Abstract
Infection caused by the Humicola sp is extremely rare. We report the first case of fungal keratitis caused by Humicola pulvericola (H. pulvericola) in a 63-year-old man with a history of exposed to hot oil two weeks ago who developed keratitis. Direct examination of confocal microscopy and corneal scrapings showed fungal hyphae and isolates were identified by morphology and by sequencing the internal transcribed spacer region of ribosomal DNA. The in vitro antifungal susceptibilities of the case strain were tested for five antifungal agents. The results showed that the infectious agent was resistant towards fluconazole, caspofungin and amphotericin B; itraconazole and voriconazole were highly effective against H. pulvericola. He was diagnosed with H. pulvericola keratitis and treated with oral itraconazole and natamycin eyedrops. After one month of treatment, the lesion gradually improved, with the best-corrected visual acuity improving to 0.8.
Introduction
Fungal aetiology is widely recognized as the primary culprits responsible for ocular morbidity, and Fusarium and Aspergillus are the most commonly encountered pathogens, especially in tropical and subtropical areas.Citation1–3 Humicola is a genus of hyphomycetes related to the family Chaetomiaceae, which frequently found in various environments such as soil, indoor spaces, and compost habitats.Citation4 Within this genus, more than 50 different species have been identified and described.Citation4 However, it is worth noting that human infections caused by Humicola species are extremely rare and have seldom been reported in scientific literature.Citation5 Here, we report first case of fungal keratitis caused by H. pulvericola in a 63-year-old man with a history of exposed to hot oil two weeks ago who developed keratitis.
Case Presentation
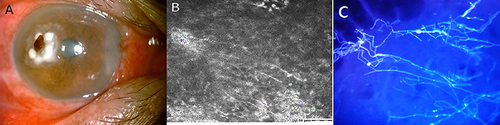
A 63-year-old man presented with pain, redness, and foreign body sensation in his right eye after being exposed to hot oil two weeks ago. His best-corrected visual acuity in the right eye was 0.2. Obvious conjunctival congestion, with white opacity on the temporal side of the cornea and visible brown foreign body in his right eye (). Confocal microscopy revealed abundant hyphae structure at the lesion of the right cornea (). No abnormality was observed in the left eye. Calcofluor white staining of corneal scrapings showed abundant septate hyaline (). Corneal scrapings were directly inoculated into 5% sheep blood agar and Sabouraud glucose agar (SDA), and incubated at 28°C and 35°C, respectively. After 2–3 days, small, white colonies, apparently of a single fungus species, appeared on the two culture media.
Figure 1 (A) Obvious conjunctival congestion, with white opacity on the temporal side of the cornea and visible brown foreign body in his right eye; (B) Confocal microscopy revealed abundant hyphae structure at the lesion of the right cornea; (C) Calcofluor white staining of corneal scrapings showed abundant septate hyphae.
The clinical isolate was subcultured on potato dextrose agar (PDA) and SDA, and incubated at 25°C and 35°C in the dark. After 7 days incubated at 25°C, the colonies on PDA and SDA were fluffy, with pale yellow aerial mycelia. At 35°C incubated 7d, the colony on PDA was yellow-brown (obverse), and a dark black color (reverse); colony on SDA was grey (obverse), and a dark black color (reverse) (). Micromorphology examination showed hyphae hyaline and chlamydospores. Chlamydospores produced laterally or intercalary on hyphae, single-cell, thick-walled, brown, globose to subglobose ( and ). It progressively became darken after 14 days and aerial hyphae gradually enrich (). Micromorphology examination showed two types of conidia: chlamydospores and acremonium-like conidiophores (). Acremonium-like conidiophores, which were small (2.5–3 µm × 1.2–1.8 µm), hyaline, aseptate, smooth, obovoid, with slightly truncate ends, arranged in long divergent chains on the apex of the flask shaped conidiogenous cells (phialides) (). The strain was confirmed by Internal transcribed spacer 1 (ITS1) sequencing, and the obtained nucleotide sequence was compared with the nearest sequence at the NCBI GenBank database. The first homology sequence presenting the highest identity was H. pulvericola rDNA region (99.81% nucleotide identity).
