Abstract
Objective:
To investigate the effectiveness, safety, and tolerability of moxifloxacin (MXF) (intravenous [IV] or sequential therapy [IV followed by oral]) under daily treatment conditions in a large number of patients with respiratory tract infections.
Design:
Patients with a diagnosis of respiratory tract infection should be treated with MXF IV and/or tablets 400 mg once daily for a duration at the physician’s discretion. For each patient, the physician documented data at an initial visit and at the end of therapy (EOT) visit and/or, in the case of sequential therapy, an interim visit when the patient switched to oral treatment.
Results:
A total of 1953 patients treated with MXF were documented and were valid for an effectiveness and safety evaluation. An improvement was observed in 98.1% (n = 1911/1949) of patients treated with MXF. Recovery was documented in 89.9% (n = 1754/1951) of the patients. At the EOT visit, severity of infection was assessed to be “relieved” or at least “improved” in 96.5% (n = 1873/1940) of the patients. Physicians assessed overall effectiveness as “good” or “very good” in 93.3% (n = 1822/1953) of all patients. The physicians’ overall tolerability rating was “very good” or “good” in 93.5% (n = 1827/1953) of all patients. The incidence rates of adverse events (AEs) and adverse drug reactions (ADRs) were 0.72% (n = 14) and 0.67% (n = 13), respectively. One serious AE “falling white blood cell count” occurred (0.05%), which was also defined as a serious ADR and resolved.
Conclusion:
MXF was generally well tolerated and highly effective in the treatment of different respiratory tract infections. The incidence of AEs and ADRs was low. The efficacy, safety, and tolerability information collected in this study confirms the clinical safety profile of MXF and its value as antibiotic treatment for respiratory tract infections.
Keywords:
Introduction
Respiratory tract infections, such as community-acquired pneumonia (CAP), acute exacerbation of chronic bronchitis (AECB), or acute bacterial sinusitis, are important causes of morbidity and mortality worldwide. The most frequent causative organism in CAP is Streptococcus pneumoniae or, less commonly, one of the atypical organisms Mycoplasma pneumoniae, Chlamydophila pneumonia, or Legionella pneumophilia.Citation1 In AECB, the primary organisms are Haemophilus influenzae, S. pneumonia, and Moraxella catarrhalis.Citation2
The antibiotic used to treat any of these respiratory tract infections should be chosen to maximize bacterial eradication and minimize the risk of antimicrobial resistance development. Of particular concern is the emergence of the multidrug-resistant S. pneumonia.Citation3,Citation4
Moxifloxacin is an 8-metoxifluoroquinolone with a broad spectrum of antibacterial activity. It is effective against Gram-positive pathogens and Gram-negative pathogens, atypical pathogens, and anaerobic bacteria. Among respiratory pathogens, most penicillin- and macrolide-resistant and β-lactamase-producing strains are sensitive to moxifloxacin.Citation5
This study was designed to obtain data on the effectiveness and safety of moxifloxacin (administered intravenously [IV] or sequentially [IV followed by oral]) under daily treatment conditions in China in a large number of patients with diagnosed bacterial respiratory tract infections.
Materials and methods
Study design
This was a prospective, noninterventional, noncontrolled, multicenter, postmarketing surveillance trial conducted between February 2006 and March 2007 in China in patients presenting at a hospital as part of a global study. The study was carried out within an approved indication in accordance with US Food and Drug Administration and European Medicines Agency guidelines and applicable local law(s) and regulation(s). Only data and observations surveyed during regular therapy with moxifloxacin were documented. No intervention in the therapeutic decisions of the investigator was allowed. Doctors were instructed to prescribe moxifloxacin for treatment of a respiratory tract infection in the usual manner in accordance with the Chinese marketing authorization as far as possible. No additional diagnostic or monitoring procedures were applied to patients. For each patient, the physician documented data at an initial visit and an end of therapy (EOT) visit and/or, in the case of sequential therapy, an interim visit when the patient switched to oral treatment. Observation parameters were fever, cough, dyspnea, and sputum, which were documented to assess symptom development. Effectiveness parameters included course of severity of infection, course of patient’s general condition, course of clinical signs and symptoms, duration until improvement and recovery, and overall assessment of effectiveness by patient and physician. Safety parameters were adverse events (AEs), including adverse drug reactions (ADRs), and global assessment of tolerability.
