Abstract
Background
Recent research on vitamin D indicates that our current understanding of the factors leading to chronic inflammation should be revised. One of the key mechanisms by which microbial immunosuppression occurs is the suppression of one of the most common endogenous cell nucleus receptors: the vitamin D receptor (VDR). Autoimmune diseases may be correlated with VDR deactivation (VDR-deac) which occurs when the receptor is no longer able to transcribe antimicrobial agents. Excess 1,25-dihydroxyvitamin D (1,25D) is not converted to 25-hydroxyvitamin D (25D); thus, high 1,25D levels may be accompanied by low 25D values.
Patients and methods
Since 1,25D promotes osteoclast activity and may thereby cause osteoporosis, fatty-degenerative osteolysis of the jaw (FDOJ), as described by our team, may also be associated with VDR-deac. In 43 patients, vitamin D conversion, immune system function and the quality of bone resorption and formation in the jawbone were related factors that may enhance chronic inflammatory processes. Here, we examine the relationship between immunology and bone metabolism among 43 FDOJ patients and those with immune system diseases (ISDs).
Results
We provide a link between FDOJ, RANTES/CCL5 overexpression and VDR-deac.
Conclusion
The clinical data demonstrate the interaction between VDR-deac and proinflammatory RANTES/CCL5 overexpression in FDOJ patients.
Introduction
The etiology of chronic inflammatory diseases, such as rheumatoid arthritis, multiple sclerosis, systemic lupus erythematosus and arteriosclerosis, has not been elucidated. It is widely acknowledged that several factors are linked to the development of such diseases, including genetic predisposition and environmental and dietary factors. Recently, modern metagenomic research on intra- and extracellular genes has shown that over the course of centuries, thousands of microbes have succeeded in establishing themselves permanently in our bodies. A clever mechanism directed to this end is the deactivation of the vitamin D receptor (VDR); intraphagocytic bacteria produce ligands that deactivate the VDR. In turn, pathogenic bacteria have developed mechanisms to alter and evade the host immune response.Citation1,Citation2 In this way, interleukin (IL)-2 and interferon gamma (IFN-γ) switch off the body’s own innate immunity which makes possible an immunogenic reaction, particularly in the case of intracellular microorganisms.Citation3 Researchers have found that autoimmune disease markers can be greatly increased in the presence of chronic mycobacterial infections.Citation4 Advances in detection techniques using improved genome-based cultivation methods are highly likely to significantly expand the number of known pathogens involved in chronic diseases.Citation5–Citation7 It is increasingly recognized that bacteria can persist as cell wall-deficient variants (otherwise known as L-forms)Citation5–Citation7 and as “persistent” forms within metagenomic bacterial communities.Citation8,Citation9 By means of the VDR, 1,25-dihydroxyvitamin D3 (1,25[OH]2D3/1,25D) regulates the immune system which is present in most immune cell types, particularly monocytes, macrophages and dendritic cells.Citation10 In general, the innate immune system is enhanced and the adaptive immune system is inhibited by 1,25D.Citation11,Citation12 Thus, an effective immune response is heavily dependent on the vitamin D endocrine system which is responsible for balancing inflammatory and anti-inflammatory processes.Citation13 The VDR transcribes over 1,400 genes which accounts for 4% of all human genes and which are no longer able to be transcribed when the VDR is deactivated. The VDR also affects parathormone and calcium-sensitive receptors and is thus essential for maintaining well-regulated calcium homeostasis in the bone. Hence, the VDR is further implicated in systemic disorders of calcium metabolism, as well as vitamin D utilization in fatty-degenerative osteolysis of the jawbone (FDOJ).Citation14
Research aims
The purpose of this article was to investigate the extent to which deactivated VDR (VDR-deac) plays an etiological role in the development of chronically altered metabolism in the jawbone in a cohort of patients with immune system diseases (ISDs). This article also attempts to provide further insight with respect to the question of whether VDR-deac and inflammatory cytokine overexpression in FDOJ areas may function as a reinforcement loop in ISDs.
Materials and methods
The study presented herein was performed as a case-control study and was deemed to be retrospective in nature. Approval was granted by Institute for Medical Diagnostics (IMD)-Berlin forensic accredited Institute DIN EN 15189/DIN EN 17025. All patients provided their written consent to participate in this study, and the samples and data were collected in the course of routine practice, i.e., regular oral surgery procedures. The study cohort of 43 patients with FDOJ comprised of five groups consisting of patients with various specialist-diagnosed ISDs: atypical facial and trigeminal pain (n=9); neurodegenerative disorders (multiple sclerosis and amyotrophic lateral sclerosis) (n=5); tumors (breast cancer and prostate cancer) (n=5); rheumatism (fibromyalgia and Lyme disease) (n=16); and chronic fatigue syndrome (n=8). The patients’ average age was 54.05 years (age range: 23–75 years). There was a gender ratio (females to males) of 25:18. A control group comprised of 19 patients without FDOJ, had an average age of 54 years (age range: 38–71 years) and a gender ratio (female to male) of 8:11. All patients were seeking to uncover the etiology of their respective ISDs, including possibly FDOJ-induced “silent inflammation” of the jawbone. The serum values of 25-hydroxyvitamin D3 (25[OH]D3/25D) and 1,25D were determined for the cohort. Following the clinically necessary excision of FDOJ, conspicuous FDOJ samples were analyzed for their cytokine content. Serum vitamin D levels were analyzed in FDOJ group (n=43). Cytokine levels in the jawbone were analyzed in FDOJ group (n=43) and the control group (n=19).
