mycoses. 2005;48(6):365–377. doi:10.1111/j.1439-0507.2005.01165.x
- Pichová I, Pavlícková L, Dostál J, et al. Secreted aspartic proteases of Candida albicans, Candida tropicalis, Candida parapsilosis and Candida lusitaniae. Inhibition with peptidomimetic inhibitors. Eur J Biochem. 2001;268(9):2669–2677. doi:10.1046/j.1432-1327.2001.02152.x
- Braun BR, Johnson AD. TUP1, CPH1 and EFG1 make independent contributions to filamentation in Candida albicans. Genetics. 2000;155(1):57–67. doi:10.1093/genetics/155.1.57
- Cleary IA, Lazzell AL, Monteagudo C, Thomas DP, Saville SP. BRG1 and NRG1 form a novel feedback circuit regulating Candida albicans hypha formation and virulence. Mol Microbiol. 2012;85(3):557–573. doi:10.1111/j.1365-2958.2012.08127.x
- Zheng X, Wang Y, Wang Y. Hgc1, a novel hypha-specific G1 cyclin-related protein regulates Candida albicans hyphal morphogenesis. EMBO J. 2004;23(8):1845–1856. doi:10.1038/sj.emboj.7600195
- Miller NS, Dick JD, Merz WG. Phenotypic switching in Candida lusitaniae on copper sulfate indicator agar: association with amphotericin B resistance and filamentation. J Clin Microbiol. 2006;44(4):1536–1539. doi:10.1128/JCM.44.4.1536-1539.2006
- Sanchez Y, Lindquist SL. HSP104 required for induced thermotolerance. Science. 1990;248(4959):1112–1115. doi:10.1126/science.2188365
- Gong Y, Li T, Yu C, Sun S. Candida albicans heat shock proteins and Hsps-associated signaling pathways as potential antifungal targets. Front Cell Infect Microbiol. 2017;7(520):520. doi:10.3389/fcimb.2017.00520
- Papon N, Courdavault V, Clastre M, Bennett RJ. Emerging and emerged pathogenic Candida species: beyond the Candida albicans paradigm. PLoS Pathog. 2013;9(9):e1003550.
- Martinez-Alvarez JA, Perez-Garcia LA, Flores-Carreon A, Mora-Montes HM. The immune response against Candida spp. and Sporothrix schenckii. Rev Iberoam Micol. 2014;31(1):62–66. doi:10.1016/j.riam.2013.09.015
- Netea MG, Brown GD, Kullberg BJ, Gow NA. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol. 2008;6(1):67–78. doi:10.1038/nrmicro1815
- Netea MG, Joosten LA, van der Meer JW, Kullberg BJ, van de Veerdonk FL. Immune defence against Candida fungal infections. Nat Rev Immunol. 2015;15(10):630–642. doi:10.1038/nri3897
- Reid DM, Gow NAR, Brown GD. Pattern recognition: recent insights from Dectin-1. Curr Opin Immunol. 2009;21(1):30–37. doi:10.1016/j.coi.2009.01.003
- Vecchiarelli A, Bistoni F, Cenci E, Perito S, Cassone A. In-vitro killing of Candida species by murine immunoeffectors and its relationship to the experimental pathogenicity. Sabouraudia. 1985;23(5):377–387. doi:10.1080/00362178585380541
- Høgåsen AK, Abrahamsen TG, Gaustad P. Various Candida and Torulopsis species differ in their ability to induce the production of C3, factor B and granulocyte-macrophage colony-stimulating factor (GM-CSF) in human monocyte cultures. J Med Microbiol. 1995;42(4):291–298. doi:10.1099/00222615-42-4-291
- Aybay C, Imir T. Tumor necrosis factor (TNF) induction from monocyte/macrophages by Candida species. Immunobiology. 1996;196(4):363–374. doi:10.1016/S0171-2985(96)80059-3
- Killick J, Morisse G, Sieger D, Astier AL. Complement as a regulator of adaptive immunity. Semin Immunopathol. 2018;40(1):37–48. doi:10.1007/s00281-017-0644-y
