Abstract
Purpose
The global spread of blaCTX-M-I extended-spectrum beta-lactamase (ESBL)-producing Salmonella spp. remains a major threat to treatment and control. Evidence of emergence and spread of this marker are lacking in Nigeria. This study investigated blaCTX-M-I ESBL production among Salmonella isolates from hospitalized patients.
Methods
Patients (158 total) made up of two groups were evaluated. Group A was composed of 135 patients with persistent pyrexia and group B was composed of 23 gastroenteritis patients and their stool samples. Samples were cultured, and isolates were identified and were subjected to antibiotic susceptibility testing by standard methods. Isolates were further screened for ESBL production, blaCTX-M-I genes and transferability by double disk synergy test, plasmid extraction, polymerase chain reaction, and conjugation experiment.
Results
Thirty-five (25.9%) Salmonella isolates were identified from group A, of which 74.3% were S. typhi, 22.9% were S. paratyphi and two (5.7%) were invasive non-typhoidal S. enteritidis. Nine Plasmodium falciparum infections were recorded, four of which were identified as co-infections with typhoidal Salmonella. Only two (8.7%) S. enteritidis samples were obtained from group B (P>0.05). A total of 24 isolates were ESBL-positive, eliciting resistance to five to seven antibiotics, and were multiple-drug resistant. ESBL production due to the blaCTX-M-I gene cluster was detected in eleven (45.8%) Salmonella isolates. Nine (81.8%) of the eleven blaCTX-M-I ESBL producers were S. typhi and two (18.2%) isolates were S. enteritidis. Four of nine S. typhi blaCTX-M-I ESBL-producing strains harbored 23 kb self-transmissible plasmid that was co-transferred with cefotaxime and augmentin resistance to Escherichia coli j53-2 transconjugants.
Conclusion
This study revealed the emergence of blaCTX-M-I S. typhi as an agent of persistent pyrexia with potential to spread to other Enterobacteriaceae in Lagos, Nigeria. Cautionary prescription and judicious use of third-generation cephalosporins, particularly cefotaxime, for the treatment of typhoid fever and routine screening for P. falciparum co-infection with ESBL-producing Salmonella in the laboratories during diagnosis of persistent pyrexia conditions in patients are recommended.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
In developing countries, diseases such as enteric fever and diarrhea are on the increase due to poor sanitation and inadequate potable water supply.Citation1 An estimated annual incidence of 540 per 100,000 for typhoid fever in developing countries and about 21 million cases worldwide had been reported.Citation2 The number of strains of Salmonella enterica serovars that have developed resistance to one or more antibacterial agent has steadily increased, probably due to continuous antibiotic pressure.Citation3 Resistance to third-generation cephalosporins (3GCs) due to acquisition and expression of bla-CTX-M-mediated, extended-spectrum β-lactamase (ESBL) enzymes among Gram-negative bacteria in the family Enterobacteriaceae is also on the increase. These enzymes are produced exclusively by Gram-negative bacteria and are active against 3GCs, especially cefotaxime, compared to bla-TEM and bla-SHV ESBLs.Citation3 These ESBL enzymes are usually plasmid-mediated, but are susceptible to cephamycins such as cefoxitin, beta-lactamase inhibitors such as clavulanate, sulbactam, tazobactam, and carbapenems.Citation4
ESBL-producing organisms are reported to account for a significant proportion of intensive care infections and mortality in children and immunocompromised patients.Citation5 Problems of ESBLs have led to limited as well as expensive treatment options, and have impacted negatively on clinical outcomes.Citation6 Growing incidences of ESBLs in Salmonella species have been identified in numerous countries of Latin America, Africa, Europe, and Asia.Citation3,Citation7,Citation8 In Nigeria, blaCTX-M-I ESBL-producing Escherichia coli, Enterobacter spp., and Klebsiella spp. have been documented.Citation9–Citation11 However, data on blaCTX-M-I-mediated ESBL-producing S. enterica serovars are currently not available in Nigeria. In a recent work carried out by our research team, we provided evidence of ESBL-bla-CTX-M-producing S. enterica serovars in Lagos.Citation12 Unfortunately, these strains were not characterized for carriage of the blaCTX-M-I gene cluster, which is associated with community-associated infections in many countries including Nigeria.Citation11,Citation13 This family of blaCTX-M-I, reported to have replaced the bla-CTX-M-2 gene cluster in the late 1990s, have now spread worldwide among many members of the Enterobacteriaceae, including Salmonella.Citation14
In recent times, increasing episodes of persistent fever among patients affected by Salmonella typhi and Plasmodium falciparum infections has been a major concern in hospitals and clinics in Lagos and other parts of Nigeria.Citation15,Citation16 This is because of the perceived clinical failure associated with the use of 3GCs, particularly cefotaxime, ceftriaxone, and cefuroxime, and increasing drug pressure with arthemisin combination therapies.Citation15,Citation16 The current World Health Organization WHO) recommendation for parasite-based diagnosis of malaria in pyrexia cases before treatment in the era of artemisinin-based combination therapy (ACT) has further made it imperative to screen for malaria parasites by microscopic or rapid diagnostic method in countries like Nigeria, where ACT has replaced monotherapies as first- and second-line treatments for uncomplicated malaria.Citation17,Citation18 In the current study, we investigated the carriage of blaCTX-M-I ESBL among Salmonella strains isolated from patients, and the possibility of Plasmodium spp. co-infection.