The use of medications for the treatment of systemic diseases was not generally regarded as an exclusion criterion. Alcohol addiction, fetal alcohol syndrome or abuse of any other drugs were additional exclusion criteria.Citation15,Citation16 Defined exclusion criteria consisted of cortisone and bisphosphonate use due to their effects on bone metabolism as well as current or recent use of vitamin D preparations, i.e., within the previous 14 days. The patients’ serum vitamin D levels were correlated with the cytokine profiles of FDOJ areas of the five patient groups.
Serum measurement of 25D and 1,25D
The analysis of 1,25D was carried out by means of the chemiluminescence immunoassay IDS-iSYS 1,25 VitDXp with the analytical device iSYS (International Decision Systems, Minneapolis, MN, USA). The analysis of 25D levels was carried out using the chemiluminescence immunoassay LIAISON® 25 OH Vitamin D TOTAL with the LIAISON® analyzer (DiaSorin, Saluggia, Italy).
Cytokine profile measurement in jawbone osteolysis
The current treatment for FDOJ lesions consists of bony cavity curettage.Citation17,Citation18 To elucidate a possible causative link between FDOJ and VDR-deac at the Munich Clinic for Integrative Dentistry (Munich, Germany), 43 patients with ISDs who were also diagnosed with FDOJ underwent surgery on the affected jaw area. Following the administration of local anesthesia, the mucoperiosteal flap was folded over and the cortical layer was removed. All patients exhibited FDOJ within the bone marrow that was similar to FDOJ samples described in the literature.Citation19,Citation20 In all 43 cases, surgery was performed on edentulous jaw areas at the sites of former wisdom teeth and the adjacent retromolar areas.
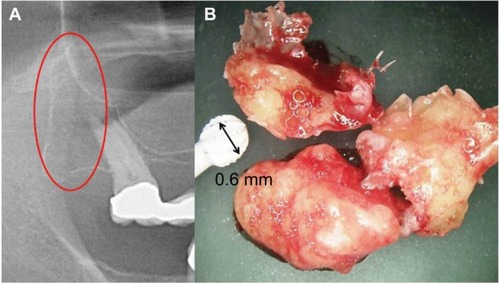
In 62 jawbone samples (43 FDOJ samples from the ISD group and 19 healthy jawbone samples), seven mediating factors were measured: fibroblast growth factor (FGF)-2, IL-1 receptor antagonist (IL-1ra), IL-6, IL-8, monocyte chemotactic protein (MCP)-1, tumor necrosis factor alpha (TNF-α) and RANTES/CCL5 (R/C). The FDOJ clumps with a volume of up to ½ cm3 were scooped out ( shows a clinical example of their morphology), and these pea-sized clumps of tissue were immediately placed in a sterile container (Sarstedt micro-tube; ref. 72.692.005) which was sealed and stored at −20°C until transportation to the laboratory (IMD-Berlin, Berlin, Germany). There, the tissue samples were mechanically comminuted, incorporated with 200 μL of protease buffer (Complete Mini Protease Inhibitor Cocktail; Hoffmann-La Roche, Basel, Switzerland) and homogenized. The homogenate was centrifuged for 15 minutes at 13,400 rpm, after which the supernatant was removed and centrifuged for another 25 minutes at 13,400 rpm. The determination of R/C was carried out in the supernatant of the tissue homogenate using the Human Cytokine/Chemokine Panel I (MPXHCYTO-60K; Millipore GmbH, Schwalbach, Germany), according to the manufacturer’s protocol and using the Luminex 200™ with xPonent® software (Luminex, Austin, TX, USA).
Statistical analysis
The measured values obtained from the FDOJ and control cohorts were subjected to descriptive statistical analyses using SAS 9.4. The median, mean and distribution of the data were determined to assess whether nonparametric or parametric tests were most appropriate. The difference between the values of FDOJ patients and the general population was assessed using Student’s t-test. Differences between the two cohorts were determined using the Wilcoxon–Mann–Whitney test. The differences between disease groups were tested with the Kruskal–Wallis test. Statistical significance was set at a P<0.05.
Results
Distribution of 1,25D and 25D in the total cohort
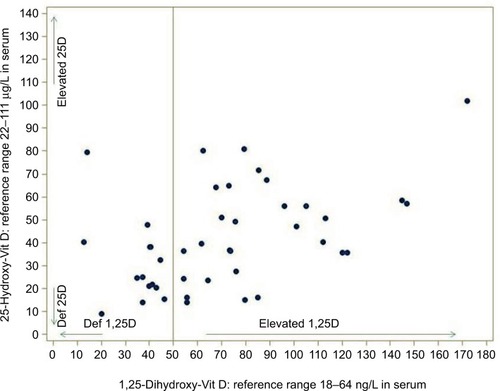
For male and female adults, the elderly, and both summer and winter values, a mean level of 22–111 μg/L was assumed for cholecalciferol 25D. The mean level of calcitriol 1,25D was assumed to be 18–64 ng/L for the same distribution (standard values were obtained from German Laborwerte Verzeichnis). In the recent literature,Citation3,Citation21 values >45 are considered as suspicious, and for this reason the upper reference value was set to 50 ng/L in our statistical analyses. As is well known, only one-thousandth of the amount of 1,25D is present in the blood when compared to 25D. The distribution of these values in the total study cohort (n=43) is shown in .
