Abstract
Purpose
Methicillin-resistant Staphylococcus aureus (MRSA) is one of the most common pathogens of community- and hospital-acquired infections, and its prevalence is increasing globally. Guiyang is the capital city of Guizhou Province, Southwest China; as the transport and tourism centre of Southwest China, Guizhou Province is bordered by Yunnan, Sichuan, Chongqing, and Guangxi Provinces. Although MRSA prevalence is increasing, little is known about its aspects in the area. The purpose of this study was to analyse MRSA molecular characteristics, antimicrobial resistance, and virulence genes in Guiyang.
Methods
In total, 209 MRSA isolates from four hospitals (2019–2020) were collected and analysed by antimicrobial susceptibility testing and molecular classification by the MLST, spa, and SCCmec typing methods. Isolate antibiotic resistance rates were detected by a drug susceptibility assays. PCR amplification was used to detect the virulence gene-carrying status.
Results
Twenty-four STs, including 4 new STs (ST7346, ST7347, ST7348, and ST7247) and 3 new allelic mutations, were identified based on MLST. The major prevalent ST type and clone complex were ST59 (49.8%) and CC59 (62.7%), respectively. Spa type t437 (42.1%) and SCCmec IV (55.5%) were identified by spa and SCCmec typing methods as the most important types. Drug sensitivity data showed that the multidrug resistance rate was 79.0%. There were significant differences in multidrug resistance rates and virulence gene-carrying rates for seb, hla, hlb, cna and bap between ST59 and non-ST59 types.
Conclusion
ST59-SCCmecIV-t437 is a major epidemic clone in Guiyang that should be monitored by local medical and health institutions. The situation differs from other adjacent or middle provinces of China, which may be due to the special geographical location of the region and the trend in antibiotic use or lifestyle. This study provides empirical evidence for local medical and health departments to prevent and control the spread of MRSA.
Introduction
Antimicrobial resistance (AMR) of clinical bacteria threatens human health. According to the latest data by the China Antimicrobial Surveillance Network (CHINET), antibiotic-resistant Staphylococcus aureus ranked third in the country’s clinical detection rate in 2021, with methicillin-resistant Staphylococcus aureus (MRSA) comprising 30% of all antibiotic-resistant S. aureus.Citation1 MRSA was once strictly associated with healthcare settings, termed hospital-acquired MRSA (HA-MRSA), including hospitals, healthcare centres and hospital staff. However, since community-acquired MRSA (CA-MRSA) infection was first reported in the 1980s, CA-MRSA infection has gradually become the major type of MRSA infection.Citation2 In recent years, the AMR of HA-MRSA and CA-MRSA has changed.Citation3 Overall, MRSA has a higher mortality rate than methicillin-sensitive S. aureus (MSSA), leading to longer hospitalizations and higher associated treatment costs. Therefore, analysis of the molecular characteristics, antimicrobial resistance, and virulence gene profiles of MRSA isolates are very important for controlling outbreaks of high-antimicrobial resistance and high-virulence strains.Citation4 Moreover, such information will lay the foundation for developing new anti-MRSA agents.
Biofilm is an important factor of MRSA pathogenicity,Citation5 involving 4 stages: initial attachment, irreversible attachment, bacterial growth and ECM generation, and biofilm maturation.Citation6 eDNA (extracellular DNA), PIA (Polysaccharide Intercellular Adhesion), and CWA (Cell Wall Associated) proteins, nucleases and proteases participate in construction of the biofilm material matrix and are regulated by different signalling pathways.Citation7 Biofilm formation is a dynamic process. Once it matures, the bacteria encased are released and spread to a new site to form another biofilm. Thus, these bacteria are the main targets of many new anti-MRSA agents.Citation8–13 Additionally, biofilms are an obstacle for antibiotic resistance treatment of MRSA bacteria inside the biofilm, which is why MRSA strains are difficult clear from the infection site. Based on this, many novel anti-MRSA agents have been investigated. Most importantly, several detection methodsCitation14,Citation15 and vaccines for biofilms via polysaccharide intercellular adhesion (PIA) antigen or PIA-rSesC have been developed.Citation16–19
A variety of molecular classification methods are helpful for determining the type of MRSA isolate. Multilocus sequence typing (MLST) consists of seven housekeeping genes (arcC, aroE, glpF, gmk, pta, tpi, and yqil).Citation20 Among them, types with one or two different alleles can be classified as the same clonal complexes (CCs).Citation21–23 Staphylococcal protein A typing (spa) is based on amplification and sequencing of the surface protein A gene. Staphylococcal chromosomal cassette mec (SCCmec) is a mobile genetic element used for typing. To date, 14 different types of SCCmec elements have been found.Citation24,Citation25
During S. aureus infection, it produces a quantity of virulence factors, often leading to toxin-mediated diseases, including toxic shock syndrome, staphylococcal food-borne diseases, and scalded skin syndrome.Citation26,Citation27 Exotoxins are classified into three categories based on known functions: cytotoxins, superantigens, and cytotoxic enzymes.Citation21,Citation28 These include haemolysin (Hl), Panton-Valentine leukocidin (PVL), staphylococcal enterotoxin (Se), toxic shock syndrome toxin-1 (TSST-1), fibronectin-binding protein (Fnb), intracellular adhesin (Ica), collagen adhesin (Cna), and biofilm-associated protein (Bap).Citation28,Citation29 These exotoxins jointly regulate the host immune system and cell adhesion and play a vital role in S. aureus infection.Citation28
In recent years, the global prevalence of MRSA has been changing, resulting in different epidemic clones and antimicrobial resistance profiles in different regions and at different times, with regional variability.Citation30 Guizhou Province is bordered by Yunnan, Sichuan, Chongqing, and Guangxi Provinces in southwestern China. However, no multicentre study on MRSA isolates has been carried out in this area. In this study, MLST, spa-type, and SCCmec methods were used for the first time to analyse MRSA isolates from provincial, city, and county hospitals to detect the main epidemic MRSA strains in Guiyang. The antimicrobial resistance and virulence genes of these isolates were also investigated. The findings provide a guiding reference for the clinical treatment of MRSA-related infections.