- Singh DK, Tóth R, Gácser A. Mechanisms of pathogenic Candida species to Evade the host complement attack. Front Cell Infect Microbiol. 2020;10:94. doi:10.3389/fcimb.2020.00094
- Dunkelberger JR, Song WC. Complement and its role in innate and adaptive immune responses. Cell Res. 2010;20(1):34–50. doi:10.1038/cr.2009.139
- Meri T, Hartmann A, Lenk D, et al. The yeast Candida albicans binds complement regulators factor H and FHL-1. Infect Immun. 2002;70(9):5185–5192. doi:10.1128/IAI.70.9.5185-5192.2002
- Chi HW, Yang YS, Shang ST, et al. Candida albicans versus non-albicans bloodstream infections: the comparison of risk factors and outcome. J Microbiol Immunol Infect. 2011;44(5):369–375. doi:10.1016/j.jmii.2010.08.010
- Khan Z, Ahmad S, Al-Sweih N, Khan S, Joseph L. Candida lusitaniae in Kuwait: prevalence, antifungal susceptibility and role in neonatal fungemia. PLoS One. 2019;14(3):e0213532. doi:10.1371/journal.pone.0213532
- Luzzati R, Amalfitano G, Lazzarini L, et al. Nosocomial candidemia in non-neutropenic patients at an Italian tertiary care hospital. Eur J Clin Microbiol Infect Dis. 2000;19(8):602–607. doi:10.1007/s100960000325
- Chow JK, Golan Y, Ruthazer R, et al. Factors associated with candidemia caused by non-albicans Candida species versus Candida albicans in the intensive care unit. Clin Infect Dis. 2008;46(8):1206–1213. doi:10.1086/529435
- Raja A, Park J. Disseminated Candida lusitaniae: nosocomial acquisition secondary to an indwelling urinary catheter. Case Rep Infect Dis. 2021;2021:6632730. doi:10.1155/2021/6632730
- Simitsopoulou M, Kyrpitzi D, Velegraki A, Walsh TJ, Roilides E. Caspofungin at catheter lock concentrations eradicates mature biofilms of Candida lusitaniae and Candida guilliermondii. Antimicrob Agents Chemother. 2014;58(8):4953–4956. doi:10.1128/AAC.03117-14
- Fowler SL, Rhoton B, Springer SC, Messer SA, Hollis RJ, Pfaller MA. Evidence for person-to-person transmission of Candida lusitaniae in a neonatal intensive-care unit. Infect Control Hosp Epidemiol. 1998;19(5):343–345. doi:10.2307/30141376
- Guinet R, Chanas J, Goullier A, Bonnefoy G, Ambroise-Thomas P. Fatal septicemia due to amphotericin B-resistant Candida lusitaniae. J Clin Microbiol. 1983;18(2):443–444. doi:10.1128/jcm.18.2.443-444.1983
- Merz WG. Candida lusitaniae: frequency of recovery, colonization, infection, and amphotericin B resistance. J Clin Microbiol. 1984;20(6):1194–1195. doi:10.1128/jcm.20.6.1194-1195.1984
- Blinkhorn RJ, Adelstein D, Spagnuolo PJ. Emergence of a new opportunistic pathogen, Candida lusitaniae. J Clin Microbiol. 1989;27(2):236–240. doi:10.1128/jcm.27.2.236-240.1989
- Desnos-Ollivier M, Moquet O, Chouaki T, Guérin AM, Dromer F. Development of echinocandin resistance in Clavispora lusitaniae during caspofungin treatment. J Clin Microbiol. 2011;49(6):2304–2306. doi:10.1128/JCM.00325-11
- Parentin F, Liberali T, Perissutti P. Polymicrobial keratomycosis in a three-year-old child. Ocul Immunol Inflamm. 2006;14(2):129–131. doi:10.1080/09273940500328487
- Huynh N, Chang HY, Borboli-Gerogiannis S. Ocular involvement in hospitalized patients with candidemia: analysis at a Boston tertiary care center. Ocul Immunol Inflamm. 2012;20(2):100–103. doi:10.3109/09273948.2011.646383