Materials and methods
Patient population and study design
A total of 158 patients who sought treatment at referral centers including Ikeja General Hospital, Lagos; Infectious Diseases Hospital Mainland, Lagos; Central Bank of Nigeria Clinics Satellite, Lagos; and Central Medical Laboratory Health Centre, Lagos from October 2010 to July 2011 were recruited for the study. Important biodata, history of vaccination, antimicrobial therapy, time of onset of illness, etc, of these patients were recorded. Ethics approval from the ethics committee of each institution was obtained prior to patients’ enrollment.
Case definition, sample processing, Plasmodium and Salmonella detection
Two categories of patients were demarcated. A total of 135 patients were assigned to group A, and had been diagnosed by a physician for persistent fever (≥37.5°C) in the previous 72 hours with or without one or more of the following symptoms: diarrhea, headache, abdominal pains, loss of appetite, vomiting, and/or nausea for 5 consecutive days. In detail, 4 mL of blood was collected from each of the patients at the early onset of symptoms. Three of the 4 mL blood samples in each case were inoculated into 27 mL of brain-heart infusion (BHI) broth (Oxoid, Basingstoke, UK) for bacteriological culture, while the remaining blood sample was used for the preparation of thick and thin blood films on grease-free slides (two per sample) for the detection and speciation of Plasmodium parasites by light microscopy. Group B was made up of 23 patients who had presented with frequent stools for 2 or more days (diarrhea). A fresh stool sample from each patient was inoculated into Cary-Blair transport medium (10 mL/tube) and was brought to the laboratory for bacteriological culture.
Bacterial agent isolation
Blood samples in the inoculated BHI broth culture bottles were incubated overnight aerobically at 37°C. Similarly, stool samples from Cary-Blair medium were inoculated into enrichment Selenite F broth (Oxoid) and were incubated at 37°C for 18–24 hours aerobically. Thereafter, sub-cultures were made onto deoxycholate citrate agar, Salmonella-Shigella agar, and MacConkey agar plates. All the culture agar plates were incubated at 37°C aerobically for 18–24 hours. In negative blood samples, sub-cultures were repeated daily from the BHI broth cultures for 7 consecutive days, after which the samples were disposed of. After overnight incubation, culture plates were examined for colonial morphology and Gram stained. Colonies were first identified by standard methods, as described in Cowan and Steel’s Manual.Citation19 The Analytical Profile Index 20E identification system (Institut Mérieux, Marcy l’Etoile, France) was used for the confirmation of the Salmonella isolates. Further identification of Salmonella species based on their somatic (O) and flagella (H) antigen characteristics was done using polyvalent antisera (Wellcome Diagnostic, London, UK).
Antimicrobial susceptibility testing
All Salmonella isolates were investigated for their in vitro susceptibilities to 13 antibiotics by disk diffusion, as described by Clinical and Laboratory Standard Institute (CLSI) guidelines.Citation20 Disks with the following preparations were used for susceptibility testing: ampicillin (25 μg), chloramphenicol (30 μg), co-trimoxazole (25 μg), tetracycline (25 μg), nalidixic acid (30 μg), ciprofloxacin (20 μg), ofloxacin (20 μg), gentamicin (10 μg), cefotaxime (30 μg), augmentin (30 μg; amoxicillin 20 μg/clavulanic acid 10 μg combination), ceftriaxone (30 μg), ceftazidime (30 μg), imipenem (30 μg), levofloxacin (10 μg), and azithromycin (15 μg) (Oxoid). The plates were incubated aerobically at 37°C for 18–24 hours. The diameter of the zones of inhibition were measured with a ruler and compared with a zone interpretation chart.Citation14 E. coli American Type Culture Collection (ATCC) 25922 was used as a control. Multidrug resistance phenotype was defined as resistance to three or more classes of antibiotics.