Materials and Methods
Clinical Bacterial Isolate Collection, Culture, and Genomic DNA Extraction
All MRSA strains were isolated and banked in 4 hospitals (including 3 tertiary teaching hospitals) in Guiyang from 2019 to 2020: The First Affiliated Hospital of Guizhou Medical University, The First People’s Hospital of Guiyang, Guihang Guiyang Hospital, and People’s Hospital of Kaiyang. All isolates were first identified using Gram staining and coagulase and catalase tests. Then, they were further identified using a Mérieux automated bacterial tester, VITEK 2 AST-GP67 Test Kit, and mecA gene testing with PCR amplification. All isolates were stored at −80°C for further experiments. After phenotype and genotype identification, 209 MRSA isolates were ultimately identified and included in the research. The sample sources of these isolates included cutaneous abscesses and wound secretions (n=76, 36.4%), sputum and pharynx swabs (n=113, 45.1%), blood (n=9, 4.3%), and others (catheter tip, pleural fluid, drainage liquid, ascites, joint fluid, and urine) (n=11, 5.3%) (according to laboratory department statistics data). It should be clarified that all clinical MRSA strains were obtained from the laboratory department in these hospitals. The clinical samples were not specifically isolated for this research. Thus, all these strains were obtained as part of the routine hospital laboratory procedure. We did not collect samples from patients directly at all. Before we took the stock strains from the laboratory department of hospital, all personal information was removed for patient protection.
All primers used in the present study were synthesized by Sangon Biotech (Shanghai) Co., Ltd., as listed in . Any strain with PENG and CFX (cefoxitin) resistance and mecA positivity was considered an MRSA strain. All strains were cultured by streaking onto sheep blood agar culture plates and growing for 16–18 h overnight. Then, single colonies were selected and inoculated into liquid medium. After 12 h of growth, MRSA DNA was extracted using a bacterial genomic DNA rapid extraction kit. The DNA obtained was dissolved in 100 µL of TE buffer and stored at −20°C. S. aureus ATCC25923, ATCC25913, and ATCC43300 were used as quality control strains.
Table 1 Primers Used in This Study
MLST, spa, and SCCmec Typing
MLST Typing
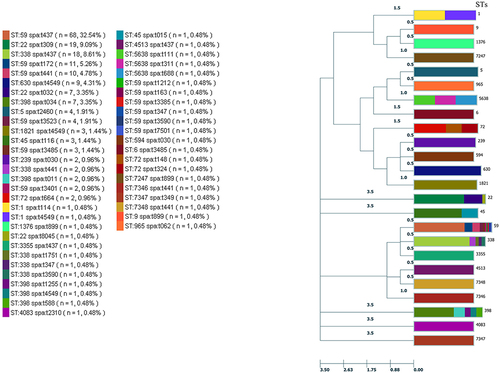
According to the standard protocol of primer design and PCR amplification conditions,Citation31 7 housekeeping gene fragments (arcC, aroE, glpF, gmk, pta, tpi, and yqil) of each MRSA isolate were amplified and sequenced (Sangon Biotech (Shanghai) Co., Ltd.). These sequences were submitted to the MLST database (https://pubmlst.org/) and analysed for allele numbers or ST type. Unique sequences of MRSA strains that could not be compared to any known ST types were submitted to the MLST database and assigned as new alleles or ST types. In this study, 4 isolates could not be assigned to any known ST; these novel alleles were submitted to the MLST database, and 3 new alleles were assigned, namely, arcC(845), glpF (900), and pta (857). (https://pubmlst.org/bigsdb?db=pubmlst_saureus_seqdefandpage=alleleInfoandlocus=arcCandallele_id=845, arcC:845; https://pubmlst.org/bigsdb?db=pubmlst_saureus_seqdefandpage=alleleInfoandlocus=glpFandallele_id=900, glpF:900; https://pubmlst.org/bigsdb?db=pubmlst_saureus_seqdefandpage=alleleInfoandlocus=ptaandallele_id=857, pta:857.). At the same time, 4 new STs were identified: ST7346, ST7347, ST7348 and ST7247 ().
Figure 1 Virulence genes and antimicrobial resistance rates of MRSA clinical isolates linked to STs. Antibiotic and virulent genes were detected in less than 3% of isolates with a particular ST; the number of MRSA isolates is given.
By clustering analysis, isolate strains with 6 identical allelic loci were defined as clonal complexes (CCs).
spa Typing
Similar as above, typing was obtained by amplifyingCitation32 and sequencing the variable region (X) of the MRSA spa gene in different strains. Then, the sequence was submitted to the spa type database (http://spatyper.fortinbras.us/) for spa typing.
SCCmec Typing
MRSA isolate strains were classified by multiplex PCRCitation24,Citation33,Citation34 and agarose gel electrophoresis for SCCmec type. According to the literature, S. aureus can be identified as I–XIV types.Citation25 Some of the MRSA isolates that could not be classified as any known SCCmec type were defined as non-type (NT).
Antibiotic Susceptibility Test
Antibiotic susceptibility testing was conducted for all S. aureus isolates using VITEK 2 AST-GP67 Test Kit (Compact system) and the Kirby-Bauer disc diffusion method (Oxoid) according to the guidelines of the Clinical and Laboratory Standard Institute (CLSI) M100-S29, 2020. The antibiotics tested were FT, SXT, CIP, CM, QDA, CMP, NOR, GEN, LEV, ERY, RIF, LZD, TET, TEC, VAN, PENG and CFX (all antibiotic abbreviations are shown in the abstract). Isolates resistant to three or more antimicrobial agents were considered multidrug-resistant strains.Citation35
Detection of Virulence Genes
The 12 virulence factor gene fragments of MRSA isolates were screened using independent PCR assays, including sea, seb, pvl (lukS-PV/lukF-PV), tsst, hla, hlb, fnbA, fnbB, icaA, icaD, cna and bap. The PCR mixtures contained 1 μL DNA template, 2 μL primers (10 μM), 12.5 μL 2×Taq Master Mix (GenStar, China), and 9.5 μL double-distilled water. All PCR products were detected by 1.0% agarose gel electrophoresis. One of the PCR products was identified as a positive control by sequencing and sequence alignment analysis. All the primers used in this study are listed in .