Data analysis
Descriptive and explorative analyses were conducted with the software system SAS® for Windows, (Version 9.1; SAS Institute, Lans, NC) According to confidentiality guidelines, each patient was assigned an individual five-digit number (pseudonymization), and only the physicians knew which patient had which number and could identify the patient. The analysis was based on the intent-to-treat (ITT) population. For qualitative variables, absolute and relative frequencies were calculated. For stratified analyses, row percents (row%) and column percents (col%) were calculated. For numeric data, the following distribution parameters were calculated: median, mean, standard deviation (SD), minimum, maximum, and 1%, 5%, 25%, 75%, 95%, and 99% percentiles. For coding of verbatim documentation, the following coding systems were applied: concomitant therapies: World Health Organization DD 2005/Q3; adverse events: MedDRA® Version 10.0.
Study population
ITT population/safety population
The definitions for the safety and the ITT populations were identical in this study. Patients were included in the ITT population if at least one dose of moxifloxacin was taken during the observational period. Seventy-four of 2027 enrolled patients were excluded from ITT analysis due to one or more of the following reasons: i) patient double entry, ii) first visit in more than 2 days before the start of the study (retrospective documentation), and iii) no symptoms at initial visit. Thus, for the ITT population as well as in the safety analysis, data of 1953 patients were analyzed.
Results
Demographics
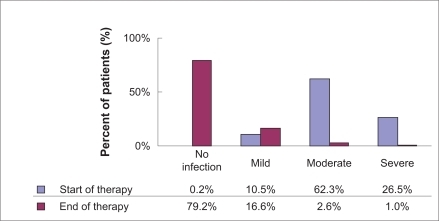
A total of 1953 patients treated with moxifloxacin were documented and were valid for effectiveness and safety evaluation. The vast majority of patients were of Asian origin (99.8%); one patient was white (0.1%) and one patient was black (0.1%). For two patients, information on race was missing (0.1%). A total of 1284 patients were male (65.7%), and 669 patients were female (34.3%). The age ranged from 14.0 to 105.0 years with a mean age of 59.6 ± 17.5 years. A total of 46.9% (n = 915/1953) of the patients suffered from CAP, and 40.1% (n = 783/1953) of the patients examined were diagnosed with AECB. Combined infections (CAP and AECB, CAP and other, AECB and other, CAP and AECB and other) were diagnosed in 3.8% (n = 75/1953) of all cases, and 15.9% (n = 310/1953) of patients were affected by other infections. Most frequent “other infections” were respiratory infection (7.4%, n = 23/310), cross-infection in hospital (4.8%, n = 15/310), and inhalation pneumonia (4.8%, n = 15/310). Of the patients, 22.4% were smokers and 19.3% past smokers, which is known to be an important etiological factor with regard to chronic bronchitis; 57.5% of the patients were nonsmokers. In terms of alcohol consumption, the majority of patients were abstinent (81.0%). The current infection was rated by the attending physicians to be “mild” in 205 patients (10.5%), “moderate” in 1217 patients (62.3%), and “severe’” in 518 (26.5%) of the patients. A total of 721 (36.9%) patients (38.2% of AECB patients and 30.7% of the CAP group) had already received at least one antibiotic treatment for the current bacterial infection before the start of moxifloxacin therapy, whereas the majority of 1186 patients (60.7%) had not yet received any antibiotic pretreatment ().
Table 1 Baseline demographics and diagnosis
Treatments
The route of moxifloxacin application was exclusively IV in 78.3% of patients, whereas moxifloxacin sequential therapy was administered in 21.6% of the patients. In the case of sequential therapy, 415 out of 421 patients (98.6%) started with moxifloxacin IV therapy and then switched to oral treatment. A minority of six patients (1.4%) changed from oral to IV treatment. A total of 99.6% of all patients treated with IV (patients treated exclusively with IV and treated by sequential therapy) received the recommended daily dose of 400 mg, six patients (0.3%) were administered 800 mg moxifloxacin treatment, and in one case (0.1%) information on moxifloxacin IV dose was missing. Oral therapy was administered as a 400 mg daily dose regimen in 99.8% of the patients, and in only one case (0.1%) was information missing.
