Abstract
Reactive oxygen species and thiol antioxidants, including glutathione (GSH), regulate innate immunity at various levels. This review outlines the redox-sensitive steps of the cellular mechanisms implicated in inflammation and host defense against infection, and describes how GSH is not only important as an antioxidant but also as a signaling molecule. There is an extensive literature of the role of GSH in immunity. Most reviews are biased by an oversimplified picture where “bad” free radicals cause all sorts of diseases and “good” antioxidants protect from them and prevent oxidative stress. While this may be the case in certain fields (eg, toxicology), the role of thiols (the topic of this review) in immunity certainly requires wearing scientist’s goggles and being prepared to accept a more complex picture. This review aims at describing the role of GSH in the lung in the context of immunity and inflammation. The first part summarizes the history and basic concepts of this picture. The second part focuses on GSH metabolism/levels in pathology, the third on the role of GSH in innate immunity and inflammation, and the fourth gives 4 examples describing the importance of GSH in the response to infections.
Oxidative damage and antioxidant defense, inflammation, and immunity
Reactive oxygen species and oxidative stress
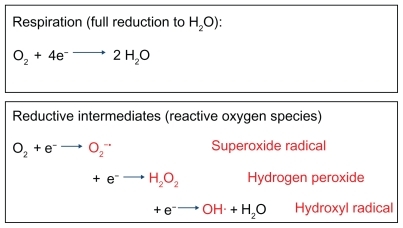
While the oxidative deterioration of lipids has been known for a long time, it was only in the late 1960s that we became aware of the importance of these chemical processes in pathology and toxicology with the finding that lipid peroxidation or rancidification occurred also in biological systems.Citation1,Citation2 The expression “oxidative stress” appears in the scientific literature in the 1970s. It implies that in aerobic organisms not only can molecular oxygen (O2) be reduced to water in the biochemical process known as respiration, which requires 4 electrons, but O2 can also be reduced to other intermediate species, between O2 and water, resulting from 1-electron reductive steps (). These intermediates are superoxide radical (O2−), hydrogen peroxide (H2O2), and hydroxyl radical (OH); they are all referred to as reactive oxygen species (ROS) and are extremely reactive because they avidly tend to reach the fully reduced state (H2O). Reactivity, in biological systems, is almost inevitably associated with toxicity, and ROS can damage various biological molecules: lipids (causing lipid peroxidation and membrane damage), DNA (causing DNA breaks), and proteins (causing, for instance, oxidation of various amino acids and inactivation of essential enzymes).
It is now thought that oxidative stress is implicated in the pathogenesis of several diseases and conditions, from aging to inflammation and carcinogenesis, if not as a primary cause of disease at least as an aggravating mechanism.
Antioxidant defenses and glutathione
The reality of oxidative stress is demonstrated by the existence, in all aerobic organisms, of several antioxidant enzymes devoted to ROS detoxification, such as peroxidase, catalase, superoxide dismutase, and peroxiredoxins. A number of small molecules also act as ROS scavengers. Unlike antioxidant enzymes, which catalyze the transformation of ROS into less toxic molecules, the concept of scavenger is that it must be an easily oxidizable target for ROS, and it must be present at concentrations high enough that the probability that a ROS reacts with the scavenger is higher than that of reacting with another target. When dealing with lipid oxidation (such as in rancidification of butter), the most important scavenger is probably vitamin E. As proteins with a sulfydryl group are often target of oxidation (−SH groups can be easily oxidized), small-molecular-weight thiols are potent scavengers.
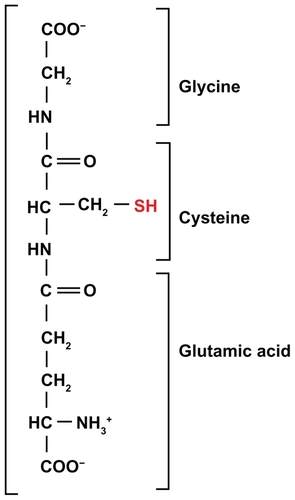
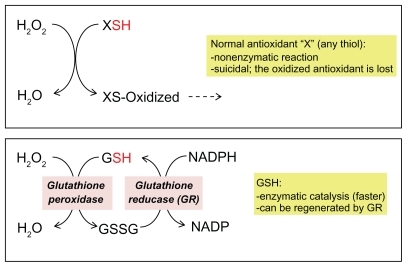
The small thiol present in highest concentration in the cytoplasm is glutathione (GSH), a tripeptide glycine-cysteine-glutamic acid (): the −SH group of its cysteine is extremely sensitive to oxidation, mainly by peroxides. Its importance is supported by the existence of a molecular machinery that makes it particularly effective. In fact, a scavenger, including any thiol antioxidant such as the common laboratory reagent beta-mercaptoethanol, would normally act as a suicidal decoy, being oxidized and thus becoming useless. However, when GSH is oxidized, it forms GSH disulfide (GSSG), and this can be re-reduced by a specific enzyme, glutathione reductase. Not only can GSH be enzymatically regenerated from GSSG, but also the reaction of GSH with the ROS (a peroxide), which already takes place with high reactivity, is catalyzed by GSH peroxidases that facilitate the inactivation of a wide range of peroxides ().