Statistical Analysis
In this study, SPSS 26.0 software was used for statistical analysis of experimental data. A P value <0.05 was considered statistically significant. WHONET software was used to analyse antimicrobial sensitivity data. Minimum spanning tree diagram analysis was performed using goeBURST software.
Ethical Approval
This study was approved by the People’s Hospital of Kaiyang, Guizhou Medical University Teaching Hospital management (20190203001).
Results
Molecular Typing of MRSA Strains in Guiyang
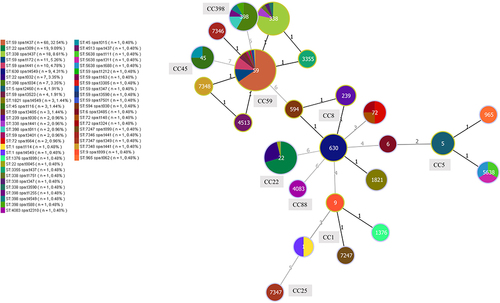
All 209 MRSA clinical isolates were successfully typed for MLST and assigned to 24 STs (sequence types). The specific results of the MLST typing are provided in . The most prevalent ST type was ST59 (49.76%, 104/209), followed by ST22 (12.9%, 27/209), ST338 (11.0%, 23/209), ST398 (5.7%, 12/209) and ST630 (4.3%, 9/209). All STs were identified as belonging to 9 clonal complexes (CCs) by goeBURST and the International MLST database. As shown in and , CC59 62.7% (131/209) was the most prevalent CC, followed by CC22 12.9% (27/209), CC8 9.1% (19/209), CC398 5.7% (12/209) and CC5 4.3% (9/209). In addition, 4 isolates could not be assigned to any known ST. These novel alleles were submitted to the MLST database, and 3 new alleles were assigned: arc (845), glpF (900), and pta (857). Four new STs were also identified: ST7346, ST7347, ST7348 and ST7247 ().
Table 2 The MLST, spa and SCCmec Typing of MRSA Isolate Strains
Figure 2 Minimum spanning tree constructed by goeBURST based on the MLST data of this study. The number between lines indicates locus differences. The size of each node corresponds to the number of strains. The colour partition of each disc corresponds to the proportion of the SPA types. Figures on the nodes are ST numbers.
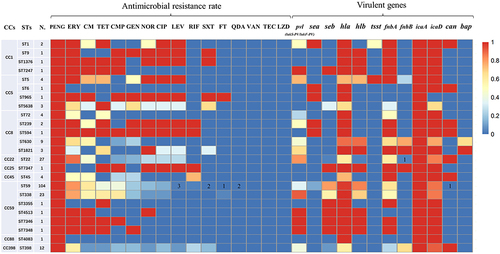
In total, 36 spa types were found by spa typing. The most prevalent was t437 42.1% (88/209), followed by t309 9.1% (19/209), t441 6.7% (14/209), t4549 6.7% (14/209) and t172 5.3% (11/207). The t032 and t034 types were detected in 7 isolates, and t2460, t3485 and t3523 were identified in four isolates each ().
SCCmec typing was performed successfully for 181 of the 209 MRSA isolates (86.6%). Six SCCmec types, namely, types I, II, III, IV, V, and XII, were detected. The most common SCCmec type was IV, which was observed in 116 isolates 55.5% (116/209), and the second most common SCCmec type was II 20.1% (42/209). Only 1 isolate was SCCmec XII 0.5% (1/209), 4 isolates were SCCmec III 1.9% (4/209), 6 isolates were SCCmec I 2.9% (6/209) and 12 isolates were SCCmec V 5.7% (12/209). In addition, 28 isolates were classified as NT (non-type) by SCCmec typing ().
Antimicrobial Susceptibility of MRSA Strains in Guiyang
According to antimicrobial sensitivity testing, all 209 MRSA clinical isolates were susceptible to VAN, TEC, and LZD. However, no MRSA isolate was susceptible to PENG or CFX. The antimicrobial resistance profiles of the MRSA isolates are shown in . The multidrug resistance (MDR) rate among all MRSA isolates was 79.0%.
Table 3 The Distribution of Antimicrobial Resistance of 209 MRSA Isolate Strains
Virulence Gene Profile of MRSA Strains in Guiyang
In total, 209 MRSA clinical isolates were amplified with primers for 12 virulence genes by PCR amplification. The detection frequency of each virulence gene is shown in . Among them, the detection frequencies of the cytotoxic genes hla, hlb, and pvl (lukS-PV/lukF-PV) were 89.0%, 60.3%, and 47.8%, respectively. The detection rates of the staphylococcal superantigen genes sea, seb, and tsst were 9.6%, 49.8%, and 2.4%, respectively. Of the genes associated with biofilm formation, the intracellular adhesion molecule genes icaA and icaD were present in 100.0% and 88.0% of the isolates, respectively. Detection rates of the fnbA, fnbB, and cna microbial surface components recognizing adhesive matrix molecules (MSCRAMMs) genes were 61.7%, 9.6%, and 23.9%, respectively. The bap gene was detected in 9 isolates (4.3%). Interestingly, 119 isolates (56.9%) carried 6 or more virulence genes ( and ).