ESBL assay
All the isolates that exhibited reduced susceptibility and/or resistance to 3GCs were screened for ESBL production, using the double disk synergy test method. This was done by placing the 3GC antibiotics, ie, ceftazidime (30 μg) and ceftriaxone (30 μg) at a distance of 15 mm (center to center) from 30 μg augmentin (20 μg amoxicillin combined with 10 μg clavulanic acid), using CLSI interpretative guidelines as the standard.Citation21 An aliquot of a 0.5 μl Klebsiella pneumoniae ATCC 700603 was used as the positive control and E. coli ATCC 25922 was used as the negative control in each test batch.
Plasmid DNA extraction
Plasmid extraction was performed by a simplified alkaline lysis method described by Cheng et al.Citation22 Briefly, overnight culture (1.5 mL) was centrifuged at 5,000 rpm for 1 minute to pellet the cells. The supernatant was gently decanted. After washing, the cell pellet was re-suspended in 300 μL Tris (tris(hydroxymethyl)aminomethane)-EDTA(ethylenediaminetetraacetic acid)-NaOH-SDS (sodium dodecyl sulfate) (TENS) buffer solution and mixed by gentle inversion (five times) within 3–5 minutes of incubation on ice to obtain a straw-like, sticky lysate. This was followed by neutralization by adding 200 μL of 5 M potassium acetate buffer (pH 5.2), followed by incubation on ice for 10 minutes. Plasmid DNA solution was recovered as supernatant after centrifugation at 10,000 rpm for 10 minutes. Plasmid pellets isolated with ice-cold absolute ethanol were washed in 70% ethanol before re-suspension in 20–40 μL Tris (10 mM)-EDTA (1 mM) buffer (pH 8.0). The plasmid DNAs were separated by electrophoresis on 0.8% agarose pre-stained with ethidium bromide (0.5 μg/mL).
Detection of blaCTX-M-I gene cluster
Genomic DNA was extracted from the Salmonella isolates by boiling method and was used as a template for the detection of ESBL blaCTX-M-I gene cluster by polymerase chain reaction (PCR). The 25 μL PCR reaction mixture contained 10 pmol of each forward and reverse primer pair for the blaCTX-M-I gene cluster, 200 μM of deoxynucleotide triphosphate, 1 μL of Taq DNA polymerase, and 1 μL of DNA solution. The PCR program consisted of an initial denaturation step of 5 minutes at 94°C, a 30 cycle period (each cycle consisting of 30 seconds at 94°C, 40 seconds at 52°C, and 50 seconds at 72°C), and then a final extension step of 5 minutes at 72°C. The primer nucleotide sequences used for the 415 bp blaCTX-M-I group gene were 5′-AAA AAT CAC TGC GCC AGT TC-3′ and 5′-AGC TTA TTC ATC GCC ACG TT-3′ for Enterobacteriaceae species, as previously reported by Woodford et al.Citation23 The 415 bp blaCTX-M-I gene was resolved on 1.5% agarose gel pre-stained with ethidium bromide (0.5 μg/mL) by electrophoresis.
Conjugation experiment
Salmonella isolates harboring the blaCTX-M-I group of ESBLs were selected for the conjugation experiment using the broth mating technique described by Chen et alCitation24 with rifampicin-resistant E. coli j53-2 as the recipient. Transconjugants were selected on a BHI agar plate containing cefotaxime (4 μg/mL) and rifampicin (200 μg/mL). Transfer of blaCTX-M-I gene, plasmids, and co-dissemination of antibiotic resistance was confirmed by PCR, plasmid isolation, and antibiotic susceptibility testing for the transconjugants and recipient, as previously done for the donor.
Statistical analyses
Data were expressed as frequencies or percentages. Fischer’s exact test was performed on 2×2 and 2×3 contingency tables of data for assessing disparity in Salmonella isolation rates between blood and stool samples, and ESBL patterns among the three S. enterica serovars isolated. Outcomes of P<0.05 were taken to be significant.