- Apsemidou A, Füller MA, Idelevich EA, Kurzai O, Tragiannidis A, Groll AH. Candida lusitaniae breakthrough fungemia in an immuno-compromised adolescent: case report and review of the literature. J Fungi. 2020;6(4):380. doi:10.3390/jof6040380
- Atkinson BJ, Lewis RE, Kontoyiannis DP. Candida lusitaniae fungemia in cancer patients: risk factors for amphotericin B failure and outcome. Med Mycol. 2008;46(6):541–546. doi:10.1080/13693780801968571
- Holzschu DL, Presley HL, Miranda M, Phaff HJ. Identification of Candida lusitaniae as an opportunistic yeast in humans. J Clin Microbiol. 1979;10(2):202–205. doi:10.1128/jcm.10.2.202-205.1979
- Steinbach WJ, Roilides E, Berman D, et al. Results from a prospective, international, epidemiologic study of invasive candidiasis in children and neonates. Pediatr Infect Dis J. 2012;31(12):1252–1257. doi:10.1097/INF.0b013e3182737427
- Tragiannidis A, Fegeler W, Rellensmann G, et al. Candidaemia in a European Paediatric University Hospital: a 10-year observational study. Clin Microbiol Infect. 2012;18(2):E27–E30. doi:10.1111/j.1469-0691.2011.03720.x
- Wawrysiuk S, Rechberger T, Futyma K, Miotła P. Candida lusitaniae - a case report of an intraperitoneal infection. Prz Menopauzalny. 2018;17(2):94–96. doi:10.5114/pm.2018.77310
- Baumgartner C, Freydiere AM, Gille Y. Direct identification and recognition of yeast species from clinical material by using albicans ID and CHROMagar Candida plates. J Clin Microbiol. 1996;34(2):454–456. doi:10.1128/jcm.34.2.454-456.1996
- Odds FC, Bernaerts R. CHROMagar Candida, a new differential isolation medium for presumptive identification of clinically important Candida species. J Clin Microbiol. 1994;32(8):1923–1929. doi:10.1128/jcm.32.8.1923-1929.1994
- Cooke VM, Miles RJ, Price RG, Midgley G, Khamri W, Richardson AC. New chromogenic agar medium for the identification of Candida spp. Appl Environ Microbiol. 2002;68(7):3622–3627. doi:10.1128/AEM.68.7.3622-3627.2002
- Deorukhkar SC, Roushani S. Identification of Candida species: conventional methods in the era of molecular diagnosis. Ann Microbiol Immunol. 2018;1(1):1002.
- Essendoubi M, Toubas D, Bouzaggou M, Pinon JM, Manfait M, Sockalingum GD. Rapid identification of Candida species by FT-IR microspectroscopy. Biochim Biophys Acta. 2005;1724(3):239–247. doi:10.1016/j.bbagen.2005.04.019
- Elie CM, Lott TJ, Reiss E, Morrison CJ. Rapid identification of Candida species with species-specific DNA probes. J Clin Microbiol. 1998;36(11):3260–3265. doi:10.1128/JCM.36.11.3260-3265.1998
- Fujita S, Lasker BA, Lott TJ, Reiss E, Morrison CJ. Microtitration plate enzyme immunoassay to detect PCR-amplified DNA from Candida species in blood. J Clin Microbiol. 1995;33(4):962–967. doi:10.1128/jcm.33.4.962-967.1995
- Michel-Nguyen A, Favel A, Chastin C, Selva M, Regli P. Comparative evaluation of a commercial system for identification of Candida lusitaniae. Eur J Clin Microbiol Infect Dis. 2000;19(5):393–395. doi:10.1007/PL00011231
- Campbell CK, Davey KG, Holmes AD, Szekely A, Warnock DW. Comparison of the API Candida system with the AUXACOLOR system for identification of common yeast pathogens. J Clin Microbiol. 1999;37(3):821–823. doi:10.1128/JCM.37.3.821-823.1999
