Abstract
Background
Candidemia caused by Candida tropicalis has more serious adverse consequences and an even higher mortality. Time to positivity (TTP) has been widely used to identify microbial species, resistant microorganisms and distinguish real pathogens and pollutants. However, few studies have demonstrated TTP as a presumptive diagnosis of C. tropicalis in patients with candidemia.
Patients and Methods
A retrospective study of 136 episodes of candidemia and simulated blood cultures with 314 episodes of confirmed Candida strains were applied to explore the role of TTPs in diagnosing C. tropicalis. TTPs were recorded as the shorter one if both aerobic and anaerobic vials were positive. Lastly, relationships were tested between TTPs and resistance and initial inocula concentration.
Results
For the retrospective study, the mean of TTPs for C. tropicalis from 136 patients with candidemia was significantly shorter than other Candida species. The area under the receiver operating characteristics (ROC) curve was 0.8896 ± 0.030 with a sensitivity of 92.86% and a specificity of 77.87%, respectively, indicating TTPs with a cut-off value of <25.50 h had a strong diagnostic power for C. tropicalis in patients with candidemia. Moreover, TTPs from 314 simulated blood cultures showed similar results as the retrospective study, demonstrating TTP is a powerful diagnostic tool in early diagnosing C. tropicalis in patients with candidemia. Additionally, our results showed no statistical significance between TTPs and initial inocula concentration and resistance of Candida species, suggesting initial inocula concentration does not impact TTPs, and TTPs may not be promising in predicting the resistance of all Candida species.
Conclusion
TTP can be employed to early distinguish C. tropicalis from other Candida species in patients with candidemia, which is extremely helpful to initiate empiric antifungal treatments to improve clinical outcomes.
Introduction
C. albicans, C. glabrata, C. tropicalis and C. parapsilosis are four major pathogens causing candidemia worldwide.Citation1,Citation2 Invasive infections caused by these species have been increasingly seen in patients with immunodeficiency, organ transplantation, cancer patients with chemotherapies, and long-term antibiotics or glucocorticoid treatments.Citation3 Although C. albicans remains the most common pathogen in candidemia, non-C. albicans species are remarkably increasing as well, accounting for more than 50% of invasive candidiasis in multiple regions.Citation4–6 C. tropicalis is one of the invasive candidiasis notably increasing in recent years and has a sharp spike, especially in Latin America and part of Asian Pacific countries including India and China.Citation4,Citation7 In addition, the resistance of C. tropicalis to azoles has deteriorated year by year as well.Citation8,Citation9 It is reported from a Chinese five-year surveillance on invasive candidiasis that the resistance rate of C. tropicalis to fluconazole has increased the prominence from less than 8% in 2010 to over 22% in 2014.Citation10 It is noteworthy that invasive infection of C. tropicalis has more serious adverse consequences and relatively higher mortality compared with other Candida species,Citation11,Citation12 and its clinical outcomes largely rely on early effective interventions including susceptible antifungal treatments.Citation5,Citation13 However, identification of C. tropicalis takes a long time. Therefore, it is extremely important to identify potential pathogens and initiate immediate empiric therapy in patients with candidemia.Citation14
Time to positivity (TTP) provided by automatic blood culture machines can be used as powerful evidence to determine microbial species,Citation15 resistance,Citation16 distinguish real pathogens and pollutants,Citation17 predict sources and prognosis of bacteremia.Citation18 Some studies have previously reported that TTP of C. glabrata is longer compared with other Candida species in patients with candidiasis in BacT/AlertCitation19 and BACTEC 9240 systems.Citation20 Mounting evidence has shown that C. glabrata tend to grow in anaerobic blood culture vials particularly,Citation19,Citation20 and an exclusive or an earlier growth in anaerobic vials is useful to predict C. glabrata.Citation21 However, whether TTP can be applied as a presumptive diagnosis for C. tropicalis is rarely reported.
The purpose of this study was to investigate the diagnostic value of TPP in making an early differential diagnosis of C. tropicalis in patients with candidemia.