The mean duration of moxifloxacin treatment was 9.1 days (SD 3.6 days) with a median of 8.0 days. Sequential therapy lasted, on average, 12.5 days (SD 3.8 days). Exclusive IV therapy was applied for, on average, 8.1 days (SD 2.9 days). The total treatment duration varied with the patients’ diagnoses: AECB therapy was slightly shorter (8.9 days, SD 3.7 days) compared with CAP therapy (9.2 days, SD 3.4 days) and the therapy for other diagnoses (9.0 days, SD 3.7 days), respectively. Combined infections were treated for an average of 8.4 days (SD 2.8 days). The majority of all documented patients treated by sequential therapy (53.2%, 224/421) received moxifloxacin treatment for a time period of 10–14 days, whereas duration for most patients treated exclusively with IV therapy was 7–9 days (41.2%, 631/1530). A small proportion of patients was treated for less than 5 days (sequential therapy: 0.5%; IV therapy: 2.8%). A total of 26.8% treated by sequential therapy and 3.3% treated by exclusive IV therapy, respectively, were treated for more than 14 days.
Effectiveness results
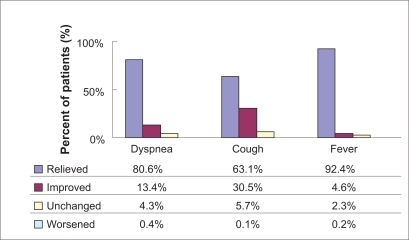
At each visit, the patients’ condition and severity of infection were assessed and recorded by the attending physician. Additionally, fever and the three most prevalent symptoms (dyspnea, cough, and sputum) were reported (). The patients’ condition and severity of infection at the start and end of therapy are displayed in and .
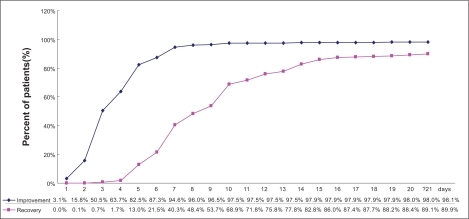
In total, there were 1949 patients recorded with data on improvement and 1951 patients recorded with data on recovery. A total of 82.5% (n = 1607/1949) of the patients had improved after 5 days of treatment; 68.9% (n = 1345/1951) patients recovered after 10 days. At the end of the observational period, an improvement was observed in 98.1% (n = 1911/1949) of the respiratory tract infection patients treated with moxifloxacin, and recovery was documented for 89.9% (n = 1754/1951) of the participating patients ().
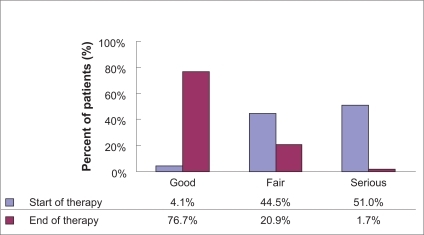
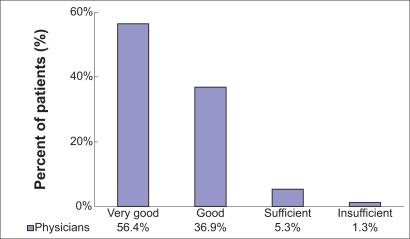
Moxifloxacin IV treatment, as well as sequential therapy (moxifloxacin IV/oral), was very effective in the treatment of respiratory tract infections. For 93.3% (n = 1822/1953) of the documented patients, effectiveness was rated as “very good” (56.4%, n = 1102/1953) and “good” (36.9%, n = 720/1953) by the attending physician. For “sufficient” and “insufficient”, 5.3% (n = 103/1953) and 1.3% (n = 25/1953) were rated individually. The overall effectiveness assessment made by the physicians is shown in . Stratification by diagnosis revealed best ratings (“very good” and “good”) in the patient group suffering from CAP (94.5%). Of the AECB patients, 92.6% had “very good” and “good” effectiveness ratings, compared with 88.1% of patients with other infections. The physicians’ final assessment of effectiveness was also stratified by severity of the infection at the initial visit: Effectiveness was assessed as “very good” and “good” in 95.1% of patients with a “mild” infection, in 94.6% of patients with a “moderate” infection, and in 89.8% of patients with a “severe” infection. In multimorbid patients with at least two concomitant diseases, effectiveness was assessed as “very good” and “good” in 91.3% of patients compared with 95.0% of patients with fewer than two concomitant diseases.