Table 4 The Frequencies of Virulence Genes of 209 MRSA Isolate Strains
Correlation and Difference Between MLST, spa Type, SCCmec Type, Antimicrobial Susceptibility, and Virulence Genes of MRSA in Guiyang
Intriguingly, there was a strong correlation between the main ST and spa types among the MRSA isolates ( and ). ST59-t437 65.4% (68/104), ST338-t437 78.3% (18/23), ST22-t309 70.4% (19/27), ST630-t4549 100.0% (9/9), ST398-t034 58.3% (7/12) and ST5-t2460 100.0% (4/4) were strongly associated types. The majority (76.2%, 89/116) of the SCCmec IV MRSA isolates were ST59, and SCCmec II was primarily associated with ST338 (31.0%, 13/42). Comprehensive analysis of MLST, spa type, and SCCmec typing the results identified ST59-SCCmec IV-t437 27.8% (58/209) as the major clone among the MRSA isolates.
The multidrug resistance of the 209 MRSA isolates mainly concentrated around classes 3–5 antimicrobials (69.9%, 146/209). CC8-t030 and CC1-t899 exhibited high-intensity multidrug resistance to 6–8 classes of antimicrobials. Among the 209 MRSA isolates, only 2 ST59 isolates were resistant to QDA. The ST59 isolates found among the 209 MRSA isolates were less resistant to LEV than CIP and NOR among fluoroquinolones. Moreover, ST45, ST72 and ST630 isolates were completely resistant to fluoroquinolones, whereas ST22, ST45, and ST630 isolates were completely sensitive to TET ().
Table 5 The Frequency Analysis of Antimicrobial Resistance Between ST59 and Non-ST59
According to the virulence gene detection test, all ST22, ST398, ST45, and ST239 isolates carried the cna gene. However, the bap gene was only detected in the 4 CC8-t4549 isolates. CC59 isolates did not carry the fnbB gene. The pvl (lukS-PV/lukF-PV) gene encompassed multiple STs, among which the ST22 (22/27) and ST338 (21/23) isolates were the two types with the highest rate of carriage (). The detection rate of the seb gene was higher than that of the sea gene, which was mainly carried by CC59 isolates. Although the detection rate of the tsst gene was low, ST5-t2460 isolates had a high rate of the tsst gene positivity.
Table 6 The Frequency Analysis of Virulence Gene Between ST59 and Non-ST59
Comparation of ST59 and Non-ST59 MRSA Strains in Guiyang
The antimicrobial resistance rate and virulence gene-carrying status of ST59 isolates and non-ST59 isolates were analysed. The MDR rate of the ST59 isolates was higher than that of non-ST59 isolates, and the ST59 isolates showed higher resistance rates to CM and CMP and lower resistance rates to CIP and LEV. Nonetheless, no significant difference in resistance rate to any other antibiotics was found between ST59 and non-ST59 isolates. Among the 12 tested virulence genes, seb, hla, and hlb were more frequent in ST59 isolates than in non-ST59 isolates. In contrast, the detection rates of pvl (lukS-PV/lukF-PV), fnbB, cna, and bap in ST59 isolates were lower. Detection rates of the other virulence genes were not significantly different between the two isolate types ().
Discussion
Increasing antibiotic resistance is a global public health problem. MRSA prevalence has increased in recent years.Citation4,Citation23,Citation27,Citation36–38 In addition, antibiotic resistance and MRSA prevalence trends change dynamically according to different medication use habits and time periods. Thus, monitoring the prevalence and antibiotic resistance as well as timely updates are valuable and essential. This study focused on the molecular characteristics, antimicrobial resistance, and virulence gene profiles of 209 MRSA isolates in Guiyang, a multi-ethnic city in Southwest China, from 2019 to 2020.
The results showed that the most common MLST type was CC59-ST59, and ST59-SCCmec IV-t437 was identified as the main common clone type in Guiyang (). According to the literature, 5 CCs are most reported among HA-MRSA strains around the world, namely, CC5, CC8, CC22, CC30, and CC45.Citation30 CC8-ST239, CC5-ST5, and CC22-ST22 are also the most common in Asian countries.Citation39 Regarding the CA-MRSA epidemic, CC5, CC8, CC22, CC30, CC59, and CC398 were the most common. CC8-ST8 (USA300) is common in the United States.Citation40 CA-MRSA comprises CC22-ST22 and CC30-ST36 in Britain,Citation41 CC30-ST30 in Australia,Citation42 and CC59-ST59 in East Asia.Citation39 CC59-ST59 is also common in Taiwan, Hong Kong, Vietnam, and Sri Lanka.Citation43 However, the main types of inland China differ. For example, predominant types are CC398-ST398 and CC5-ST5 in Shanghai (eastern China), CC59-ST338 and CC8-ST239 in Guangdong (southern China), CC8-ST239 in Wuhan (central China), CC5-ST5, CC8-ST239, and CC59-ST59 in Zhejiang (south-eastern China), and CC45-ST45 in Hainan (southern China). Thus, the prevalence of the dominant MRSA type varies across the world and are associated with specific geographical regions.
According to previous reports, most HA-MRSA isolates carry SCCmec I, II, or III elements, and CA-MRSA isolates carry SCCmec IV or V elements.Citation44,Citation45 In this study, HA-MRSA (I+II+III) accounted for 52 strains (24.88%) and CA-MRSA (IV+V+XII) for 129 (61.72%). The remaining 28 non-type strains could not be discriminated by the method used (). Therefore, the main MRSA isolate in the study area was CA-MRSA. It is therefore reasonable that most people become infected with MRSA before they visit the hospital. Notably, 15 strains of CC59-ST338, which belongs to CA-MRSA, were detected,Citation4 though it was found to be SCCmec II. Hence, it is controversial to identify HA-MRSA or CA-MRSA by the ST method with the SCCmec method. More accurate identification methods need to be explored in the future. In addition, 4 new uncommon MLST types were identified in the study region.