Results
Out of the 135 samples screened in group A, 35 Salmonella isolates (25.9%) were made up of three serotypes; S. typhi (71.4%), S. paratyphi (22.9%), and two (5.7%) of Salmonella enteritidis. Nine Plasmodium spp. were identified. Three strains of S. typhi and a strain of S. paratyphi were isolated from patients with Plasmodium sp. and complications. Thirteen of the 23 stool samples from group B were confirmed positive for bacterial pathogens, made up of eleven strains of E. coli and two strains of S. enteritidis (). Further analysis revealed non-significant difference (P>0.05) in the observed disparity in the isolation rate of S. enterica isolates or S. enteritidis isolates between culture-positive stool and blood samples from the two groups of patients studied.
Table 1 Percentage distribution of pathogens isolated according to clinical samples
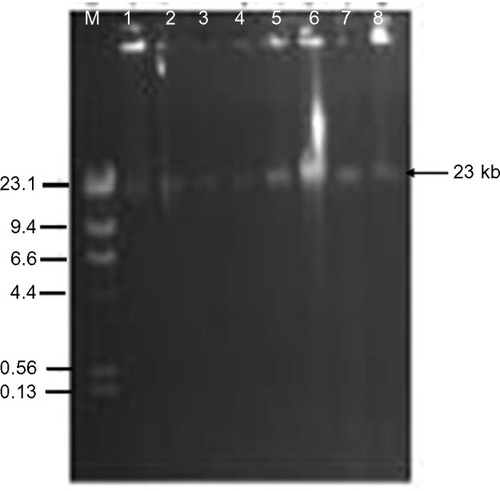
Antibiotic resistance patterns of the isolated 37 S. enterica serovars are presented in . A total of 24 isolates were ESBL producers, elicited resistance to 5–7 antibiotics, and accounted for eleven (78.6%) of the 14 antibiotic resistance patterns observed. On the whole, 75.7% of the recovered S. enterica serovars isolated were multidrug resistant. PCR analysis revealed ESBL production due to blaCTX-M-I gene cluster in eleven (45.8%) S. enterica serovars. These strains also accounted for 76.9% (ten of 13 cases) of augmentin resistance observed. Carriage of blaCTX-M-I was also significantly associated with cefotaxime resistance (). However, all the Salmonella isolates were sensitive to imipenem, levofloxacin, and azithromycin. Of the eleven blaCTX-M-I ESBL producers detected, nine (81.8%) were S. typhi and two (18.2%) were S. enteritidis. Non-production of ESBL by 24%, 75%, and 25% of S. typhi, S. paratyphi, and S. enteritidis (P=0.03), respectively, was also observed (). Conjugation experiments revealed a 23 kb self-transmissible plasmid and co-transfer of cefotaxime and augmentin resistance to E. coli j53-2 transconjugants by four of the eleven blaCTX-M-I-producing donor serovars, all of which were S. typhi strains ( and ).
Table 2 Antibiotic resistance patterns of Salmonella enterica serovars producing bla-CTX-M-1 extended-spectrum beta lactamase
Table 3 Antibiotic resistance and plasmid transfer by conjugation from the donor Salmonella typhi isolates to the Escherichia coli j53-2 recipient
Figure 1 Distribution of Salmonella enterica serovars producing extended spectrum beta lactamases.
Notes: P<0.05 was significant. S. enterica serovars as per ESBL patterns 2×3 contingency table, Fischer’s exact test.
Abbreviation: ESBL, extended spectrum beta lactamase.
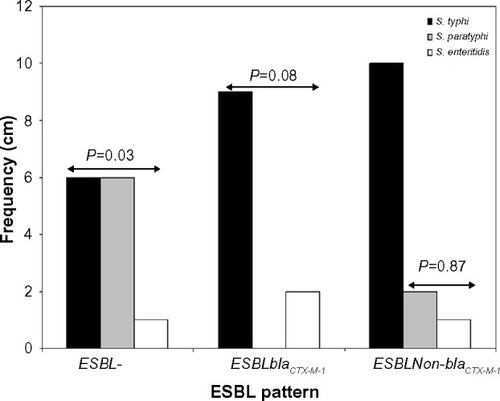
Figure 2 Plasmid transfer by conjugation from donor Salmonella typhi strains to recipient Escherichia coli j53-2. Lanes 1, 3, 5, and 7 are S. typhi donors Lag-003, -004, -007, and -010. Lanes 2, 4, 6, and 8 are E. coli j53-2 transconjugants. Lane M represents Lamda DNA Hind III markers.
Abbreviation: DNA, deoxyribonucleic acid.