The ratio of 25-hydroxy-(cholecalciferol) and 1,25-dihydroxy-(calcitriol) vitamin D3 in determining the deactivation of the vitamin D receptor
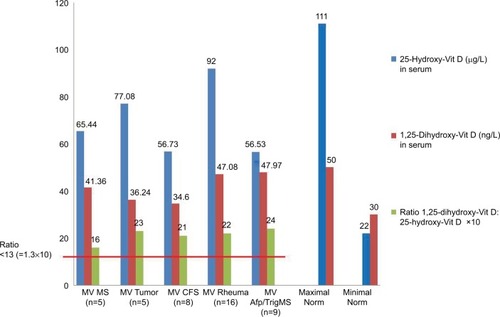
We calculated the ratio of 1,25D (measured in ng/L) and 25D (measured in μg/L) in 43 patients with systemic immunological disorders (), and clinically verified the presence of FDOJ according to recommendations presented in the literature.Citation21 This may pose a problem, as there is a thousandfold difference in the concentrations of the two vitamins in question. The direct detection of VDR-deac is not yet possible in standard laboratories, however, since it is a cell nucleus receptor that is found in the mitochondria. In practice, a simple reliable measurement is necessary to determine the status of the complex system of vitamin D regulation. It is possible to infer from the discussion in the relevant literature that a ratio >1.3 may be considered as a reference value for VDR-deac.Citation22–Citation24 The hypothesis that the mean value of the ratios in the FDOJ group is ≤1.3 may be rejected (P<0.001). Furthermore, the descriptive analysis indicates that 34 patients with ISDs from the FDOJ study cohort (n=43) showed VDR-deac. As illustrates, in all five disease groups the mean ratio was >1.3. Based on the Kruskal–Wallis test (P=0.6805), it may be assumed that the main tendencies of the disease groups do not differ. All the individual groups as well as the cohort as a whole had values >1.3. This shows that, from the cohort, VDR-deac was present in 34 patients with ISDs and clinically verified FDOJ.
Figure 3 In all five disease groups, the 25D value is above the minimum threshold while the 1,25D value falls within the standard range. (Vitamin D values are shown as mean values.) The distribution of the VDR ratio is greater than the assumed maximum of 1.3 (×10 in the graph) in all five disease groups.
Multiplex assay analysis of FDOJ
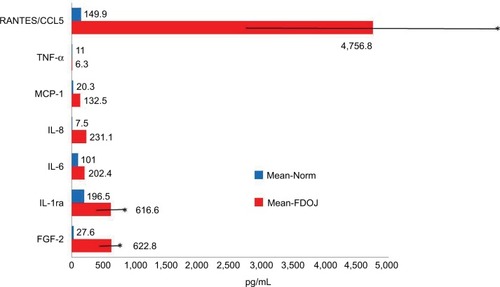
The multiplex assay analysis of the control group of 19 healthy jawbone samples indicated the following three cytokines (in pg/mL): IL-1ra, 195.5 (SD ±569); FGF-2, 27.6 (SD ±59); and R/C, 149.9 (SD ±127). There were no corresponding values found in the literature for these mediators in healthy jawbone. The mean values obtained for the FDOJ cohort were noticeably higher than for the group of healthy jawbone samples (in pg/mL): FGF-2, 622.8; IL-1ra, 616.6; and R/C, 4,590.8 (SD ±2,536.35). In , the hyperactivated signal transduction of R/C in the 43 FDOJ samples of the cohort is shown (red bars) in comparison to the 19 healthy jawbone samples (blue bars). Of particular note, IL-6, IL-8 and TNF-α have extremely low values. These cytokines are regarded as the “ignitors of humoral defense”, and their absence may explain the cryptic and asymptomatic character of FDOJ.
Figure 4 Comparison of the levels of seven cytokines obtained from 19 healthy jawbone samples with those from FDOJ samples from 43 patients with ISDs.
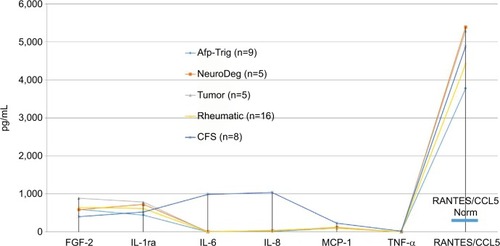
The cytokine profiles obtained from the FDOJ areas of all five groups demonstrate a common pattern (): on one hand, there is a total paralysis of the immune system; on the other, there is a one-sided derailment of FGF-2, IL-1ra and MCP-1, and, in particular, the extreme overexpression of R/C in all five ISD groups.
Figure 5 Distribution of seven cytokines in the FDOJ samples obtained from five disease groups: atypical facial and trigeminal pain (n=9); neurodegenerative disorders (multiple sclerosis and amyotrophic lateral sclerosis) (n=5); tumors (breast cancer and prostate cancer) (n=5); rheumatism (fibromyalgia and Lyme disease) (n=8); and chronic fatigue syndrome (n=8). There was significant overexpression of R/C in all groups; there were no statistically significant differences between the individual groups (Kruskal–Wallis).