Materials and Methods
Retrospective Clinical Study
All patients with bloodstream infections were recorded in the clinical system from the Department of Microbiology Laboratory, the First Affiliated Hospital of Chengdu Medical College from January 1, 2015 to December 31, 2020. After exclusion of bacteremia, polymicrobial, filamentous fungemia, Cryptococcus, no recordings of TTPs, and duplicated strains, only patients with monomicrobial candidemia were enrolled in this retrospective study. Both their demographic information and TTPs were collected for further analysis. The blood culture vials used in the retrospective clinical study were Fastidious Antimicrobial Neutralization Media Aerobic & Fastidious Antimicrobial Neutralization Media Anaerobic (bioMérieux, France, containing activated carbon adsorbent). Around 8 mL −10 mL of blood was inoculated into each vial according to the manufacturer’s instruction, but the exact amount was not recorded for each patient. All blood cultures were performed by BacT/Alert 3D120 system (bioMérieux, France). TTPs were recorded as time intervals between the beginning to an automatic alert signal appeared (indicating growth of organism) during the blood cultures. The shorter one was deemed as the TTP if both aerobic and anaerobic vials were positive. If a patient had persistent candidemia with multiple positive blood cultures, TTPs were recorded only for the first round.
Simulated Blood Cultures
Multiple impactors can affect TTP, including inoculation volume and transportation time. In order to test the accuracy of data obtained from the retrospective analysis, another 314 confirmed Candida spp. strains were applied for simulated blood cultures as an internal control. All these strains were isolated from the Department of Microbiology Laboratory of the First Affiliated Hospital of Chengdu Medical College during the fourth quarter of 2021 (Table S1). Those Candida spp. strains isolated from various clinical specimens, such as blood, urine, abscess, ascites, etc. They were inoculated into blood culture vials with an aerobic and anaerobic environment, respectively. The names of the vials were Fastidious Antimicrobial Neutralization Media PLUS Aerobic/Anaerobic (bioMérieux, France) containing adsorbent of resin. All the performances were followed according to a protocol from Yarbrough et al.Citation22 Briefly, 1000 colony-forming unit (CFU) was used as a final inoculation dose for each vial. Vials for blood cultures were incubated in BacT/Alert 3D120 system. Cultures were stopped until positive growths were detected. The maximum culture time was 120 hours. TTPs were recorded as the same method before. Vials with positive results were consequently sub-cultured to a chocolate agar plate and a sabouraud agar plate, respectively, for further validation of Candida colonies by identification of the pure growth and expected colony morphologies. The recovered growth colonies in the sub-cultures were confirmed by matrix-assisted laser desorption ionization time of flight (MALDI-TOF) mass spectrometry (bioMérieux, France).
Antifungal Susceptibility Testing
Susceptibility tests of Candida spp. to antifungal antibiotics were performed on ATB ™ Fungus 3 stripe (bioMérieux, France). Minimum inhibitory concentrations (MICs) for all antifungal drugs were recorded after 24 hours of incubation. The MIC breakpoints for Candida spp. were referred to the Clinical and Laboratory Standards Institute (CLSI).Citation23,Citation24 C. albicans ATCC 24433 and C. parapsilosis ATCC 22019 were used for quality controls. All results were tested within determined ranges.
Statistical Analysis
All data analyses were performed by GraphPad Prism 5. TTPs between C. tropicalis and other Candida species were compared by Student’s t-test. Proportions were compared by X2 or Fisher’s exact test. The diagnostic values and cut-off values of TTPs for C. tropicalis were evaluated by the receiver operating characteristic (ROC) curve. p<0.05 was considered to be statistically significant.
Results
Demographic Features of 136 Patients with Candidemia in the Retrospective Study
A total of 5031 episodes of bloodstream infection were selected at the beginning of the retrospective study. After excluding bacteremia, polymicrobial, filamentous fungemia, Cryptococcus, no recordings of TTPs, and duplicated strains, a total of 136 episodes of monomicrobial candidemia were finally enrolled in this study (). It was shown that C. albicans (n = 45; 33.09%), C. parapsilosis (n = 32; 23.53%), C. glabrata (n = 32; 23.53%), and C. tropicalis (n = 14; 10.29%) were four major pathogens in patients with monomicrobial candidemia.
Figure 1 Flow chart showing the monomicrobial candidemia selection process.