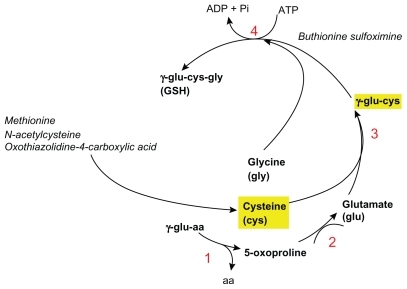
The key role of GSH as an antioxidant is demonstrated by many studies showing that experimental depletion of GSH levels – which can be achieved with various chemicals the most used of which is buthionine sulfoximine (BSO) an inhibitor of GSH synthesis – has a worsening effect in many disease models. Conversely, repleting GSH levels with precursors of its synthesis such as N-acetyl-cysteine (NAC) or 2-oxothiazolidine-4-carboxylic acid has protective effects. Cysteine precursors, rather than cysteine itself, are used because they are more cell-permeable, and can be given orally.
Innate immunity and inflammation, two faces of the same biological coin
The first line of immune defense against pathogens, before adaptive immunity (antibodies, T cell responses) develops, is called innate immunity. This is a complex set of responses triggered when specific cells (macrophages, phagocytes, dendritic cells) recognize even a yet unknown pathogen by some characteristics common to most pathogens (these “signatures” are called pathogen-associated molecular patterns) through a family of pathogen recognition receptors. This activates a response that involves production of ROS, a major bactericidal mechanism, and of soluble mediators (cytokines) whose role is to amplify the host response by recruiting and activating other immune system cells.
This aspect of the innate immune response is also known, from a different perspective, as the inflammatory response. Basically, the very same mechanisms and mediators of host defense are implicated in the pathogenesis of (noninfectious) inflammatory diseases, and inhibition of these mechanisms is the key to the mechanism of action of anti-inflammatory drugs.
Many noninfectious diseases of the respiratory system, including asthma, chronic obstructive pulmonary disease (COPD), cystic fibrosis, idiopathic pulmonary fibrosis (IPF), and oxygen toxicity, have an inflammatory component. Inflammation is also implicated in the lung toxicity of ozone, asbestos, silica, cigarette smoke, and particulate matter. An exaggerated inflammatory response is also involved in the pathogenesis or complications of pulmonary infections such as tuberculosis, severe acute respiratory syndrome, influenza, and acute respiratory distress syndrome (ARDS).