Physicians recorded that 96.8% (n = 1890/1953) of patients were “very satisfied” (58.2%, n = 1137/1953) or “satisfied” (38.6%, n = 753/1953) with the therapeutic effect of moxifloxacin. “Not satisfied” was rated in only 2.9% (n = 56/1953) of patients.
Safety and tolerability
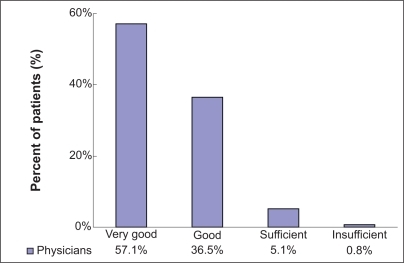
During this open-label moxifloxacin postmarketing surveillance study of 1953 patients in China, moxifloxacin was principally well tolerated. The physicians’ overall tolerability rating was “very good” (57.1%, n = 1115/1953) or “good” (36.5%, n = 712/1953) in 93.5% (n = 1827/1953) of all patients. The physicians rated tolerability as “sufficient” in 5.1% and as “insufficient” in 0.8% of the patients individually ().
Pretreatment (95.6% “very good” or “good”) as well as lower morbidity (fewer than two concomitant diseases, 94.4% “very good” or “good”) revealed better results regarding tolerability compared with patients without pretreatment (90.6% “very good” or “good”) and patients with higher morbidity (two or more concomitant diseases, 92.5%).
The incidence rates of AEs and ADRs were 0.72% (n = 14/1953) and 0.67% (n = 13/1953), respectively. Symptoms such as nausea and vomiting, which are well known in antibiotic treatment and also with quinolones, were predominant (each with 0.15%). One serious AE occurred (0.05%), which was also defined as a serious ADR. In this case, an 82-year-old male patient encountered “falling white blood cell count”, which led to “hospitalization necessary or prolonged”, but the event finally resolved.
In total, there were 33 AEs. Out of those, 29 were considered ADRs. Twenty-eight of the AEs (24 of 29 ADRs) were resolved or improved by the end of the observational period. In other cases, information on event outcome was missing. summarizes AEs and ADRs.
Table 2 Incidence of adverse events (AEs) and adverse drug reactions (ADRs) by MedDRA system organ classes and preferred terms
Discussion
Infections occur in the respiratory tract more frequently than in any other site. Acute respiratory tract infections are the most common illnesses in humans and are associated with significant morbidity and mortality. Antibiotics are commonly prescribed empirically for respiratory tract infections in adults and children in primary care.
The inappropriate prescribing of antibiotics has the potential to cause drug-related AEs, escalate the prevalence of antibiotic-resistant organisms in the community, and increase primary care consultation rates for minor illness (Standing Medical Advisory Committee 1998). How to use antibiotics in the treatment of respiratory tract infection appropriately is a big challenge for prescribers. Fortunately, there are national and international guidelines for the treatment of respiratory tract infections, eg, the European Respiratory Society’s lower respiratory tract infections guidelines.Citation6 In China, guidelines were also established to guarantee the appropriate use of antibiotics in respiratory tract infection patients, especially CAP and AECB patients. Moxifloxacin is recommended in both Chinese guidelinesCitation7,Citation8 for treating CAP and AECB. For CAP, moxifloxacin is recommended for preliminary empirical use in the following patients: young to middle-aged adults without underlying disease, elderly people or those with underlying disease, patients who have been hospitalized but not admitted to the intensive care unit (ICU), those using a combination with other antibacterials in ICU for Pseudomonas aeruginosa infection risk, and those with aspiration factor. For AECB, moxifloxacin is recommended for empirical use in Grade III and IV patients without P. aeruginosa infection risk. More and more physicians have experience in using moxifloxacin in China; nevertheless, there is still the need to collect and analyze local data, especially with a large sample size in clinical practice, in order to gather the newest efficacy data in treatment with moxifloxacin.
In our study, 2027 subjects were enrolled and 1953 were included in the statistical analysis. The mean age of all patients was 59.6 years (SD 17.5 years). A patient age over 45 years should be considered a major factor for defining the presence of chronic bronchitis. Indeed, AECB patients were more frequently older patients (80.8% of them were aged 50 years and older), whereas CAP patients were nearly evenly distributed in the age range of 30–79 years.