The demographic features of 136 episodes of monomicrobial candidemia are listed in . Patients with urinary tract infection, renal insufficiency, and antibiotic use were significantly different in proportion of C. albicans, C. glabrata, C. tropicalis and C. parapsilosis bloodstream infection (p < 0.05). The results demonstrated that C. glabrata bloodstream infection was more likely to occur in patients with urinary tract infection (73.33%) or renal insufficiency (80.00%). Compared with other Candida species, patients with candidemia caused by C. albicans were less treated with broad-spectrum antibiotics (29.55%).
Table 1 Demographic Features of 136 Patients with Candidemia
Diagnostic Value of TTP for C. tropicalis
As shown in , the mean TTP of C. tropicalis was 21.26 ± 3.20 h, which was the shortest among Candida species (C. albicans: 34.82 ± 15.55 h, C. glabrata: 66.84 ± 30.87 h, C. parapsilosis: 33.44 ± 8.32 h, C. guilliermondii: 31.00 ± 7.65 h, C. sake: 59.75 ± 19.55, and C. krusei: 30.75 ± 7.76 h). TTPs were statistically different between each type of Candida species (shown in , ** p<0.01, ***p<0.001).
Table 2 TTPs of Different Candida species Included in the Retrospective Study
Figure 2 (A) Scatter plot of time to positivity (TTP) sorted by different Candida species, **p < 0.01, ***p < 0.001. (B) Receiver operating characteristic curves of time to positivity as a diagnostic test for C. tropicalis in patients with candidemia.
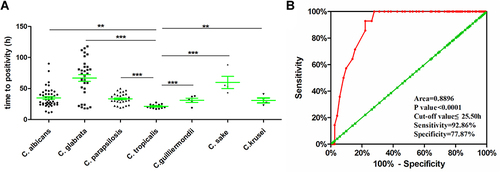
The diagnostic value of TTP for C. tropicalis in candidemia and its optimal cut-off values were determined by the receiver operating characteristic (ROC) curve (). Area under the ROC curve (AUC) demonstrated that TTP had a strong diagnostic value to identify C. tropicalis from other Candida species (AUC 0.8896 ± 0.030; 95% confidence interval 0.831–0.949). Based on the results of AUC and ROC curve, TTP < 25.50 h was the best optimal cut-off value to identify C. tropicalis from other Candida species in candidemia. Accordingly, when predicting C. tropicalis with a TTP of <25.50 h, the sensitivity and specificity were 92.86% and 77.87%, respectively; the positive and negative predictive values were 34.15% and 98.96%, respectively.
To verify the accuracy of the retrospective data, another 314 Candida strains were applied as an internal control to exclude other impactors, such as inoculation volume, transportation time, etc. The information regarding the age, gender, sample source, and susceptibility to antifungal agents were shown in Table S1. Strains collected for simulated blood cultures comprised 161 C. albicans (51.3%), 53 C. glabrata (16.9%), 33 C. parapsilosis (10.5%), 51 C. tropicalis (16.2%), and 16 other Candida species. All the results were consistent with the data obtained from our retrospective study (Table S2 and Figure S1). The mean of TTP for C. tropicalis was the shortest compared with other Candida species (18.32 ± 1.70 versus 27.35 ± 6.34, p < 0.0001). The AUC was 0.933 ±0.015, suggesting that TTP had a strong diagnostic value for C. tropicalis (p<0.0001) in simulated blood cultures. According to characteristics of ROC and AUC, the recommended cut-off value of C. tropicalis was <19.99 h, which generated sensitivity and specificity of 92.16% and 92.02%, respectively; the positive and negative predictive values were 69.12% and 98.37%, respectively.
Even though TTPs have been widely used for diagnosing Candida species, there are still some controversial issues. One current controversy is whether different concentrations of Candida species in the blood may have effects on TTP. Thus, we determined the effect of concentration of initial inocula of Candida species on TTP. Twenty Candida strains (selected from the above 314 strains randomly, including 8 C. albicans, 5 C. glabrata, 4 C. tropicalis and 3 C. parapsilosis) were inoculated with different concentrations (1000 CFU/vial, 5000 CFU/vial and 25000 CFU/vial) into vials (including anaerobic and aerobic vials), then the vials were incubated in BacT/Alert 3D120 system. It was found that TTPs tended to be shorter in vials with a higher concentration of initial inocula compared with lower one, but no statistical difference was found in each Candida species by comparisons among three different concentrations (0.06 ≤ p ≤ 0.72, Table S3).