Discussion
3GCs remain the most commonly prescribed class of antibiotics for case management of typhoidal and non-typhoidal salmonellosis in many countries of the world, including NigeriaCitation12,Citation25 In this study, 37 Salmonella isolates, which included 35 (25.9%) from blood of patients with persistent pyrexia and two (8.7%) S. enteritidis from stool samples of patients with gastroenteritis were isolated to yield a prevalence of 20.8% salmonellosis. S. typhi was further found to account for 71.4% of persistent pyrexia cases due to Salmonella isolates producing blaCTX-M-I ESBLs that were mediated by a 23 kb plasmid. These phenotypic and molecular characteristics were observed in 75.6% of Salmonella isolates that were multidrug resistant.
The findings from the current study indicated that the epidemiology of Salmonella as a public health burden in Lagos has not changed from the previous reports from LagosCitation12,Citation15,Citation26 and from other parts of Nigeria.Citation16 Interestingly, in this study, some of the bacteremic strains of S. typhi that produced blaCTX-M-I ESBL can be used to explain why persistent pyrexia occurs in affected patients despite them receiving treatment with 3GCs. The implication of this is the potential for spread of emerging blaCTX-M-I-producing S. typhi in Lagos, which will add to the prevailing public health burdens in the state. In our previous study in the same environment, we found 53.1% of Salmonella isolates, composed of Salmonella typhimurium, S. enteritidis, S. paratyphi, and Salmonella choleraesuis from gastroenteritis cases to be blaCTX-M-I positive.Citation12 The present result found eleven (nine S. typhi and two S. enteritidis) of 37 Salmonella isolates recovered to be blaCTX-M-I-positive, with four S. typhi isolates transferring this genetic marker to E. coli j53-2. This finding indicates that other groups of bla-CTX-M exist among clinical Salmonella isolates in Lagos and that blaCTX-M-I carriage may be an emerging trend with pathogenic advantage to cause persistent pyrexia in patients with typhoid fever. The blaCTX-M-I rate of 45.6% observed in this study is higher than the 7.7% detected among other clinical enteric pathogens from Abeokuta, Nigeria by Akinduti et al.Citation27 It is also higher than the 6% rate recently reported among clinical Salmonella isolates in France.Citation28 In previous studies on blaCTX-M-I gene variants in Lagos, blaCTX-M-15 was detected in 17 urinary pathogens of K. pneumoniae by Soge et alCitation29 and in Enterobacter cloacae and Pantoea agglomerans isolates by Aibinu et al.Citation30 Also in south-eastern Nigeria, Iroha et alCitation11 reported the carriage of blaCTX-M-I ESBL among 44 clinical isolates of E. coli from two hospitals in 2012. These findings indicate increasing spread of blaCTX-M-I among Enterobacteriaceae members in Nigeria.
Continuous surveillance of blaCTX-M-I-producing pathogens is needed to guide preventive interventions in Nigeria. BlaCTX-M-I gene cluster carriage is associated with increased resistance to cefotaxime. Therefore, the clinical practice of switching Salmonella bacteremic and febrile patients who failed treatment with ceftriaxone, cefuroxime, or ceftazidime empirically to cefotaxime also has the risk of treatment failure in this environment. For such patients, this study recommends the use of imipenem or levofloxacin, or azithromycin for case management. The clinical effectiveness of these antibiotics for treating multidrug-resistant infections has been reported in African countries such as Tanzania,Citation31 in North and South America,Citation32 Nepal,Citation33 and India.Citation34 These antibiotics are also included in the drug formulary of many hospitals in Lagos and other states in Nigeria, where they are used for case management of inpatients and outpatients affected by other bacterial infections.Citation9–Citation12 The results of the conjugation experiment not only showed the blaCTX-M-I gene carried by S. typhi was plasmid-mediated, but also revealed the potential for rapid spread of this genetic marker to other members of Enterobacteriaceae such as E. coli, which are often encountered in polymicrobial infections in Lagos and other regions of Nigeria.Citation35
Similar plasmid-mediated transfer of blaCTX-M-I and resistance to antibiotics such as cefotaxime, aminoglycosides, and beta-lactamase inhibitor-containing antibiotics such as augmentin, as demonstrated in the present study, have been documented by previous investigators from other countries of the world.Citation31,Citation36–Citation38 However, disparity can be seen in the molecular size of the plasmid transferred. Jin and LingCitation36 reported the presence of bla-CTX-M genes on 62 kb, 70 kb, and 92 kb harbored by S. enteritidis and S. typhimurium from Hong Kong, while Mshana et alCitation31 found a 231 kb plasmid to be responsible for the carriage of bla-CTX-M-15 gene among S. enterica isolates from Tanzania. In a study carried out by Bado et alCitation37 in Uruguay, where the first description of blaCTX-M-I gene cluster in S. enteritidis was reported, a 195 kb plasmid was incriminated as a vector. In Germany, Fischer et alCitation38 reported the transferability of blaCTX-M-15 among Salmonella isolates from horse and swine through 95 kb IncF and Inc1I plasmids. In Lagos, an earlier study by Soge et alCitation29 revealed the presence of the blaCTX-M-I gene on plasmids of sizes 58–320 kb in K. pneumoniae.