Figure 2 (A) The colonies on PDA and SDA after 7 days incubated at 25°C and 35°C; (B and C) Micromorphology examination showed hyphae hyaline and chlamydospores. Chlamydospores produced laterally or intercalary on hyphae, chlamydospores produced laterally or intercalary on hyphae, single-cell, thick-walled, brown, globose to subglobose.
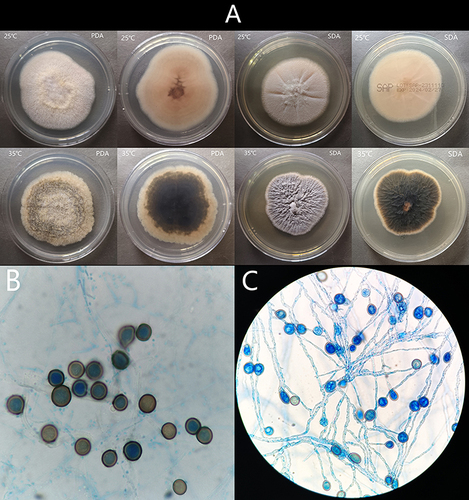
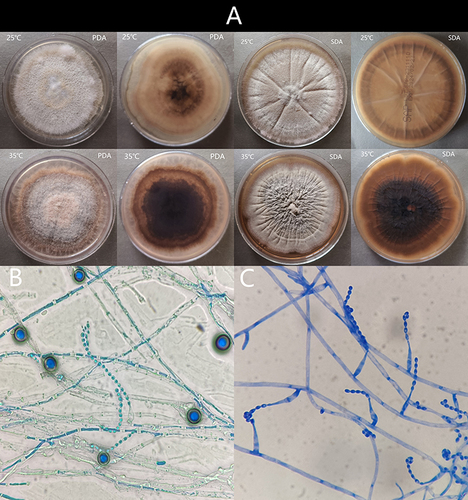
Figure 3 (A) The colonies on PDA and SDA after 14 days incubated at 25°C and 35°C. (B) Micromorphology examination showed two types of conidia: chlamydospores and acremonium-like conidiophores; (C) Acremonium-like conidiophores, which were small (2.5–3 µm × 1.2–1.8 µm), hyaline, aseptate, smooth, obovoid, with slightly truncate ends, arranged in long divergent chains on the apex of the flask shaped conidiogenous cells (phialides).
Antifungal susceptibility testing was performed according to CLSI 38 methods,Citation6 and the concentration of suspension was 0.5 Maxwell turbidity. The incubator temperature was 28°C and reading results after 48 h. Determining minimum inhibitory concentrations (MICs) for fluconazole was 8 µg/mL, amphotericin B was >32 µg/mL, itraconazole was 0.25µg/mL and voriconazole was 0.5µg/mL. Minimum effective concentration (MEC) for caspofungin was >32 µg/mL. The results showed that the infectious agent was resistant towards fluconazole, caspofungin and amphotericin B; itraconazole and voriconazole were highly effective against H. pulvericola.
The patient was diagnosed with H. pulvericola keratitis and treated with oral itraconazole and natamycin eyedrops. After one month treatment, the lesion gradually improved, with the best-corrected visual acuity improving to 0.8.