Finally, the relationships were further tested between TTPs and susceptibility of Candida species to common antifungal drugs (amphotericin B, fluconazole, 5-fluorocytosine, and voriconazole). The results showed TTPs were not significantly different between resistant and susceptible strains to those four different antifungal agents (p > 0.05), suggesting that TTPs may not be potential to predict the susceptibility or resistance of each Candida species to amphotericin B, 5-fluorocytosine, fluconazole and voriconazole (shown in Table S4).
Discussion
Candidemia is an extremely dangerous clinical condition and carries a high mortality.Citation25,Citation26 Timely clinical interventions will prominently save patients’ lives. Therefore, an early and rapid diagnosis of potential pathogens is particularly important. Besides of blood cultures, several other methods can also be employed for the identification of candidemia.Citation27,Citation28 Mannan antigen and its antibody can be used for diagnosing candidemia, it has a specificity of 90%, but its sensitivity is around 60%.Citation29 Moreover, mannan antigen and its antibody are cleared quickly in the blood and cannot be used to diagnose specific Candida species.Citation30 1-3-β-D-glucan, a component of the cell wall of many pathogenic fungi, can be employed as an important auxiliary diagnosis of candidemia. However, since it has an extensive expression on various fungi, it has a high sensitivity but a low specificity to diagnose the specific species of Candida in candidemia.Citation30 Therefore, blood culture to isolation then to identification is still a widely accepted and commonly used method for diagnosis of specific Candida species in candidemia. However, since cultures of Candida species take a long time, it is challenging to make a prompt diagnosis of the exact species. As different Candida species have different susceptibilities to antifungal drugs, reasonable prediction of Candida species and choosing proper antifungal therapeutics can be extremely important and prominently reduce the mortality of candidemia.Citation31 In this study, data from a retrospective clinical study were firstly used to investigate relationships between TTPs and Candida species in patients with candidemia. It was found that different Candida species had different TTPs. C. glabrata had the longest TTPs, with a mean of 66.84 ± 30.87 h. While C. tropicalis had the shortest TTP, with a mean of 21.26 ± 3.20 h, which was consistent with the previous study.Citation32 In their report, TTPs of C. glabrata and C. tropicalis were 62.68 h and 22.14 h, respectively. However, another study,Citation33 in which TTPs were slightly different from ours, reported that TTPs of C. glabrata and C. tropicalis were 53.4 h (the longest) and 28.3 h (the second shortest), respectively, and C. krusei had the shortest TTP (23.3 h). The major reason for these differences might be due to a small sample size of C. krusei enrolled in both their and our studies (both were only four strains of C. krusei included).
TTPs might be affected by inoculation volume (volume of blood drawn into vials) and transportation time (from ward to microbiology laboratory) as well. To exclude these effects, another 314 confirmed Candida strains were performed for simulated blood cultures, TTPs were recorded and analyzed accordingly. TTPs of C. glabrata, C. parapsilosis, C. krusei, C. sake, C. albicans, and C. tropicalis were 32.57 ± 5.77h, 28.59 ± 6.13 h, 27.52 ± 5.62 h, 25.91 ± 3.47 h, 25.41 ± 5.66 h, and 18.32 ± 1.70 h, respectively. These TTPs were consistent with but significantly shorter compared to the results obtained from our retrospective study. We speculated many factors could contribute to these differences, such as concentrations of Candida spp. (colony-forming units) in patients’ blood, inoculation volume of blood, transportation time (from ward to microbiological laboratory), and different vials (vials used for our retrospective study were Fastidious Antimicrobial Neutralization Media containing activated charcoal, and vials for simulated blood cultures were Fastidious Antimicrobial Neutralization Media PLUS with adsorbent of resin). All in all, TTP of C. tropicalis was the shortest in both clinical retrospective study and simulated blood cultures.