Nevertheless, it is sufficient to suggest that multiple self-transmissible plasmids are involved in the spread of blaCTX-M-I across the different regions and countries of the world. Their active roles for dissemination of cefotaxime resistance appear to vary according to geographical region, reservoir of Salmonella isolates (ie, food animals or pets of humans), time of study, and their genetic environment. The latter can be used to explain why blaCTX-M-I carriage accounted for 76.9% resistance to augmentin, as seen in the current study. This was further confirmed by co-transfer of resistance to antibiotics to E. coli j53-2 by conjugation. Studies conducted in other countries have reported carriage of augmentin resistance markers such as ampC, aminoglycoside modification genes, and metallo beta lactamase genes on blaCTX-M-I plasmids.Citation29–Citation31,Citation36–Citation39
Furthermore, additional antibiotic resistance conferred by this genetic marker may also set the stage for the emergence of pan-resistant S. typhi in Nigeria, as recently documented in some Asian countries.Citation40 The emergence of multidrug-resistant typhoidal and non-typhoidal Salmonella have caused life-threatening invasive disease outbreaks in children and adults in many African countries, including Zaire,Citation41 Malawi,Citation42 and Kenya.Citation43 Similarly, multidrug-resistant Salmonella serotypes have been widely prevalent in Kuwait and India.Citation44,Citation45
It is worthy to note in this study that four Salmonella isolates with P. falciparum-associated co-infection were detected, and that S. typhi mono-infection was characterized by ceftriaxone and cefotaxime resistance; the current study has further revealed the relevance of blaCTX-M-I ESBL testing for rational use of both ACT and antibiotics for good outcomes in patients with persistent pyrexia-associated Salmonella or Plasmodium, or both. In this context, the reported risk of poor treatment outcomes in patients admitted in hospitals with laboratories that do not perform tests for detection of ESBLs and do not report ESBL producers as resistant to cephalosporins is now apparent in Lagos.Citation46 The potential spread of blaCTX-M-I-producing S. typhi to other states of the country and other neighboring countries is also a possibility.
The inability to confirm the variants of blaCTX-M-I gene cluster carried by the positive S. typhi strains, which is essential to infer clonal relatedness of these strains, is one of the limitations of this study. Molecular typing techniques such as multilocus sequence typing and pulse-field gel electrophoresis to reveal information about the upstream and downstream genetic environment of the recovered blaCTX-M-I plasmid of the positive S. typhi isolates is essential to assess their levels of homogeneity or heterogeneity. Such information will also be needed to further understand mechanisms of dissemination and extra-antibiotic resistance mechanisms of blaCTX-M-I plasmids among S. typhi isolates in our environment.
Despite these limitations, this study has revealed the emergence of resistance to 3GC antibiotics due to acquisition and expression of plasmid-borne blaCTX-M-I gene cluster among S. typhi strains, and this emergence calls for cautionary parenteral use of cefotaxime and other 3GCs for the treatment of typhoid fever in Lagos, Nigeria. A need for routine screening for P. falciparum co-infection with ESBL-producing Salmonella in the laboratories during diagnosis of persistent pyrexia conditions in patients is recommended.
Acknowledgments
We are grateful to all the staff of the public hospitals used for this work. We are equally grateful to the Staff of the Department of Microbiology of Lagos State University and the Nigerian Institute of Medical Research, Yaba for their technical support.
Disclosure
The authors report no conflicts of interest in this work.