Discussion
The hyphomycete genus Humicola initially identified two species within this genus, which were H. fuscoatra (the type species) and H. grisea.Citation4,Citation7 Over the years, the number of described species in Humicola has exceeded 50,Citation7,Citation8 but there has never been a comprehensive revision of the genus, nor a thorough phylogenetic analysis conducted. Most Humicola species are typically found in soil, although some have been isolated from compost, decaying plant materials, indoor environments, and even the fur of cats.Citation9,Citation10 These species possess potential applications as bio-organic fertilizers or as biological control organisms for plant diseases, including amelioration of arsenic phytotoxicity and use Humicola sp to produce novel insecticidal compound.Citation11,Citation12 However, it is important to note that certain Humicola species have been reported to have detrimental effects on human health. Disease caused by Humicola species is rare, with two cases of allergic reactions caused by Humicola fuscoatra,Citation13,Citation14 a case of Humicola-associated peritonitis,Citation5 as well as a Humicola trauma-related invasive fungal infection.Citation15
Humicola is commonly recognized as an asexual genus in the Chaetomiaceae family.Citation4 The microscopic morphology of Humicola species may be indistinguishable from other hyphomycete genera with similar thick-walled conidia, such as Chaetomiaceae, Mycothermus, Staphylotrichum, and Trichocladium.Citation4 Some Humicola species can produce two types of conidia: large, dark, globose to subglobose chlamydospores borne singly on vegetative hyphae and small hyalin; and acremonium-like conidiophores, which can distinguish this genus from other similar genus. However, acremonium-like conidiophores only occasionally present and some species do not produce acremonium-like conidiophores.Citation4 In our case, H. pulvericola colony on PDA and SDA was fluffy, with pale yellow aerial mycelia incubated at 25°C. At 35°C, the colony on PDA was yellow-brown (obverse) and a dark black color (reverse); colony on SDA was grey (obverse), and a dark black color (reverse). H. pulvericola colony micromorphology examination showed chlamydospores within a week and produced two types of conidia after 14 days culture: chlamydospores and acremonium-like conidiophores, which can distinguish this genus from other similar genus and members within Humicola genus. DNA sequencing is a crucial method for identification Humicola species. Two cases of Humicola fuscoatra allergic reactions were diagnosed by challenge with antigen inhalation.Citation13,Citation14 The culture isolate of Humicola-associated peritonitis was identified by morphology and ITS sequencing.Citation5 The fungus of trauma-related invasive fungal infection was identified as Humicola species by DNA sequencing.Citation15 In our case, the isolate produced chlamydospores and acremonium-like conidiophores, which were typical features of Humicola. Subsequently, ITS1 sequencing confirmed it as H. pulvericola. Although characteristics of acremonium-like conidiophores production by H. pulvericola have been described, this was only used as a supplement for identification, because culture-based phenotypic methods are insensitive and slow, requiring 2–4 weeks to form typical structures. It is suggested that ITS rDNA sequencing serves as a valuable molecular biological technique for Humicola species identification.
Currently, there is a lack of recommended treatment options available for Humicola infections. A case of peritoneal dialysis associated peritonitis caused Humicola sp. was successfully treated with amphotericin B and voriconazole antifungal therapy, along with multiple operative interventions.Citation5 Humicola trauma-related invasive infection emphasized aggressive surgical management combined with antifungal drug therapy can optimize patient outcomes.Citation15 In our case, in vitro drug sensitivity test showed that H. pulvericola was resistant towards fluconazole, caspofungin and amphotericin B; itraconazole and voriconazole were highly effective against H. pulvericola. According to the MIC values, we successfully treated with oral itraconazole and natamycin eyedrops.
Conclusion
In conclusion, we report first case of fungal keratitis caused by H. pulvericola and successfully treated with itraconazole antifungal therapy for one month. Given the taxonomy of the genus Humicola is unclear and its similarity with Chaetomiaceae family in morphology, it is recommended to use molecular techniques for species identification. Correct delineation of the species and antifungal susceptibility testing will be improving clinical prognosis.
Abbreviations
ITS, Internal transcribed spacer; SDA, Sabouraud glucose agar; PDA, potato dextrose agar; MICs, minimum inhibitory concentrations; MEC, Minimum effective concentration.