- Arendrup M, Horn T, Frimodt-Møller N. In vivo pathogenicity of eight medically relevant Candida species in an animal model. Infection. 2002;30(5):286–291. doi:10.1007/s15010-002-2131-0
- Lockhart SR, Iqbal N, Cleveland AA, et al. Species identification and antifungal susceptibility testing of Candida bloodstream isolates from population-based surveillance studies in two U.S. cities from 2008 to 2011. J Clin Microbiol. 2012;50(11):3435–3442. doi:10.1128/JCM.01283-12
- Barchiesi F, Tortorano AM, Di Francesco LF, Cogliati M, Scalise G, Viviani MA. In-vitro activity of five antifungal agents against uncommon clinical isolates of Candida spp. J Antimicrob Chemother. 1999;43(2):295–299. doi:10.1093/jac/43.2.295
- Favel A, Michel-Nguyen A, Peyron F, et al. Colony morphology switching of Candida lusitaniae and acquisition of multidrug resistance during treatment of a renal infection in a newborn: case report and review of the literature. Diagn Microbiol Infect Dis. 2003;47(1):331–339. doi:10.1016/S0732-8893(03)00094-4
- Pfaller MA, Diekema DJ, Andes D, et al. Clinical breakpoints for the echinocandins and Candida revisited: integration of molecular, clinical, and microbiological data to arrive at species-specific interpretive criteria. Drug Resist Updat. 2011;14(3):164–176. doi:10.1016/j.drup.2011.01.004
- Abruzzo GK, Flattery AM, Gill CJ, et al. Evaluation of the echinocandin antifungal MK-0991 (L-743,872): efficacies in mouse models of disseminated aspergillosis, candidiasis, and cryptococcosis. Antimicrob Agents Chemother. 1997;41(11):2333–2338. doi:10.1128/AAC.41.11.2333
- Asner SA, Giulieri S, Diezi M, Marchetti O, Sanglard D. Acquired multidrug antifungal resistance in Candida lusitaniae during therapy. Antimicrob Agents Chemother. 2015;59(12):7715–7722. doi:10.1128/AAC.02204-15
- Dunkel N, Blass J, Rogers PD, Morschhäuser J. Mutations in the multi-drug resistance regulator MRR1, followed by loss of heterozygosity, are the main cause of MDR1 overexpression in fluconazole-resistant Candida albicans strains. Mol Microbiol. 2008;69(4):827–840. doi:10.1111/j.1365-2958.2008.06309.x
- Krcmery V Jr, Oravcova E, Spanik S, et al. Nosocomial breakthrough fungaemia during antifungal prophylaxis or empirical antifungal therapy in 41 cancer patients receiving antineoplastic chemotherapy: analysis of aetiology risk factors and outcome. J Antimicrob Chemother. 1998;41(3):373–380. doi:10.1093/jac/41.3.373
- Rex JH, Walsh TJ, Sobel JD, et al. Practice guidelines for the treatment of candidiasis. Infectious Diseases Society of America. Clin Infect Dis. 2000;30(4):662–678. doi:10.1086/313749
- Bouchara JP, Zouhair R, Leb S, et al. In-vivo selection of an azole-resistant petite mutant of Candida glabrata. J Med Microbiol. 2000;49(11):977–984. doi:10.1099/0022-1317-49-11-977
- Vargas K, Messer SA, Pfaller M, et al. Elevated phenotypic switching and drug resistance of Candida albicans from human immunodeficiency virus-positive individuals prior to first thrush episode. J Clin Microbiol. 2000;38(10):3595–3607. doi:10.1128/JCM.38.10.3595-3607.2000
- Odds EC. Switch of phenotype as an escape mechanism of the intruder. Mycoses. 1997;40(Suppl 2):9–12. doi:10.1111/j.1439-0507.1997.tb00556.x