References
- AkinyemiKOIwalokunBAFoliFOshodiKCokerAOPrevalence of multiple drug resistance and screening of enterotoxin gene (stn) in Salmonella enterica serovars from water sources in Lagos, NigeriaPublic Health2011125657121277605
- CrumpJALubySPMintzEDThe global burden of typhoid feverB World Health Organ200482346353
- GoqueTMBaqueroFCantonRIncreasing prevalence of ESBL-producing Enterobacteriaceae in EuropeEuro Surveill2008134719051
- JacobyAGMunoz-PriceSLThe new β-lactamasesN Engl J Med200535238039115673804
- MenasheGBorerAYagupskyPClinical significance and impact on mortality of extended-spectrum beta lactamase-producing Enterobacteriaceae isolates in nosocomial bacteremiaScand J Infect Dis20013318819311303808
- NdugulileFJureenRHarthugSUrassaWLangelandNExtended spectrum β-lactamases among gram-negative bacteria of nosocomial origin from an intensive care unit of a tertiary health facility in TanzaniaBMC Infect Dis200551615642116
- TassiosPTGazouliMTzelepiESpread of a Salmonella typhimurium clone resistant to expanded-spectrum cephalosporins in three European countriesJ Clin Microbiol199937113774377710523600
- WinokurPLCantonRCasellasJMLegakisNVariations in the prevalence of strains expressing an extended-spectrum beta-lactamase phenotype and characterization of isolates from Europe, the Americas, and the Western Pacific regionClin Infect Dis200132S94S10311320450
- AibinuIEOhaegbulamVCAdenipekunEAOgunsolaFTOdugbemiTOMeeBJExtended-spectrum beta-lactamase enzyme in clinical isolates of Enterobacter species from Lagos, NigeriaJ Clin Microbiol2003412197220012734278
- IrohaIRAmadiESAgabusACOjiAESusceptibility pattern of extended spectrum beta-lactamase producing Klebsiella pneumoniae from clinical isolatesInt J Microbiol200853437
- IrohaIREsimoneCONeumannSFirst description of Escherichia coli producing CTX-M-15-extended spectrum beta lactamase (ESBL) in out-patients from south eastern NigeriaAnn Clin Microbiol Antimicrob2012111922824236
- AkinyemiKOIwalokunBAAgboyinuJAOgunyemiOFasureAKEmergence of third generation cephalosporins and typing by rapid amplified polymorphic DNA among clinical Salmonella isolates from Lagos, NigeriaBrit Microbiol Res J20146668677
- WoertherPLBurdetCChachatyEAndremontATrends in human fecal carriage of extended-spectrum β-lactamases in the community: toward the globalization of CTX-MClin Microbiol Rev20132674475824092853
- RadiceMPowerPDi ConzaJGutkindGEarly dissemination of CTX-M-derived enzymes in South AmericaAntimicrob Agents Chemother20124660260411796390
- AkinyemiKOOshundareYOOyeyinkaOGCokerAOA retrospective study of community-acquired Salmonella infections in patients attending public hospitals in Lagos, NigeriaJ Infect Dev Ctries20126538739522610704
- FashaeKOgunsholaFAerestrupFMHendriksenRSAntimicrobial susceptibility and serovars of Salmonella from chickens and humans in Ibadan, NigeriaJ Infect Dev Ctries2010448449420818100
- World Health OrganizationGuidelines for the Treatment of Malaria2nd edGenevaWorld Health Organization2010
- World Health OrganizationThe Implementation of the Global Malaria Control Strategy: WHO Technical Report Series No 839GenevaWorld Health Organization1993
- Cowan and Steel’s Manual for the identification of Medical Bacteria3rd ednBarrowGIFelthamRKACambridge University Press1993331
- National Committee for Clinical Laboratory StandardsPerformance Standards for Antimicrobial Susceptibility Testing: Ninth Informational Supplement, Version 19Wayne, PANational Committee for Clinical Laboratory Standards1999
- Clinical Laboratory Standards InstitutePerformance Standards for Antimicrobial Susceptibility Testing: 19th Informational Supplement M100S19Wayne, PAClinical Laboratory Standards Institute2009
- ChengSZhaoSWhiteDGMengJCharacterization of multiple antimicrobial resistant Salmonella serovars isolated from retail meatsAppl Environ Microbiol2004701714711619
- WoodfordNFaganEJEllingtonLJMultiplex PCR for rapid detection of genes encoding CTX-M extended-spectrum β-lactamasesJ Antimicrob Chemother20065715415516284100
- ChenLChenZLLiuJHZengZLMaJYJiangHXEmergence of RmtB methylase-producing Escherichia coli and Enterobacter cloacae isolates from pigs in ChinaJ Antimicrob Chemother20075988088517353219