Antimicrobial sensitivity tests showed that all 209 MRSA isolates were susceptible to VAN, TEC, and LZD, but none of the MRSA isolates were susceptible to PENG and CFX. The findings suggest that VAN, TEC, and LZD remain the most effective antimicrobial agents for MRSA isolates in this area. The high resistance rates to ERY, CM, and GEN are consistent with the results of CHINET in 2020. However, the resistance rate to LEV, RIF and SXT in this study was lower than that to CIP and NOR and 20.2% lower than data according to CHINET in 2020. This might be due to different antibiotic usages and health care plans in different regional hospitals.Citation46 In general, the ability of MRSA to adapt changes under the bacteriostatic pressure of antibiotics, which might lead to certain differences in LEV, RIF, and SXT resistance rates ().
Here, the study found the multidrug resistance rate to be 79.0% (165/209). Among them, CC8-t030 and CC1-t899 exhibited high-intensity multidrug resistance against 6–8 classes of antibiotics. Such a situation has been reported to be related to the misuse of antibiotics.Citation47,Citation48 Additionally, ST59 shows a wide spectrum of antimicrobial resistance, though only 2 ST59 isolates were resistant to QDA. Interestingly, there are few reports about resistance of MRSA strains to QDA.Citation35 It was also found that ST45, ST72, and ST630 isolates were completely resistant to fluoroquinolones; ST22, ST45, and ST630 isolates were completely sensitive to TET. These results suggest a certain correlation between MLST strain type and antimicrobial susceptibility.Citation49 Furthermore, antibiotic resistance rates to CM and CMP were higher and to CIP and LEV lower in non-ST59 isolates than in ST59 isolates (). This indicates that ST59 has a high antimicrobial resistance rate, especially to CM and CMP, in Guiyang. According to the above results, it was concluded that the antimicrobial resistance rate of MRSA strains was higher; ST59 isolates are the main MRSA type and exhibit unique characteristics in Guiyang.
Virulence factors are the main factors involved in the colonization and pathogenicity of pathogenic bacteria and facilitate invasive infection,Citation36,Citation50,Citation51 causing a strong inflammatory response and promoting infection syndrome.Citation4,Citation38,Citation51 In addition, virulence factors help bacteria form biofilm for stable attachment, ensuring pathogen survival and infection. This study examined 12 virulence genes, with different detection rates. Among them, the detection rates of the icaA, hla, icaD, fnbA and hlb genes were 100.0%, 89.0%, 88.0%, 61.7% and 60.3%, respectively (). It has been suggested that MRSA strains might have strong haemolytic ability and cell adhesion ability in the study area.Citation52
In our virulence gene assay, rates of cna, fnbB, and bap gene detection were lower than those of other virulence genes, but they were particularly associated with certain types. All ST22, ST398, ST45, and ST239 isolates carried the cna gene. The bap gene was detected only in four CC8-t4549 isolates. CC59 isolates did not carry the fnbB gene. This information provides an important basis to further study the mechanism of bacterial adhesion and biofilm formation. The cytotoxin pvl (lukS-PV/lukF-PV) gene was detected in multiple STs. PVL protein can cause tissue necrosis and leukocyte destruction.Citation53 Among these isolates, ST22 81.5% (22/27) and ST338 91.3% (21/23) had higher carriage rates. This was the same as reported for pvl (lukS-PV/lukF-PV)-positive CC22-MRSA isolates in Kuwaiti hospitalsCitation54 but different from pvl (lukS-PV/lukF-PV)-negative ST22-MRSA isolates reported in Urumqi ().Citation55 This indicates some differences in the pvl (lukS-PV/lukF-PV) gene -carrying rate of ST22-MRSA isolates from different regions and possibly differences in their invasive abilities.
The seb gene was mainly detected in CC59 isolates. According to reports, SEB also promotes systemic S. aureus infection.Citation56 Therefore, the systemic infection rate caused by ST59 might be higher. Although the detection rate of the tsst gene was lower, the ST5-t2460 isolates had a higher rate of the tsst gene positivity (). Toxic shock syndrome (TSS) is an acute systemic disease affecting different organ systems of the body, resulting in severe disease.Citation27,Citation28 If a patient is infected with the ST5-t2460 isolate, clinicians should be highly vigilant about systemic infection and implement emergency measures in advance. Analysis of ST59 and non-ST59 isolates showed that the seb, hla, and hlb genes were more frequent in ST59 isolates than in non-ST59 isolates (). This suggested that ST59 might have strong haemolytic toxicity and systemic infection capacity. According to the above detection of virulence genes carried by STs, we speculate the existence of a relationship between virulence gene profile and MLST type.
Conclusion
This study revealed the molecular characteristics, antimicrobial resistance, and virulence gene-carrying status of MRSA isolates in Guiyang. Due to the special geographical environment and the habit of antibiotic use, ST59-SCCmec IV-t437 was found to be the main epidemic clone type, with a wide spectrum of antimicrobial resistance, many virulence genes, and strong adaptability and pathogenicity. Public health departments should be aware of and monitor this strain.
Abbreviations
CHINET, China Antimicrobial Surveillance Network; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus; HA-MRSA, hospital-acquired methicillin-resistant Staphylococcus aureus; CA-MRSA, community-acquired methicillin-resistant Staphylococcus aureus; MLST, multilocus sequence typing; CCs, clonal complexes; spa, Staphylococcal protein A; SCCmec, Staphylococcal chromosomal cassette mec; NT, non-type; STs, sequence types; MDR, multidrug resistance; MSCRAMMs, microbial surface components recognizing adhesive matrix molecules; CLSI, Clinical and Laboratory Standard Institute; FT, nitrofurantoin; SXT, trimethoprim/sulfamethoxazole; CIP, ciprofloxacin; CM, clindamycin; QDA, quinupristin-dalfopristin; CMP, chloramphenicol; NOR, norfloxacin; GEN, gentamicin; LEV, levofloxacin; ERY, erythromycin; RIF, rifampicin; LZD, linezolid; TET, tetracycline; TEC, teicoplanin; VAN, vancomycin; PENG, penicillin G; CFX, cefoxitin; sea, seb, Staphylococcal enterotoxin genes; pvl (lukS-PV/lukF-PV), Panton-Valentine leukocidin gene; tsst, toxic shock syndrome toxin gene; hla, hlb, haemolysin genes; fbA, fnbB, fibrinogen-binding protein genes; icaA, icaD, intracellular adhesion molecules genes; cna, collagen adhesion gene; bap, biofilm-associated protein gene.