The major current debate on TTP in patients with candidemia are impactors like the natural growth rate among different Candida species and the number of pathogens in the blood.Citation32,Citation34,Citation35 The number of pathogens in the blood is closely associated with clinical features (eg, the source or the severity of infection).Citation36 To answer this important question, we systematically evaluated the relationships between TTPs and the concentration of initial inocula. Our results demonstrated that the concentration of initial inocula had no significant effect on TTPs. Therefore, we believed that TTPs were mainly associated with the natural growth rate in Candida species, but not number of pathogens in blood. In addition, we also analyzed the relationships between TTPs and resistance of Candida species. We found no significant correlations between TTPs and resistance of each Candida species.
Secondly, the ROC curve was used to determine the diagnostic value of TTP for C. tropicalis in both retrospective clinical data and simulated blood cultures. In the retrospective study, AUC was 0.8896 ± 0.030 with a 95% confidence interval of 0.831–0.949, which was higher than other reports for making a diagnosis of C. glabrata by TTPs,Citation19,Citation27,Citation28 suggesting that TTP is a powerful tool to identify C. tropicalis from other Candida species. When TTP < 25.50 h was used as a cut-off value, the sensitivity and specificity of TTP for diagnosing C. tropicalis were 92.86% and 77.87%, and the positive and negative predictive values were 34.15% and 98.96%, respectively. Both specificity and sensitivity were prominently higher than previous reports on diagnosing C. glabrata by TTPs.Citation27,Citation28 Consistent with our retrospective clinical data, TTPs in simulated blood cultures had a high diagnostic value for C. tropicalis, the AUC was 0.933 ± 0.015 with a 95% confidence interval of 0.904–0.962, the optimal cut-off value was TTP < 19.99 h. Those data suggest that TTP has a higher diagnostic value for C. tropicalis compared with C. glabrata.
There are some limitations in this study as well. Firstly, TTPs might be affected by multiple factors, including blood inoculation volume, culture conditions, time intervals between sample inoculation to reception time in the lab, prior antifungal treatments, and pathogen numbers in patients’ blood, etc. However, our simulated blood cultures confirmed that these factors had little effect on the diagnostic value of TTP for C. tropicalis. Secondly, durations of candidemia were not recorded before blood specimens were collected and cultured, which may also have an impact on TTPs. Finally, this study was a single-center research with limited clinical samples, the results generated in our study might be different from other clinical centers due to different brands of vials and testing machines. Thus, multi-center studies with a large number of samples are required to further validate our results in the future.
Conclusion
In conclusion, our study shows that TTP is an ideal diagnostic tool to facilitate early identification of C. tropicalis from other Candida species in patients with candidemia, which can aid clinicians in timely treating patients with empirical antifungal therapies before final identification of the specific Candida species.
Ethics Approval and Consent to Participate
Protocols of human tests (clinical samples) and microbiological research were approved by the Scientific Research Ethics Committee of the Institutional Review Board (IRB) of Clinical Medical College and the First Affiliated Hospital of Chengdu Medical College. We have confirmed that all methods were performed in accordance with the relevant guidelines and regulations. For the collection of clinical isolates, written informed consent was conducted in accordance with the Declaration of Helsinki, and sufficient time was provided for questions and answers before signing written informed consent.