RANTES/CCL5 expression and the vitamin D ratio
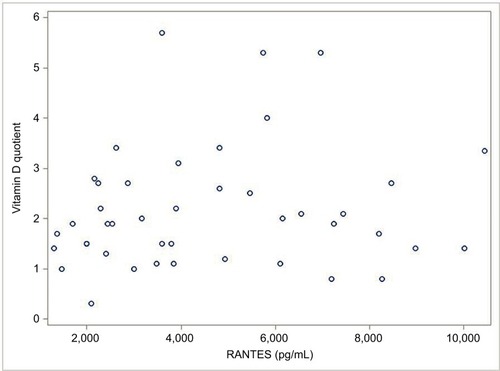
Based on the aims of this study, the correlation (value =0.13) was calculated between R/C expression in the FDOJ samples and VDR-deac. Using Spearman’s correlation, it was found that this relationship was not significant (P=0.39). The scatter plot diagram between R/C and VDR-deac is shown in . (See the “Limitations” section for various possible causes.)
Discussion
The discussion of the study findings presented addresses four factors concerning the possible etiological contribution of ISDs: the serum content of 1,25D (ng/L); the serum content of 25D (μg/L); the resulting calculated ratio; and the over-expression of R/C (in pg/mL) in FDOJ. As noted previously, microbes – including Mycobacterium tuberculosis, Borrelia and Epstein-Barr virus (EBV) – downregulate VDR activity. In the event, thereby, that the physiologically active 1,25D form exceeds normal values, this may result in a corresponding reduction of 25D. Consequently, more vitamin D experts are beginning to reconsider vitamin D supplementation among the general population.Citation25
Serum levels of 1,25D and 25D in the FDOJ cohort
Despite the recent surge in vitamin D supplementation, the number of cases of chronic diseases has increased and is expected to continue to rise.Citation26 Interpretation of the results presented in provides further supporting evidence with respect to this phenomenon. The FDOJ–ISD cohort exhibit the following relationships: the mean value of 1,25D of 71.11 ng/L (SD ±36.0) exceeds the maximum normal reference value of 50 ng/L. At the same time, the mean value of 25D of 41.4 μg/L (SD ±36.0) falls in the lower part of the reference range of 111–22 μg/L (). Accordingly, an excess of the active hormone 1,25D, in contrast to 25D, is present. Autoimmune diseases often correlate with high 1,25D values and low 25D values (which is associated with a presumed “vitamin D deficiency”), and this is also the case in the FDOJ study cohort exclusively affected by ISDs: low 25D levels are inversely correlated with high 1,25D levels. Consistent with the disease patterns exhibited by the FDOJ cohort, abnormally low levels of the metabolite 25D are associated with general mortality and an increased incidence of at least 40 different chronic infections.Citation27 Certainly, vitamin D intake is often purported to confer immunosuppressive effects. For instance, according to Arnson et al, “Vitamin D affects the immune system on many levels and via a number of mechanisms. Vitamin D has several immunosuppressive properties and, on the whole, has such an effect”.Citation28
The phenomenon of deactivation of vitamin D receptor
The clinical evidence available for a wide range of autoimmune diseases shows that the innate immune function can be induced by restoring VDR function.Citation29 Bacteria-induced ligands of the VDR deactivate this function. The infiltration of microorganisms into the cell is known to occur in the case of viruses. It is less known, however, whether bacteria can migrate into the cell by shape changing. The conversion of bacteria into so-called “cell wall-deficient forms” is a condition for their uptake within the cells. The bacteria that most effectively infiltrate the cells at the VDR are well-known agents of chronic infections (e.g., tuberculosis; borelliosis; Chlamydia infections; infections caused by all forms of herpes virus, EBV, cytomegaly virus; and Aspergillus sp. infections). Intracellular bacteria can modulate cytokine productionCitation30 and in monocytes and macrophages cytokine activation markedly inhibits 1,25D/VDR gene transcription. Capnines are the active substances produced by these microbes and are capable of disabling the VDR. If VDR-deac is present, it is increasingly likely that the body will not attack its own tissues in autoimmune diseases; rather, antibodies are produced that are directed against certain parts of the metagenome communities of microbes.Citation31 Intracellular microbes living in nuclear cells can interfere with DNA transcription and repair mechanisms, allowing them to trigger many of the dysfunctions associated with autoimmune diseases. Microbe immunosuppression succeeds as a result of VDR suppression.Citation32 Defects in VDR signal transduction have previously been associated with bacterial infections and chronic inflammation.Citation33 As early as 2010, Proal et alCitation31 reported that VDR influences at least 1,400 genes, many of which are associated with autoimmune disorders and cancer.Citation34 In 2005, Wang et alCitation35 used in silico emulation to demonstrate that the sulfonolipid capnin, which is created by the biofilm bacterial species of the genera Cytophaga, Capnocytophaga, Sporocytophaga and Flexibacter, could bind to the VDR and thereby reduce its activity.Citation33 Published models predict that as the increased concentrations of 1,25D accumulate in the nucleated cells, they will increasingly occupy the ligand-binding pockets of these receptors, thus displacing their endogenous ligands.Citation36
The connection between deactivated VDR and FDOJ: disrupted vitamin D metabolism and pathological morphology in the jawbone
Mandibular bone remodeling is mediated by inflammatory factors such as cytokines and chemokines. Collectively, our data strongly point toward R/C being an important molecule for communication between osteoclasts and osteoblasts, and shed new light on the functions of these chemokines in osteoblast biology. Vitamin D and its receptor metabolism also play an important role in bone resorption in immune-mediated osteoporosis. In the case of FDOJ, previously documented by our team,Citation14 questions arise concerning not only the effects but also the systemic causes of local areas of FDOJ. Our data show that there is no statistically significant correlation between FDOJ severity, expressed by the overexpression of R/C, and VDR-deac. Nevertheless, the presence of VDR-deac in 34 FDOJ–ISD patients (79% of the study cohort), in addition to insufficient wound healing and chronic cytokine stimulation by root-filled teeth,Citation37,Citation38 may provide further answers with respect to the disorders of jawbone metabolism that arise in FDOJ. The aforementioned effects of VDR-deac may provide a further explanation for the chronic “silent inflammation” in the jaw, here referred to as FDOJ, that we have repeatedly reported.Citation14,Citation18,Citation37 At the same time, the chemokine R/C is partly responsible for many ISDs – rheumatism, breast cancer, Hashimoto’s thyroiditis, melanomas, multiple sclerosis, Amyotrophyic lateral sclerosis and so on; it serves as a key pathogenic element, being overexpressed by up to 35-fold in the affected jaw area.