Author Contributions
Su-Wen Yang designed and implemented the experiments and drafted the manuscript. Bing Wang designed, analysed and interpreted the data, aided in the manuscript preparation, and assisted in the research design. Jing Li, Xue Zhao, Yan Zhu, and Qian Sun collected, identified, and performed the antibiotic testing and analysed the data. Xiao-Jun Wen and Hong-Mei Liu analysed the data, assisted in the design, and provided funding. All authors assisted in editing and approved the final manuscript. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no competing interests in this work.
Acknowledgments
We would like to thank the Department of Microbial Immunology, the First Affiliated Hospital of Guizhou Medical University, the First People’s Hospital of Guiyang, Guihang Guiyang Hospital, and People’s Hospital of Kaiyang for providing the clinical bacteria.
Additional information
Funding
References
- Cheung GYC, Bae JS, Otto M. Pathogenicity and virulence of Staphylococcus aureus. Virulence. 2021;12:547–569. doi:10.1080/21505594.2021.1878688
- Siddiqui AH, Koirala J. Methicillin Resistant Staphylococcus Aureus. Treasure Island (FL): StatPearls; 2022.
- Palavecino EL. Clinical, epidemiologic, and laboratory aspects of methicillin-resistant Staphylococcus aureus infections. Methods Mol Biol. 2020;2069:1–28.
- Jin Y, Zhou W, Yin Z, et al. The genetic feature and virulence determinant of highly virulent community-associated MRSA ST338-SCCmec Vb in China. Emerg Microbes Infect. 2021;10:1052–1064. doi:10.1080/22221751.2021.1914516
- Mousavi SF, Mirzaei B, Shaghaghi B, et al. Phenotypic and genotypic features of first biofilm forming nasopharyngeal colonized Streptococcus pneumoniae isolates. Iran J Microbiol. 2017;9:200–207.
- Lu L, Hu W, Tian Z, et al. Developing natural products as potential anti-biofilm agents. Chin Med. 2019;14:11. doi:10.1186/s13020-019-0232-2
- Schilcher K, Horswill AR. Staphylococcal biofilm development: structure, regulation, and treatment strategies. Microbiol Mol Biol Rev. 2020;2020:84.
- Wang C, Wei PW, Song CR, et al. Evaluation of the antimicrobial function of Ginkgo biloba exocarp extract against clinical bacteria and its effect on Staphylococcus haemolyticus by disrupting biofilms. J Ethnopharmacol. 2022;298:115602. doi:10.1016/j.jep.2022.115602
- Wang B, Wei PW, Yao Y, et al. Functional and expression characteristics identification of Phormicins, novel AMPs from Musca domestica with anti-MRSA biofilm activity, in response to different stimuli. Int J Biol Macromol. 2022;209:299–314. doi:10.1016/j.ijbiomac.2022.03.204
- Wang B, Song CR, Zhang QY, et al. The fusaric acid derivative qy17 inhibits Staphylococcus haemolyticus by disrupting biofilm formation and the stress response via altered gene expression. Front Microbiol. 2022;13:822148. doi:10.3389/fmicb.2022.822148
- Peng-Wei W, Chao-Rong S, Xu W, et al. A potential milk preservative----Phormicin C-NS, sorbic acid-modified housefly antimicrobial peptide, inhibits Candida albicans hypha and biofilm formation. Lwt. 2022;168:113883. doi:10.1016/j.lwt.2022.113883
- Wang B, Yao Y, Wei P, et al. Housefly Phormicin inhibits Staphylococcus aureus and MRSA by disrupting biofilm formation and altering gene expression in vitro and in vivo. Int J Biol Macromol. 2021;167:1424–1434. doi:10.1016/j.ijbiomac.2020.11.096
- Wang B, Wei PW, Wan S, et al. Ginkgo biloba exocarp extracts inhibit S. aureus and MRSA by disrupting biofilms and affecting gene expression. J Ethnopharmacol. 2021;271:113895. doi:10.1016/j.jep.2021.113895
- Naimi HM, Rasekh H, Noori AZ, et al. Determination of antimicrobial susceptibility patterns in Staphylococcus aureus strains recovered from patients at two main health facilities in Kabul, Afghanistan. BMC Infect Dis. 2017;17:737. doi:10.1186/s12879-017-2844-4
- Shahmoradi M, Faridifar P, Shapouri R, et al. Determining the biofilm forming gene profile of Staphylococcus aureus clinical isolates via multiplex colony PCR method. Rep Biochem Mol Biol. 2019;7:181–188.