- Peyron F, Favel A, Calaf R, Michel-Nguyen A, Bonaly R, Coulon J. Sterol and fatty acid composition of Candida lusitaniae clinical isolates. Antimicrob Agents Chemother. 2002;46(2):531–533. doi:10.1128/AAC.46.2.531-533.2002
- Ghannoum MA, Rice LB. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev. 1999;12(4):501–517. doi:10.1128/CMR.12.4.501
- Kamai Y, Kubota M, Kamai Y, Hosokawa T, Fukuoka T, Filler SG. Contribution of Candida albicans ALS1 to the pathogenesis of experimental oropharyngeal candidiasis. Infect Immun. 2002;70(9):5256–5258. doi:10.1128/IAI.70.9.5256-5258.2002
- Gil-Bona A, Reales-Calderon JA, Parra-Giraldo CM, Martinez-Lopez R, Monteoliva L, Gil C. The cell wall protein Ecm33 of Candida albicans is Involved in chronological life span, morphogenesis, cell wall regeneration, stress tolerance, and host–cell interaction. Front Microbiol. 2016;7:64. doi:10.3389/fmicb.2016.00064
- Kempf M, Apaire-Marchais V, Saulnier P, et al. Disruption of Candida albicans IFF4 gene involves modifications of the cell electrical surface properties. Colloids Surf B: Biointer. 2007;58(2):250–255. doi:10.1016/j.colsurfb.2007.03.017
- Gomez MJ, Torosantucci A, Arancia S, Maras B, Parisi L, Cassone A. Purification and biochemical characterization of a 65-kilodalton mannoprotein (MP65), a main target of anti-Candida cell-mediated immune responses in humans. Infect Immun. 1996;64(7):2577–2584. doi:10.1128/iai.64.7.2577-2584.1996
- Nobile CJ, Mitchell AP. Regulation of cell-surface genes and biofilm formation by the C. albicans transcription factor Bcr1p. Curr Biol. 2005;15(12):1150–1155. doi:10.1016/j.cub.2005.05.047
- Burt ET, Daly R, Hoganson D, Tsirulnikov Y, Essmann M, Larsen B. Isolation and partial characterization of Hsp90 from Candida albicans. Ann Clin Lab Sci. 2003;33(1):86–93.
- Chen CG, Yang YL, Shih HI, Su CL, Lo HJ. CaNdt80 is involved in drug resistance in Candida albicans by regulating CDR1. Antimicrob Agents Chemother. 2004;48(12):4505–4512. doi:10.1128/AAC.48.12.4505-4512.2004
- Araújo D, Henriques M, Silva S. Portrait of Candida species biofilm regulatory network genes. Trends Microbiol. 2017;25(1):62–75. doi:10.1016/j.tim.2016.09.004
- Kenno S, Speth C, Rambach G, et al. Candida albicans factor H binding molecule Hgt1p – a low glucose-induced transmembrane protein is trafficked to the cell wall and impairs phagocytosis and killing by human neutrophils. Front Microbiol. 2019;9:3319. doi:10.3389/fmicb.2018.03319
- Swidergall M, Ernst AM, Ernst JF. Candida albicans mucin Msb2 is a broad-range protectant against antimicrobial peptides. Antimicrob Agents Chemother. 2013;57(8):3917–3922. doi:10.1128/AAC.00862-13
- Marcil A, Gadoury C, Ash J, Zhang J, Nantel A, Whiteway M. Analysis of PRA1 and its relationship to Candida albicans- macrophage interactions. Infect Immun. 2008;76(9):4345–4358. doi:10.1128/IAI.00588-07
- Leidich SD, Ibrahim AS, Fu Y, et al. Cloning and disruption of caPLB1, a phospholipase B gene involved in the pathogenicity of Candida albicans. J Biol Chem. 1998;273(40):26078–26086. doi:10.1074/jbc.273.40.26078
- Sun JN, Solis NV, Phan QT, et al. Host cell invasion and virulence mediated by Candida albicans Ssa1. PLoS Pathog. 2010;6(11):e1001181. doi:10.1371/journal.ppat.1001181