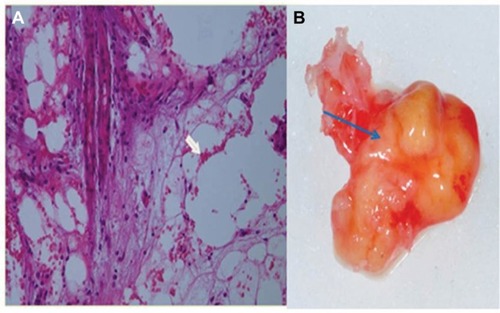
We consider, therefore, the research findings of VDR deactivation in the development of FDOJ to be illuminating. VDR-deac also alters the balance of vitamin D-controlled metabolism in the jawbone which can lead to osteolysis of the trabecular structures and to the fatty transformation of medullar spaces. Morphologically, FDOJ thus presents as fatty clumps ( and ). The medullary bone lesion evident in FDOJ is microscopically similar to aseptic, ischemic osteonecrosis.Citation17 FDOJ appears to represent the transition between the acute inflammation of a surgical dental wound and a chronically inflamed, disturbed area of the jaw.
Figure 7 (A) A cluster of dead fatty cells (white arrow) with small inflammatory cells observed in the medullary cavity of the jaw. (B) An FDOJ tissue sample with complete fatty transformation of the cancellous portion of the jawbone (blue arrow).
Bisphosphonate-related osteonecrosis of the jaw (BRONJ) has been increasingly suspected as a potential complication of bisphosphonate therapy, and the controversy with regard to the association between osteonecrosis of the jaw and bisphosphonates is a recent and growing problem. While FDOJ is normally painless and without visible bone loss, BRONJ is characterized by bone death wherein bone is destroyed in the course of turnover.Citation41 In contrast, the upregulation of R/C secretion from osteoblasts in response to the metabolism of FDOJ promotes osteoclast migration, but may also induce migration of additional preosteoblasts to the resorption site via a paracrine mechanism.Citation42 In this way, the process of bone modeling in the case of FDOJ is derailed but not connected to bone loss.
Systemic signal transduction by hyperactivated RANTES/CCL5 from FDOJ areas
At the cellular level, chronic inflammation is characterized by the infiltration of immunoinflammatory cells into the target tissue which usually precedes tissue damage. In areas of chronic inflammation, the production of cytokines by infiltrating and local tissue cells overwhelms and exceeds the regulatory mechanisms resulting in direct or indirect tissue destruction via the activation of immune and inflammatory cells. An imbalance between cytokines and their respective inhibitors is characteristic of chronic inflammatory states. Cytokines are involved in the induction of acute inflammatory events and later in the transition or persistence of chronic inflammation. This means that cytokine-producing mechanisms must be controlled in order to maintain healthy conditions.Citation39 FDOJ exhibits a metabolic derailment and an associated chronic inflammatory input in the affected jaw region, which overexpresses R/C by up to 35 times. This compromises the maintenance of regular cell signaling, thus causing persistent immune-mediated, metabolic and hemodynamic disturbances. These chronic stimuli can progress into diseases associated with local inflammation such as rheumatism, neurodegenerative diseases, tumors, chronic fatigue syndrome and facial pain ( and ). FDOJ is characterized by these metabolic and immunologic dysfunctions, with both adipocytes and macrophages being important cellular sensors and also effectors of metabolic derailments in bone metabolism. FDOJ constitutes its own unique inflammatory phenomenon, since the cell response is not bacterially or virally triggered but rather is initiated by persistent metabolic derailments. The entity responsible for these abacterial and aviral cell responses is primarily the proinflammatory chemokine (R/C). As a result, systemically relevant, dysregulated “signaling pathways” are activated as shown in the five groups of ISDs described in this study.