- Mirzaei B, Moosavi SF, Babaei R, et al. Purification and evaluation of Polysaccharide Intercellular Adhesion (PIA) antigen from Staphylococcus epidermidis. Curr Microbiol. 2016;73:611–617. doi:10.1007/s00284-016-1098-5
- Mirzaei B, Babaei R, Valinejad S. Staphylococcal vaccine antigens related to biofilm formation. Hum Vaccin Immunother. 2021;17:293–303. doi:10.1080/21645515.2020.1767449
- Mirzaei B, Babaei R, Zeighami H, et al. Staphylococcus aureus putative vaccines based on the virulence factors: a mini-review. Front Microbiol. 2021;12:704247. doi:10.3389/fmicb.2021.704247
- Mirzaei B, Mousavi SF, Babaei R, et al. Synthesis of conjugated PIA-rSesC and immunological evaluation against biofilm-forming Staphylococcus epidermidis. J Med Microbiol. 2019;68:791–802. doi:10.1099/jmm.0.000910
- O’Hara FP, Suaya JA, Ray GT, et al. spa typing and multilocus sequence typing show comparable performance in a macroepidemiologic study of Staphylococcus aureus in the United States. Microb Drug Resist. 2016;22:88–96. doi:10.1089/mdr.2014.0238
- Bai X, Wang H, Xin Y, et al. Prevalence and characteristics of Shiga toxin-producing Escherichia coli isolated from retail raw meats in China. Int J Food Microbiol. 2015;200:31–38. doi:10.1016/j.ijfoodmicro.2015.01.018
- Chao G, Bao G, Cao Y, et al. Prevalence and diversity of enterotoxin genes with genetic background of Staphylococcus aureus isolates from different origins in China. Int J Food Microbiol. 2015;211:142–147. doi:10.1016/j.ijfoodmicro.2015.07.018
- Chizimu JY, Solo ES, Bwalya P, et al. Genetic diversity and transmission of multidrug-resistant Mycobacterium tuberculosis strains in Lusaka, Zambia. Int J Infect Dis. 2022;114:142–150. doi:10.1016/j.ijid.2021.10.044
- Bhowmik D, Das BJ, Pandey P, et al. An array of multiplex PCR assays for detection of Staphylococcal chromosomal cassette mec (SCCmec) types among staphylococcal isolates. J Microbiol Methods. 2019;166:105733. doi:10.1016/j.mimet.2019.105733
- Urushibara N, Aung MS, Kawaguchiya M, et al. Novel staphylococcal cassette chromosome mec (SCCmec) type XIV (5A) and a truncated SCCmec element in SCC composite islands carrying speG in ST5 MRSA in Japan. J Antimicrob Chemother. 2020;75:46–50. doi:10.1093/jac/dkz406
- Becker K, Sunderkotter C. Hautinfektionen durch MRSA Epidemiologie und Klinik [Skin infections with MRSA. Epidemiology and clinical features]. Hautarzt. 2012;63:371–380. German. doi:10.1007/s00105-011-2255-1
- Hidron AI, Low CE, Honig EG, et al. Emergence of community-acquired meticillin-resistant Staphylococcus aureus strain USA300 as a cause of necrotising community-onset pneumonia. Lancet Infect Dis. 2009;9:384–392. doi:10.1016/S1473-3099(09)70133-1
- Tam K, Torres VJ, Fischetti VA. Staphylococcus aureus secreted toxins and extracellular enzymes. Microbiol Spectr. 2019;7. doi:10.1128/microbiolspec.GPP3-0039-2018
- Zhao H, Xu S, Yang H, et al. Molecular typing and variations in amount of tst gene expression of TSST-1-producing clinical Staphylococcus aureus isolates. Front Microbiol. 2019;10:1388. doi:10.3389/fmicb.2019.01388
- Lakhundi S, Zhang K. Clinical, epidemiologic, and Staphylococcus aureus: molecular characterization, evolution, and epidemiology. Clin Microbiol Rev. 2018;31. doi:10.1128/CMR.00020-18
- Enright MC, Day NP, Davies CE, et al. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol. 2000;38:1008–1015. doi:10.1128/JCM.38.3.1008-1015.2000
- Koreen L, Ramaswamy SV, Graviss EA, et al. spa typing method for discriminating among Staphylococcus aureus isolates: implications for use of a single marker to detect genetic micro- and macrovariation. J Clin Microbiol. 2004;42:792–799. doi:10.1128/JCM.42.2.792-799.2004
- Boye K, Bartels MD, Andersen IS, et al. A new multiplex PCR for easy screening of methicillin-resistant Staphylococcus aureus SCCmec types I-V. Clin Microbiol Infect. 2007;13:725–727. doi:10.1111/j.1469-0691.2007.01720.x
- Zhang K, McClure JA, Conly JM. Enhanced multiplex PCR assay for typing of staphylococcal cassette chromosome mec types I to V in methicillin-resistant Staphylococcus aureus. Mol Cell Probes. 2012;26:218–221. doi:10.1016/j.mcp.2012.04.002
- Yan XM, Wang J, Tao XX, et al. A conjugative MDR pMG1-like plasmid carrying the lsa(E) gene of enterococcus faecium with potential transmission to Staphylococcus aureus. Front Microbiol. 2021;12:667415. doi:10.3389/fmicb.2021.667415
- Madera S, McNeil N, Serpa PH, et al. Prolonged silent carriage, genomic virulence potential and transmission between staff and patients characterize a neonatal intensive care unit (NICU) outbreak of methicillin-resistant Staphylococcus aureus (MRSA). Infect Control Hosp Epidemiol. 2022;1–7. doi:10.1017/ice.2022.48
- Yoshimura J, Yamakawa K, Umemura Y, et al. Impact of beta-lactamase detection reagent on rapid diagnosis of ESBL-producing pathogens using urine samples of patients with Gram-negative bacteriuria. Int J Infect Dis. 2021;113:18–22. doi:10.1016/j.ijid.2021.09.059
- Algammal AM, Enany ME, El-Tarabili RM, et al. Prevalence, antimicrobial resistance profiles, virulence and enterotoxins-determinant genes of MRSA isolated from subclinical bovine mastitis in Egypt. Pathogens. 2020;9:362. doi:10.3390/pathogens9050362
- Song JH, Hsueh PR, Chung DR, et al. Spread of methicillin-resistant Staphylococcus aureus between the community and the hospitals in Asian countries: an ANSORP study. J Antimicrob Chemother. 2011;66:1061–1069. doi:10.1093/jac/dkr024
- Challagundla L, Luo X, Tickler IA, et al. Range expansion and the origin of USA300 North American epidemic methicillin-resistant Staphylococcus aureus. mBio. 2018;9. doi:10.1128/mBio.02016-17
- Hsu LY, Harris SR, Chlebowicz MA, et al. Evolutionary dynamics of methicillin-resistant Staphylococcus aureus within a healthcare system. Genome Biol. 2015;16:81. doi:10.1186/s13059-015-0643-z
- Nimmo GR, Coombs GW. Community-associated methicillin-resistant Staphylococcus aureus (MRSA) in Australia. Int J Antimicrob Agents. 2008;31:401–410. doi:10.1016/j.ijantimicag.2007.08.011
- Mun YS, Hwang YJ. Novel spa and multi-locus sequence types (MLST) of Staphylococcus Aureus samples isolated from clinical specimens in Korean. Antibiotics. 2019;8:202. doi:10.3390/antibiotics8040202
- Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009;7:629–641. doi:10.1038/nrmicro2200
- Zhu P, Jiang Y, Wang Y. Molecular typing and drug resistance of methicillin-resistant Staphylococcus aureus in Zhejiang province. 2014.