Author Contributions
All authors made a significant contribution to the manuscript, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, Kullberg BJ. Invasive candidiasis. Nat Rev Dis Primers. 2018;4:18026. doi:10.1038/nrdp.2018.26
- Medina N, Soto-Debran JC, Seidel D, et al. MixInYeast: a multicenter study on mixed yeast infections. J Fungi. 2020;7(1):13. doi:10.3390/jof7010013
- Pfaller MA, Andes DR, Diekema DJ, et al. Epidemiology and outcomes of invasive candidiasis due to non-albicans species of Candida in 2496 patients: data from the Prospective Antifungal Therapy (PATH) registry 2004–2008. PLoS One. 2014;9(7):e101510. doi:10.1371/journal.pone.0101510
- Pfaller MA, Diekema DJ, Gibbs DL, et al. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: a 10.5-year analysis of susceptibilities of Candida Species to fluconazole and voriconazole as determined by CLSI standardized disk diffusion. J Clin Microbiol. 2010;48(4):1366–1377. doi:10.1128/JCM.02117-09
- Kullberg BJ, Arendrup MC. Invasive Candidiasis. N Engl J Med. 2015;373(15):1445–1456. doi:10.1056/NEJMra1315399
- Arendrup MC, Patterson TF. Multidrug-resistant candida: epidemiology, molecular mechanisms, and treatment. J Infect Dis. 2017;216(suppl_3):S445–S451. doi:10.1093/infdis/jix131
- Pfaller MA, Messer SA, Woosley LN, Jones RN, Castanheira M. Echinocandin and triazole antifungal susceptibility profiles for clinical opportunistic yeast and mold isolates collected from 2010 to 2011: application of new CLSI clinical breakpoints and epidemiological cutoff values for characterization of geographic and temporal trends of antifungal resistance. J Clin Microbiol. 2013;51(8):2571–2581. doi:10.1128/JCM.00308-13
- Arastehfar A, Hilmioglu-Polat S, Daneshnia F, et al. Recent Increase in the prevalence of fluconazole-non-susceptible candida tropicalis blood isolates in Turkey: clinical implication of azole-non-susceptible and fluconazole tolerant phenotypes and genotyping. Front Microbiol. 2020;11:587278. doi:10.3389/fmicb.2020.587278
- Wang Y, Fan X, Wang H, et al. Continual decline in azole susceptibility rates in candida tropicalis over a 9-year period in China. Front Microbiol. 2021;12:702839. doi:10.3389/fmicb.2021.702839
- Fan X, Xiao M, Liao K, et al. Notable increasing trend in azole non-susceptible Candida tropicalis causing invasive candidiasis in China (August 2009 to July 2014): molecular epidemiology and clinical azole consumption. Front Microbiol. 2017;8:464. doi:10.3389/fmicb.2017.00464
- Munoz P, Giannella M, Fanciulli C, et al. Candida tropicalis fungaemia: incidence, risk factors and mortality in a general hospital. Clin Microbiol Infect. 2011;17(10):1538–1545. doi:10.1111/j.1469-0691.2010.03338.x
- Tadec L, Talarmin JP, Gastinne T, et al. Epidemiology, risk factor, species distribution, antifungal resistance and outcome of candidemia at a single French hospital: a 7-year study. Mycoses. 2016;59(5):296–303. doi:10.1111/myc.12470
- Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62(4):e1–e50. doi:10.1093/cid/civ933
- Hof H. Developments in the epidemiolgy of invasive fungal infections - implications for the empiric and targeted antifungal therapy. Mycoses. 2008;51(Suppl 1):1–6. doi:10.1111/j.1439-0507.2008.01522.x
- Martinez JA, Pozo L, Almela M, et al. Microbial and clinical determinants of time-to-positivity in patients with bacteraemia. Clin Microbiol Infect. 2007;13(7):709–716. doi:10.1111/j.1469-0691.2007.01736.x
- Lai CC, Wang CY, Liu WL, et al. Time to blood culture positivity as a predictor of drug resistance in Acinetobacter baumannii complex bacteremia. J Infect. 2011;63(1):96–98. doi:10.1016/j.jinf.2011.05.009
- Kassis C, Rangaraj G, Jiang Y, Hachem RY, Raad I. Differentiating culture samples representing coagulase-negative staphylococcal bacteremia from those representing contamination by use of time-to-positivity and quantitative blood culture methods. J Clin Microbiol. 2009;47(10):3255–3260. doi:10.1128/JCM.01045-09
- Orihuela-Martin J, Rodriguez-Nunez O, Morata L, et al. Performance of differential time to positivity as a routine diagnostic test for catheter-related bloodstream infections: a single-centre experience. Clin Microbiol Infect. 2020;26(3):383e381–383 e387. doi:10.1016/j.cmi.2019.07.001
- Kim SH, Yoon YK, Kim MJ, Sohn JW. Clinical impact of time to positivity for Candida species on mortality in patients with candidaemia. J Antimicrob Chemother. 2013;68(12):2890–2897. doi:10.1093/jac/dkt256
- Cobos-Trigueros N, Morata L, Torres J, et al. Usefulness of time-to-positivity in aerobic and anaerobic vials to predict the presence of Candida glabrata in patients with candidaemia. J Antimicrob Chemother. 2013;68(12):2839–2841. doi:10.1093/jac/dkt285
- Foster N, Symes C, Barton R, Hobson R. Rapid identification of Candida glabrata in Candida bloodstream infections. J Med Microbiol. 2007;56(Pt 12):1639–1643. doi:10.1099/jmm.0.47406-0
- Yarbrough ML, Wallace MA, Burnham CD, Diekema DJ. Comparison of microorganism detection and time to positivity in pediatric and standard media from three major commercial continuously monitored blood culture systems. J Clin Microbiol. 2021;59(7):e0042921. doi:10.1128/JCM.00429-21
- Clinical and Laboratory Standards Institute. M60Ed1E Performance Standards for Antifungal Susceptibility Testing of Yeasts. Wayne, PA.: CLSI; 2017.