Systemic cross-linking of VDR deactivation and osteolysis in the jawbone
Mice lacking the cathelicidin gene, which is reliably transcribed by the VDR, exhibit longer wound-healing times than their wild types.Citation33 This effect is regulated by the VDR only in humans and non-human primates, as the mouse cathelicidin gene does not possess a VDR binding site at the promoter.Citation34 Thus, VDR-deac has a negative effect on wound healing. It may, therefore, be medically and systematically necessary to study the relationship between VDR-deac and bone metabolism in FDOJ. A previous study investigating the relationship between R/C and 1,25D levels established yet a further connection: R/C secretion from osteoblasts was inhibited by hormones such as 25D and dexamethasone.Citation42 This means that if the effect of 1,25D is switched off intra-cellularly (i.e., if VDR-deac is present), the R/C level would increase with a potentially negative immunomodulating effect on ISD. High 1,25D values promote osteoclast activity.Citation43 The fact that excess 1,25D contributes to the successive formation of FDOJ corresponds with the observation that FDOJ and “vitamin D deficiency” are related. The research has shown that in response to 1,25D, normal osteoclasts increase their production of acid hydrolases and subsequently increase their cell count. This means that osteoclast-mediated bone resorption is increased as a function of 1,25D. High 1,25D values, in turn, are the result of chronic inflammation in conjunction with VDR-deac. Therefore, it is the chronic inflammatory process itself that causes osteoporosis and not “vitamin D deficiency”. Low 25D values are only a consequence of the aforementioned contexts. Attempts to promote healing in FDOJ cases with excessive vitamin D supplementation are thus immunologically counterproductive. From the interrelations cited in the present study, it may be concluded that VDR-deac is a possible cause of the severe osteolysis observed in 34 of the 43 patients with FDOJ and of the extreme R/C overexpression. An almost complete absence of “ignitors of the immune system” (TNF-α and IL-6) in FDOJ areas () is another reason for the ailing defense present in those regions. Simultaneously, the VDR-deac prevents 1,25D from contributing to the expression of antimicrobial peptides (AMPs), such as cathelicidin and beta-defensin, which help to eliminate pathogens.Citation44,Citation45 In general, the innate immune system is stimulated by 1,25D, and the adaptive immune system is inhibited.Citation46,Citation47 Thus, an effective immune response is highly dependent on the vitamin D3 endocrine system responsible for the balance of proinflammatory and anti-inflammatory elements. This relationship is illustrated by Blaney et alCitation48 in a study of 100 patients with autoimmune and chronic diseases: 85% of patients showed 1,25D levels that were >46.2 ng/L without hypercalcemia. Furthermore, large and reliable studies conducted with Danish population data found that the mean 1,25D value in a normal population was 29 ng/L with a standard deviation of 9.5. In addition, a review of the literatureCitation49 confirmed the association of elevated 1,25D levels with bone metabolism. It has been found that elevated 1,25D regulates VDR activity in the small intestine. This, in turn, transcribes and multiplies the genes that transport calcium and phosphorous across the intestinal epithelium. The mucosal response, and calcium and phosphorus resorption, is thus dependent on a competently activated VDR, while increased 1,25D reduces VDR competence. The fact that calcium and phosphorous resorption may be inhibited when VDR activity is impaired by increased 1,25D is illustrated by a study of Crohn’s disease patients with elevated 1,25D levels and low bone mineral density.Citation50 It was concluded that treating the underlying chronic inflammation improved the metabolic bone disease. Brot et alCitation51 found that elevated 1,25D levels were associated with markedly reduced bone density and bone content as well as increased bone turnover. At levels >42 ng/L, 1,25D stimulates osteoclast activity in the bone. This leads to osteoporosis development, tooth fractures and soft tissue calcification.Citation52 Accordingly, it was found that a combination of high 1,25D and low 25D levels is associated with the lowest bone mineral levels and poorest bone health.Citation50 The schematic overview in illustrates the interconnection between VDR-deac, autoimmune and systemic diseases, and FDOJ.
Figure 8 Interconnection between deactivated VDR, autoimmune and systemic diseases, disturbed bone metabolism and fatty-degenerative osteolysis of the jawbone with an immunological amplification loop.
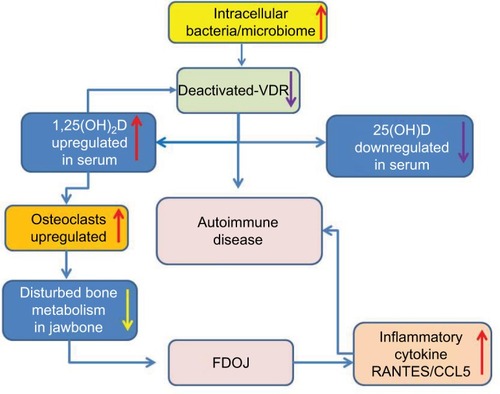
According to the existing theories on VDR-deac, our data indicate that both systemic problems and local FDOJ may be related to VDR-deac. Clinically, there may be an amplification loop for both factors: VDR-deac may trigger the development of FDOJ and ISDs; subsequently, FDOJ – which arises as a result of VDR-deac – also leads to ISD development via chronic R/C overexpression. The discussion concerning the close relationship between bone metabolism, bone cells and the immune system opens up a new interdisciplinary research field known as “osteoimmunology”.Citation53 The data presented here highlight the complexity of interwoven pathways and the shared mechanisms involved in the cross talk between the immune and bone systems. Emerging new homeostatic networks define an interdisciplinary field of osteoimmunology in which other organs and systems are functionally interconnected.Citation54 The conjunction of R/C overexpression in FDOJ and the role of microbiota contributes to a proinflammatory cytokine milieu which drives bone resorption, leading to changes in bone density and in bone phenotype.Citation55 Based on the overexpression of the chemokine R/C in the FDOJ cases presented here, further studies investigating maxilla–mandibular osteoimmunology are recommended.
Conclusion
During the course of evolution, reactions to microbes have resulted in finely tuned immune responses.Citation56 The present study brings the discussion on the subjects of the microbiome, as well as intracellular infections, and dysregulations of bone metabolism and signaling pathways into focus. We detail the interconnection between deactivated VDR, autoimmune and systemic diseases, disturbed bone metabolism and FDOJ osteolysis. In doing so, attention is drawn to the question of whether systemic cross-linking of VDR-deac and FDOJ-derived R/C overexpression may be responsible for the development of otherwise inexplicable systemic inflammatory reactions. We found that VDR-deac and metabolic regulation in the mineral matrix of the jawbone, combined with systemic dysregulated signal transduction, play a critical role in the development of the inflammatory condition FDOJ.