The overall effectiveness assessment showed that for 1822 (93.3%) of 1953 documented patients, effectiveness was rated as “very good” and “good” by the attending physician. Liu and Landen reported in 2007 that in their postmarketing surveillance of moxifloxacin in treating respiratory tract infection, improvement was observed in 69.1% of the patients after 3 days of moxifloxacin treatment and in 90.4% after 5 days.Citation9 Full recovery had occurred in 71.3% by 7 days and in 86.8% by 10 days. Physicians rated moxifloxacin as “very good” or “good” in 92% of patients for effectiveness. The results are similar in both studies, which demonstrated moxifloxacin to be of good effectiveness in treating respiratory tract infection.Citation9
For safety consideration, moxifloxacin treatment was well tolerated, with physicians rating overall tolerability as “very good” and “good” in 93.5% of patients. The physicians rated tolerability to be “sufficient” in 5.1% and to be “insufficient” in 0.8% of the patients. Pretreatment (95.6%) as well as lower morbidity (fewer than two concomitant diseases, 94.4%) corresponded to better results regarding tolerability compared with “no” pretreatment (90.6%) and higher morbidity (two or more concomitant diseases, 92.5%). The incidence rates of AEs and ADRs were 0.72% and 0.67%, respectively. Symptoms such as nausea and vomiting, which are well known in antibiotic treatment, were predominant (0.15%, respectively). These low numbers underline the very good tolerability and safety of moxifloxacin. Similar results were acquired from Liu and Landen’s study.Citation9 Physicians rated moxifloxacin as “very good” or “good” in 90.8% of the patients for tolerability. A total of 129 AEs occurred in 74 (1.9%) patients and mainly involved either the gastrointestinal or nervous system. All events were mild or moderate, and most resolved or improved after stopping treatment.Citation9
This study involved 150 investigators and enrolled a large number of patients in China. The results are helpful for physicians in clinical practice for treating AECB. Although this study lacked bacteriological testing and did not evaluate bacterial outcome, in medical practice, antibiotics are evaluated on the basis of clinical signs and symptoms. Nevertheless, this study is a single-arm, open-label study, and bias is inevitable when evaluating final effectiveness. Stricter studies in clinical practice are needed to further investigate this area. In general, the data of this study provide a large sample of evidence from China for rational antibiotic usage in treating respiratory tract infections in the future.
Conclusion
In this observational study, moxifloxacin was generally well tolerated and highly effective in the treatment of different respiratory tract infections requiring initial IV therapy. The incidence of AEs and ADRs was low. The safety and tolerability information collected in this study confirms the clinical safety profile of moxifloxacin and its benefit as a valuable choice of antibiotic treatment for respiratory tract infections.
Acknowledgment/Disclosure
Bayer Healthcare Company Ltd provided funding for this study and for preparation of the manuscript.
References
- WoodheadMCommunity-acquired pneumonia in Europe: causative pathogens and resistance patternsEur Respir J Suppl20023620s27s12168744
- American Thoracic Society, European Respiratory SocietyStandards for the Diagnosis and Management of Patients with COPD http://www.thoracic.org/clinical/copd-guidelines/resources/copddoc.pdf. Accessed January 13, 2011.
- ScheldWMMaintaining fluoroquinolones class effectiveness: review of influencing factorsEmerg Infect Dis200391912533274
- LonksJRGarauJMedeirosAAImplications of antimicrobial resistance in the empirical treatment of community-acquired respiratory tract infections: the case of macrolidesJ Antimicrob Chemother200250Suppl S2879212556438
- BlondeauJMA review of the comparative in vitro activities of 12 antimicrobial agents, with a focus on five new respiratory quinolonesJ Antimicrob Chemother199943Suppl B11110382869
- WoodheadMBlasiFEwigSGuidelines for the management of adult lower respiratory tract infectionsEur Respir J2005261138118016319346
- LiuYDiagnosis and treatment guideline for community-acquired pneumoniaChin J Tuberc Respir Dis200629654
- YaoWDiagnosis and treatment guideline for chronic obstructive pulmonary diseaseChin J Tuberc Respir Dis20073015
- LiuLYLandenHTreatment of respiratory tract infections with moxifloxacin: results of postmarketing surveillance in ChinaInt J Clin Pract2007611509151517635617