Ethics Approval and Patient Consent
Written informed consent was obtained from the patient for the publication of the case details. The study was approved by the medical ethics committee of The People’s Hospital of Guangxi Zhuang Autonomous Region.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflict of interest in this work.
Acknowledgments
We would like to acknowledge the reviewers for their helpful comments on this paper.
Data Sharing Statement
The datasets used and analysed during the current study available from the corresponding author on reasonable request.
Additional information
Funding
References
- Manikandan P, Abdel-Hadi A, Randhir Babu Singh Y, et al. Fungal keratitis: epidemiology, rapid detection, and antifungal susceptibilities of fusarium and aspergillus isolates from corneal scrapings. Biomed Res Int. 2019;2019:6395840. doi:10.1155/2019/6395840
- Mahmoudi S, Masoomi A, Ahmadikia K, et al. Fungal keratitis: an overview of clinical and laboratory aspects. Mycoses. 2018;61(12):916–930. doi:10.1111/myc.12822
- Menard M, Shah YS, Stroh IG, et al. Microbial profile and clinical outcomes of fungal keratitis at a single-center tertiary care hospital. Clin Ophthalmol. 2022;16:389–399. doi:10.2147/OPTH.S346227
- Wang XW, Yang FY, Meijer M, et al. Redefining Humicola sensu stricto and related genera in the Chaetomiaceae. Stud Mycol. 2019;93:65–153. doi:10.1016/j.simyco.2018.07.001
- Burns N, Arthur I, Leung M, et al. Humicola sp. as a cause of peritoneal dialysis-associated peritonitis. J Clin Microbiol. 2015;53(9):3081–3085. doi:10.1128/JCM.01253-15
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi. 3rd ed. CLSI Standard M38. Clinical and Laboratory Standards Institute; 2017.
- Wang XW, Houbraken J, Groenewald JZ, et al. Diversity and taxonomy of Chaetomium and chaetomium-like fungi from indoor environments. Stud Mycol. 2016;84:145–224. doi:10.1016/j.simyco.2016.11.005
- Wang XW, Lombard L, Groenewald JZ, et al. Phylogenetic reassessment of the Chaetomium globosum species complex. Persoonia. 2016;36:83–133. doi:10.3767/003158516X689657
- Jiang Y-L, Y-M W, J-J X, Geng Y-H, Wang H-F, Zhang T-Y. Four new <I> Humicola species from soil in China. Mycotaxon. 2016;131(2):269–275. doi:10.5248/131.269
- Seifert KA, Gams W. The genera of Hyphomycetes - 2011 update. Persoonia. 2011;27:119–129. doi:10.3767/003158511X617435
- Tripathi P, Khare P, Barnawal D, et al. Bioremediation of arsenic by soil methylating fungi: role of Humicola sp. strain 2WS1 in amelioration of arsenic phytotoxicity in Bacopa monnieri L. Sci Total Environ. 2020;716:136758. doi:10.1016/j.scitotenv.2020.136758
- Tabata N, Tomoda H, Iwai Y, Omura S. Xanthoquinodin B3, a new anticoccidial agent produced by Humicola sp. FO-888. J Antibiot. 1996;49(3):267–271. doi:10.7164/antibiotics.49.267
- Kita T, Nishi K, Fujimura M, et al. A case of hypersensitivity pneumonitis caused by Humicola fuscoatra. Respirology. 2003;8(1):95–98. doi:10.1046/j.1440-1843.2003.00434.x
- Ogawa H, Fujimura M, Tofuku Y. Isolated chronic cough with sputum eosinophilia caused by Humicola fuscoatra antigen: the importance of environmental survey for fungus as an etiologic agent. J Asthma. 2002;39(4):331–336. doi:10.1081/JAS-120002290
- Gonte MR, Ranganathan KL, Helliwell LA. Humicola Trauma-related Invasive Fungal Infection in an Immunocompetent Patient. Plast Reconstr Surg Glob Open. 2022;10(11):e4568. doi:10.1097/GOX.0000000000004568