- Clinical and Laboratory Standards Institute. M59 Epidemiological Cutoff Values for Antifungal Susceptibility Testing. 1st ed. Wayne, PA: CLSI; 2016.
- Arastehfar A, Daneshnia F, Hafez A, et al. Antifungal susceptibility, genotyping, resistance mechanism, and clinical profile of Candida tropicalis blood isolates. Med Mycol. 2020;58(6):766–773. doi:10.1093/mmy/myz124
- Arastehfar A, Shaban T, Zarrinfar H, et al. Candidemia among Iranian patients with severe COVID-19 admitted to ICUs. J Fungi. 2021;7(4):280. doi:10.3390/jof7040280
- Zarrinfar H, Kaboli S, Dolatabadi S, Mohammadi R. Rapid detection of Candida species in bronchoalveolar lavage fluid from patients with pulmonary symptoms. Braz J Microbiol. 2016;47(1):172–176. doi:10.1016/j.bjm.2015.02.001
- Arastehfar A, Daneshnia F, Kord M, et al. Corrigendum: comparison of 21-Plex PCR and API 20C AUX, MALDI-TOF MS, and rDNA sequencing for a wide range of clinically isolated yeast species: improved identification by combining 21-Plex PCR and API 20C AUX as an alternative strategy for developing countries. Front Cell Infect Microbiol. 2019;9:176. doi:10.3389/fcimb.2019.00176
- Mikulska M, Calandra T, Sanguinetti M, et al. The use of mannan antigen and anti-mannan antibodies in the diagnosis of invasive candidiasis: recommendations from the Third European Conference on Infections in Leukemia. Crit Care. 2010;14(6):R222. doi:10.1186/cc9365
- Ellepola AN, Morrison CJ. Laboratory diagnosis of invasive candidiasis. J Microbiol. 2005;43:65–84.
- Lei J, Xu J, Wang T. In vitro susceptibility of Candida spp. to fluconazole, itraconazole and voriconazole and the correlation between triazoles susceptibility: results from a five-year study. J Mycol Med. 2018;28(2):310–313. doi:10.1016/j.mycmed.2018.03.005
- Huang L, Zhang YY, Sun LY. Time to positivity of blood culture can predict different Candida species instead of pathogen concentration in candidemia. Eur J Clin Microbiol Infect Dis. 2013;32(7):917–922. doi:10.1007/s10096-013-1826-8
- Cobos-Trigueros N, Kaasch AJ, Soriano A, et al. Time to positivity and detection of growth in anaerobic blood culture vials predict the presence of Candida glabrata in candidemia: a two-center European cohort study. J Clin Microbiol. 2014;52(8):3082–3084. doi:10.1128/JCM.01198-14
- Paquette K, Sweet D, Stenstrom R, et al. Neither blood culture positivity nor time to positivity is associated with mortality among patients presenting with severe manifestations of sepsis: the FABLED cohort study. Open Forum Infect Dis. 2021;8(7):ofab321. doi:10.1093/ofid/ofab321
- Hsieh YC, Chen HL, Lin SY, Chen TC, Lu PL. Short time to positivity of blood culture predicts mortality and septic shock in bacteremic patients: a systematic review and meta-analysis. BMC Infect Dis. 2022;22(1):142. doi:10.1186/s12879-022-07098-8
- Schroeder M, Weber T, Denker T, et al. Epidemiology, clinical characteristics, and outcome of candidemia in critically ill patients in Germany: a single-center retrospective 10-year analysis. Ann Intensive Care. 2020;10(1):142. doi:10.1186/s13613-020-00755-8