Limitations
The limitations of the cross-sectional study presented herein, in which vitamin D3 levels, cytokine levels and FDOJ samples were collected concurrently, lie in the small sample size employed. Futhermore, confounders were not controlled. These include the duration of any underlying disease, the duration of FDOJ, multimorbidity, medication use and the administration of other therapies. Bias may also be present in the determination of the VDR ratio. For instance, values were collected only once for each participant and at different seasonal times, which is limiting given that vitamin D levels are subject to natural fluctuations throughout the year.
Future directions
In a cohort of 43 patients with FDOJ, on the one hand, and ISDs, on the other, this study was able to demonstrate two phenomena that present similar effects to those associated with ISDs: aseptic osteolysis in the jawbone and the VDR-deac. Although the evidence for causality presented remains insufficient, our findings contribute to the expansion of the current understanding of chronic aseptic osteolysis of the jawbone, while also relating it to the immune system.
Acknowledgments
English-language translation and editing of this manuscript were done by Natasha Gabriel.
Disclosure
The authors report no conflicts of interest in this work.
References
- WoolardMDFrelingerJAOutsmarting the host: bacteria modulating the immune responseImmunol Res200841318820218592144
- DermineJFDesjardinsMSurvival of intracellular pathogens within macrophagesProtoplasma19992101–21124
- ProalADAlbertPJBlaneyGPLindsethIABenediktssonCMarshallTGImmunostimulation in the era of the metagenomeCell Mol Immunol2011821322521278764
- LiuPTStengerSLiHToll-like receptor triggering of a vitamin D-mediated human antimicrobial responseScience200631157681770177316497887
- WenLWongFSHow can the innate immune system influence autoimmunity in type 1 diabetes and other autoimmune disorders?Crit Rev Immunol200525322525016048437
- FredricksDNReimanDAInfectious agents and the etiology of chronic idiopathic diseasesCurr Clin Top Infect Dis1998181802009779355
- PordeusVSzyper-KravitzMLevyRAVazNMShoenfeldYInfections and autoimmunity: a panoramaClin Rev Allergy Immunol200834328329918231878
- ShoenfeldYIsenbergDAThe mosaic of autoimmunityImmunol Today104123126
- WaterhouseJCPerezTHAlbertPJReversing bacteria-induced vitamin D receptor dysfunction is key to autoimmune diseaseAnn N Y Acad Sci2009117375776519758226
- WhiteJHVitamin D signaling, infectious diseases, and regulation of innate immunityInfect Immun20087693837384318505808
- HayesCNasholdFSpachKPedersenLThe immunological functions of the vitamin D endocrine systemCell Mol Biol (Noisy-le-grand)200349227730012887108
- TopilskiIFlaishonLNavehYHarmelinALevoYShacharIThe anti-inflammatory effects of 1,25-dihydroxyvitamin D3 on Th2 cells in vivo are due in part to the control of integrin-mediated T lymphocyte homingEur J Immunol20043441068107615048717
- ManginMSinhaRFincherKInflammation and vitamin D: the infection connectionInflammRes20146310803819
- LechnerJvon BaehrVRANTES and fibroblast growth factor 2 in jawbone cavitations: triggers for systemic disease?Int J Gen Med2013627729023637551
- LauAvon DossowVSanderMMacGuillMLanzkeNSpiesCAlcohol use disorder and perioperative immune dysfunctionAnesth Analg2009108391692019224804
- FeltesBCde Faria PoloniJNunesIJBonattoDFetal alcohol syndrome, chemo-biology and OMICS: ethanol effects on vitamin metabolism during neurodevelopment as measured by systems biology analysisOMICS201418634436324816220
- OnoKSymposium: recent advances in avascular necrosisClin Orthop Rel Res19922772138
- LechnerJMayerWImmune messengers in neuralgia inducing cavitational osteonecrosis (NICO) in jaw bone and systemic interferenceEur J Integr Med201027177
- RatnerEJLangerBEvinsMLAlveolar CAVITATional osteopathosis – manifestations of an infectious process and its implication in the causation of chronic painJ Periodontol198657105936032945921
- WangMJiaoXMengQLocalization method in the diagnosis of the pathological jaw bone cavityI Acta Acad Med Sichuan198213341344
- MarshallTGVDR nuclear receptor is key to recovery from cognitive dysfunctionDays of molecular medicine, Abstract presentation417–192008Karolinska InstituteSweden
- ReinhardtTAHorstRLSelf-induction of 1,25-dihydroxyvitamin D3 metabolism limits receptor occupancy and target tissue responsivenessJ Biol Chem19892642715917159212550427
- ChristakosSDhawanPVerstuyfAVerlindenLCarmelietGVitaminDmolecular mechanism of action, and pleiotrophic effectsPhysiol Rev201696136540826681795
- CaltonEKKeaneKNNewsholmePSoaresMJThe impact of vitamin D levels on inflammatory status: a systematic review of immune cell studiesPLoS One20151011e014177026528817
- TsengLControversies in vitamin D supplementationeScholar- ship2003 Available from: http://www.escholarship.org/uc/item/4m84d4fn#page-1Accessed May 7, 2013