- ArletGBarrettTJButayePCloeckaertAMulveyMRWhiteDGSalmonella resistant to extended-spectrum cephalosporins: prevalence and epidemiologyMicrobes Infect200681945195416714134
- AkinyemiKOSmithSIOyefoluAOCokerAOMultidrug resistance in Salmonella enterica serovar typhi isolated from patients with typhoid fever complications in Lagos, NigeriaPublic Health200511932132715733694
- AkindutiPAOluwadunAIwalokunBAOnagbesanOMEjiludeOCommunity-acquired CTX-M beta lactamase isolates from Abeokuta, NigeriaBrit Microbiol Res J20145351358
- HaenniMSarasEMétayerVMédailleCMadecJYHigh prevalence of blaCTX-M-1/IncI1/ST3 and blaCMY-2/IncI1/ST2 plasmids in healthy urban dogs in FranceAntimicrob Agents Chemother2014585358536224982072
- SogeOOAdeniyiBARobertsMCNew antibiotic resistance genes associated with CTX-M plasmids from uropathogenic Nigerian Klebsiella pneumoniaeJ Antimicrob Chemother20065851048105316997844
- AibinuIPfeiferYPetersFEmergence of bla(CTX-M-15), qnrB1 and aac(6′)-Ib-cr resistance genes in Pantoea agglomerans and Enterobacter cloacae from Nigeria (sub-Saharan Africa)J Med Microbiol20126116516721921107
- MshanaSEGerwingLMindeMOutbreak of a novel Enterobacter sp. carrying blaCTX-M-15 in a neonatal unit of a tertiary care hospital in TanzaniaInt J Antimicrob Agents201138326526921752606
- BiedenbachDJTolemanMWalshTRJonesRNAnalysis of Salmonella spp. with resistance to extended-spectrum cephalosporins and fluoroquinolones isolated in North America and Latin America: report from the SENTRY Antimicrobial Surveillance Program (1997–2004)Diagn Microbiol Infect Dis2006541132116290025
- PathakA1MarothiYKekreVMahadikKMacadenRLundborgCHigh prevalence of extended-spectrum β-lactamase-producing pathogens: results of a surveillance study in two hospitals in Ujjain, IndiaInfect Drug Resist20125657322570555
- PokharelBMKoiralaJDahalRKMishraSKKhadgaPKTuladharNRMultidrug-resistant and extended-spectrum beta-lactamase (ESBL)-producing Salmonella enterica (serotypes Typhi and Paratyphi A) from blood isolates in Nepal: surveillance of resistance and a search for newer alternativesInt J Infect Dis20061043446816978898
- IwalokunBAGbenleGOSmithSIOgunledunAAkinsindeKAOmonigbehinEAEpidemiology of shigellosis in Lagos, Nigeria: trends in antimicrobial resistanceJ Health Popul Nutr20011918319011761772
- JinYLingJMCTX-M-producing Salmonella spp. in Hong Kong: an emerging problemJ Med Microbiol200655Pt 91245125016914655
- BadoIGarcía-FulgueirasVCordeiroNFFirst human isolate of Salmonella enterica serotype Enteritidis harboring bla-CTX-M-14 in South AmericaAntimicrob Agents Chemother2012562132213422290976
- FischerJRodríguezIBaumannBbla-CTX-M-15-carrying Escherichia coli and Salmonella isolates from livestock and food in GermanyJ Antimicrob Chemother2014692951295825074857
- Baba Ahmed-Kazi TaniZArletGNews of antibiotic resistance among Gram-negative bacilli in AlgeriaPathol Biol (Paris)201462169178 French24819127
- TadesseGA meta-analysis of the proportion of antimicrobial resistant human Salmonella isolates in EthiopiaBMC Pharmacol Toxicol2014155125213011
- MilledgeJCalisJCGrahamSMThe effect of HIV infection or neonate bacterial meningitis in Blantyre, MalawiArch Dis Child2003881112111814670782
- KaruikiSRevathiGKariukiNMultidrug-resistant non-typhoidal Salmonella infection in Africa: zoonotic or anthroponotic transmissionJ Med Microbiol20065558559116585646
- AmatyaNMShrasthaBLekhakBEtiological agents of bacteraemia and antibiotic susceptibility pattern in Kathmandu Model hospitalJNMA J Nepal Med Assoc20074611211818274566
- DimitrovTUdoEEAlbaksamiOClinical and microbiological investigations of typhoid fever in an infectious disease hospital in KuwaitJ Med Microbiol20075653854417374897
- ShuklaITiwanRAgarwalMPrevalence of Extended spectrum beta-lactamase producing Klebsiella pneumoniae in a tertiary care hospitalIndian J Med Microbiol200422879117642702
- KrugerTSzaboDKeddyKHInfections with Non-typhoidal Salmonella species producing TEM-63 or a novel TEM enzyme, TEM-131, in South AfricaAntimicrob Agents Chemother2004484263427015504851