- Fu Y, Xiong M, Li X, et al. Molecular characteristics, antimicrobial resistance and virulence gene profiles of Staphylococcus aureus isolates from Wuhan, Central China. Infect Drug Resist. 2020;13:2063–2072. doi:10.2147/IDR.S249988
- Guo Y, Wang B, Rao L, et al. Molecular characteristics of rifampin-sensitive and -resistant isolates and characteristics of rpoB gene mutations in methicillin-resistant Staphylococcus aureus. Infect Drug Resist. 2021;14:4591–4600. doi:10.2147/IDR.S336200
- Wang W, Liu F, Baloch Z, et al. Genotypic characterization of methicillin-resistant Staphylococcus aureus isolated from pigs and retail foods in China. Biomed Environ Sci. 2017;30:570–580. doi:10.3967/bes2017.076
- Pulingam T, Parumasivam T, Gazzali AM, et al. Antimicrobial resistance: prevalence, economic burden, mechanisms of resistance and strategies to overcome. Eur J Pharm Sci. 2022;170:106103. doi:10.1016/j.ejps.2021.106103
- Sinsinwar S, Jayaraman A, Mahapatra SK, et al. Anti-virulence properties of catechin-in-cyclodextrin-in-phospholipid liposome through down-regulation of gene expression in MRSA strains. Microb Pathog. 2022;167:105585. doi:10.1016/j.micpath.2022.105585
- Jing S, Ren X, Wang L, et al. Nepetin reduces virulence factors expression by targeting ClpP against MRSA-induced pneumonia infection. Virulence. 2022;13:578–588. doi:10.1080/21505594.2022.2051313
- Lavecchia A, Chiara M, De Virgilio C, et al. Staphylococcus arlettae genomics: novel insights on candidate antibiotic resistance and virulence genes in an emerging opportunistic pathogen. Microorganisms. 2019;7:580. doi:10.3390/microorganisms7110580
- Wu S, Huang J, Zhang F, et al. Prevalence and characterization of food-related methicillin-resistant Staphylococcus aureus (MRSA) in China. Front Microbiol. 2019;10:304. doi:10.3389/fmicb.2019.00304
- Boswihi SS, Udo EE, Monecke S, et al. Emerging variants of methicillin-resistant Staphylococcus aureus genotypes in Kuwait hospitals. PLoS One. 2018;13:e0195933. doi:10.1371/journal.pone.0195933
- Yuan W, Liu J, Zhan Y, et al. Molecular typing revealed the emergence of pvl-positive sequence type 22 methicillin-susceptible Staphylococcus aureus in Urumqi, Northwestern China. Infect Drug Resist. 2019;12:1719–1728. doi:10.2147/IDR.S202906
- Bae JS, Da F, Liu R, et al. Contribution of staphylococcal enterotoxin B to Staphylococcus aureus systemic infection. J Infect Dis. 2021;223:1766–1775. doi:10.1093/infdis/jiaa584
- Zhang K, McClure JA, Elsayed S, Louie T, Conly JM. Novel multiplex PCR assay for characterization and concomitant subtyping of staphylococcal cassette chromosome mec types I to V in methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 2005;43(10):5026–33. doi: 10.1128/JCM.43.10.5026-5033.2005
- Zhang K, McClure JA, Conly JM. Corrigendum to “Enhanced multiplex PCR assay for the typing of staphylococcal cassette chromosome mec types I to V in methicillin-resistant Staphylococcus aureus”. Mol Cell Probes. 2019;45:68. doi: 10.1016/j.mcp.2019.03.004
- Alli OA, Ogbolu DO, Shittu AO, Okorie AN, Akinola JO, Daniel JB. Association of virulence genes with mecA gene in Staphylococcus aureus isolates from Tertiary Hospitals in Nigeria. Indian J Pathol Microbiol. 2015;58(4):464–71. doi: 10.4103/0377-4929.168875
- Li X, Fang F, Zhao J, Lou N, Li C, Huang T, Li Y. Molecular characteristics and virulence gene profiles of Staphylococcus aureus causing bloodstream infection. Braz J Infect Dis. 2018;22(6):487–494. doi: 10.1016/j.bjid.2018.12.001
- Zhang K, McClure JA, Elsayed S, Louie T, Conly JM. Novel multiplex PCR assay for simultaneous identification of community-associated methicillin-resistant Staphylococcus aureus strains USA300 and USA400 and detection of mecA and Panton-Valentine leukocidin genes, with discrimination of Staphylococcus aureus from coagulase-negative staphylococci. J Clin Microbiol. 2008;46(3):1118–22. doi: 10.1128/JCM.01309-07
- Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA. 1998;279(19):1537–41. doi: 10.1001/jama.279.19.1537
- Haddad O, Merghni A, Elargoubi A, Rhim H, Kadri Y, Mastouri M. Comparative study of virulence factors among methicillin resistant Staphylococcus aureus clinical isolates. BMC Infect Dis. 2018;18(1):560. doi: 10.1186/s12879-018-3457-2