- ShelbyJNeeds great, evidence lacking for people with multiple chronic conditionsScribd42013 Available from: https://chro-nicillnessrecovery.org/component/search/?searchword=Shelby%20J.&searchphrase=all&Itemid=48Accessed May 7, 2013
- MelamedMLMichosEDPostWAstorB25-hydroxyvitamin D levels and the risk of mortality in the general populationArch Intern Med20081681629163718695076
- ArnsonYAmitalHShoenfeldYVitamin D and autoimmunity: new aetiological and therapeutic considerationsAnn Rheum Dis20076691137114217557889
- ArasakiKReport on a case of systemic sarcoidosis treated according to the Marshall ProtocolThe 26th Conference of the Japan Society of Sarcoidosis and Other Granulomatous DiseasesOctober 6 2006Tokyo, Japan
- WangKXChenLHelicobacter pylori L-form and patients with chronic gastritisWorld J Gastroenterol20041091306130915112347
- ProalADAlbertPJMarshallTGAutoimmune disease and the human metagenomeNelsonKEMetagenomics of the Human BodyNew YorkSpringer2010231275
- SunJVitamin D and mucosal immune functionCurr Opin Gastroenterol201026659159520639756
- AuvynetCRosensteinYMultifunctional host defense peptides: antimicrobial peptides, the small yet big players in innate and adaptive immunityFEBS J2009276226497650819817855
- WeiRChristakosSMechanisms underlying the regulation of innate and adaptive immunity by vitamin DNutrients201578251826026404359
- WangTTTavera-MendozaLELaperriereDLarge-scale in silico and microarray-based identification of direct 1,25-dihydroxyvitamin D3 target genesMol Endocrinol200519112685269516002434
- MarshallTGVitamin D discovery outpaces FDA decision makingBioessays200830217318218200565
- ProalADAlbertPJMarshallTGDysregulation of the vitamin D nuclear receptor may contribute to the higher prevalence of some autoimmune diseases in womenAnn NY Acad Sci2009117325225919758159
- LechnerJvon BaehrVChemokine R/C as an unknown link between wound healing in the jawbone and systemic disease: is prediction and tailored treatments in the horizon?EPMA J201561025987906
- LechnerJvon BaehrVStimulation of proinflammatory cytokines by volatile sulfur compounds in endodontically treated teethInt J Gen Med2015109109118
- KirkpatrickCJFuchsSPetersKBrochhausenCHermannsMIUngerREVisions for regenerative medicine: interface between scientific fact and science fictionArtif Organs20063082282717026583
- GuttaRLouisPJBisphosphonates and osteonecrosis of the jaws: science and rationaleOral Surg Oral Med Oral Pathol Oral Radiol Endod2007104218619317448709
- YanoSFunctional Expression of β-Chemokine receptors in osteoblasts: role of regulated upon activation, normal T cell expressed and secreted (RANTES) in osteoblasts and regulation of its secretion by osteoblasts and osteoclastsEndocrinology2013146523242335
- GriseMAMarksSCJrMacKayCAPopoffSNEffects of 1,25 dihydroxyvitamin D on osteoclast number and cytochemistry in normal and osteopetrotic (os) rabbitsAm J Anat199018932612662148052
- LaiYGalloRLAMPed up immunity: how antimicrobial peptides have multiple roles in immune defenseTrends Immunol200930313114119217824
- WangTNestelFBourdeauVCutting edge: 1,25-di- hydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expressionJ Immunol200417352909291215322146
- HayesCNasholdFSpachKPedersenLThe immunological functions of the vitamin D endocrine systemCell Mol Biol (Noisy-le-grand)200349227730012887108
- TopilskiIFlaishonLNavehYHarmelinALevoYShacharIThe anti-inflammatory effects of 1,25-dihydroxyvitamin D3 on Th2 cells in vivo are due in part to the control of integrin-mediated T lymphocyte homingEur J Immunol20043441068107615048717
- BlaneyGPAlbertPJProalADVitamin D metabolites as clinical markers in autoimmune and chronic diseaseAnn NY Acad Sci2009117338439019758177
- AbreuMTKantorovichVVasiliauskasEAMeasurement of vitamin D levels in inflammatory bowel disease patients reveals a subset of Crohn’s disease patients with elevated 1,25- dihydroxyvitamin D and low bone mineral densityGut20045381129113615247180
- IshizukaSKuriharaNMiuraDVitamin D antagonist, TEI-9647, inhibits osteoclast formation induced by 1alpha,25- dihydroxyvitamin D3 from pagetic bone marrow cellsJ Steroid Biochem Mol Biol200489–901–5331334
- BrotCJørgensenNMadsenORJensenLBSørensenOHRelationships between bone mineral density, serum vitamin D metabolites and calcium:phosphorus intake in healthy peri-menopausal womenJ Intern Med1999245550951610363752
- VanderschuerenDPyeSRO’NeillTWActive vitamin D (1,25-dihydroxyvitamin D) and bone health in middle-aged and elderly men: the European Male Aging Study (EMAS)J Clin Endocrinol Metab2013983995100523386642
- HorowitzMChoiYOsteoimmunology: interactions of the bone and immune systemEndocr Rev200829440344018451259
- GinaldiLDe MartinisMOsteoimmunology and beyondCurr Med Chem201623333754377427604089
- HsuEPacificiRFrom Osteoimmunology to osteomicrobiology: How the microbiota and the immune system regulate boneCalcif Tissue Int2017 Epub 2017 Oct 27
- MangalamAKTanejaVDavidCSHLA class II molecules influence susceptibility versus protection in inflammatory diseases by determining the cytokine profileJ Immunol2013190251351823293357