Glutathione metabolism in disease
GSH synthesis and its precursors
GSH is synthesized from its 3 amino acids with a biosynthetic pathway shown in . A study in humans, using radioisotopes,Citation3 has demonstrated that the availability of cysteine,and its precursor methionine, is the rate-limiting factor in GSH synthesis. In general, it is assumed that the two main limiting factors are the levels of cysteine and of the enzyme gamma-glutamyl-cysteine synthetase (also known as glutamate-cysteine ligase).Citation4
Genetic GSH deficiency and immunity
Glutathione synthetase (GS) deficiency (oxoprolinuria) is a rare autosomal recessive disorder affecting about 70 patients in the world. Patients with severe GS deficiency show, among other conditions, an increased susceptibility to bacterial infections.Citation5,Citation6
One case report showed that neutrophils from a child with GS deficiency undergo oxidative damage upon phagocytosis, indicating that GSH is important in defending the neutrophils from the ROS they produce as part of their microbicidal armamentarium.Citation7 Of note, neutrophils from GS-deficient patients, despite normal phagocytosis and increased release of hydrogen peroxide, are less efficient in killing bacteria, indicating a helper role for GSH in the bactericidal activity.Citation8
Acquired GSH deficiency and immunity
Many pathological conditions are associated with decreased GSH levels (). This could be due to several reasons. For instance, oxidative stress could cause GSH loss though oxidation. Another important aspect is nutrition, as it was shown that, even when dietary protein intake is sufficient for maintaining nitrogen balance, it may not be sufficient for maintaining cellular GSH, particularly in conditions of oxidative stress.Citation9 GSH deficiency could also arise from metabolic problems. For instance, in AIDS patients, a decrease in gamma-cystathionase activity in the liver was reported.Citation10
Table 1 Lung diseases associated with glutathione deficiency
The use of stable radioisotopes allowed the characterization of cysteine metabolism and GSH synthesis in septic patients. This study showed that sepsis decreases blood GSH synthesis by 60%.Citation3 Of note, these patients had an intake of sulfur amino acids below that recommended by the World Health Organization.Citation3 Studies in animal models have reported an increased requirement for cysteine in sepsis, probably due to an increased turnover of GSH, as GSH synthesis accounts for 40% of the increased cysteine utilization.Citation11,Citation12 These and other studies show that even when the dietary intake of cysteine is sufficient for protein synthesis (nitrogen balance), it may not be sufficient to maintain adequate GSH levels.Citation9 One should also bear in mind that infectious and inflammatory conditions are associated with an increased production of acute-phase proteins by the liver, and it was estimated that they account significantly for the increased utilization of sulfur amino acids, thus competing with GSH.Citation13,Citation14
GSH as a regulator of innate immunity
Anti-inflammatory role of GSH in lung diseases
The idea that GSH may play an anti-inflammatory role became popular in the 1990s with a study by Schreck et alCitation15 showing that antioxidants inhibit, whereas ROS activate, the transcription factor NF-κB that commands the transcription of several inflammatory genes,Citation16 a role that has been confirmed in the lung.Citation17
In fact, a protective role of GSH against inflammatory pathologies of the lung has been demonstrated by the protective effect of different GSH-precursors in various animal models ().
Table 2 Protective effect of glutathione repletion in lung diseases
Along the same line, depleting endogenous GSH with BSO had a worsening effect in some of the models above, including chemically induced pulmonary edema,Citation21 cigarette smoke,Citation31 carrageenan-induced pleurisy,Citation32 and endotoxin-induced pulmonary inflammation.Citation33
It can bee seen that there is an overlap between the list of pulmonary diseases associated with inflammation and those where GSH repletion is protective, indirectly supporting the hypothesis of an anti-inflammatory role of GSH.
An increased susceptibility to sepsis-induced ARDS and lethalityCitation34 was observed in mice lacking the transcription factor Nrf2, which has among its target genes the GSH biosynthetic enzyme gamma-glutamylcysteine synthase.Citation35 IPF is an example of lung disease in which normalizing the GSH deficit has positive effects. Patients with IPF have a 4-fold lower GSH concentration in the epithelial lining fluid of the normal lower respiratory tract;Citation36 moreover, the administration of the GSH precursor NAC not only restores GSH levelsCitation37 but, in association with other drugs used for the treatment of IPF, it also improves end points such as vital capacity and single-breath carbon monoxide diffusing capacity.Citation22
GSH is essential for innate and adaptive immune functions
The functions of GSH are not only inhibitory as described above for the inflammatory response. In fact, GSH is essential for some functions of the immune system, both innate and adaptive, including T-lymphocyte proliferation,Citation38,Citation39 phagocytic activity of polymorphonuclear neutrophils (PMN),Citation40 and dendritic cell functions,Citation41 and is also important for the first step of adaptive immunity, consisting of the antigen presentation by antigen-presenting cells (APC). Indeed, cell-mediated immunity requires that protein antigens be first degraded in the endocytic vesicles of APCs (eg, macrophages, dendritic cells), so that the smaller peptides can be presented on the cell surface by the major histocompatibility complex to activate proliferation of antigen-specific T cells. One of the first steps in antigen degradation and processing is the reduction of disulfide bonds,Citation42 which requires GSH.Citation43 It should also be noted that although GSH inhibits the production of most inflammatory cytokines, it is required to maintain an adequate interferon-gamma (IFN-gamma) production by dendritic cells,Citation44 which is essential for the host defense against intracellular pathogens such as mycobacteria.Citation45
This essential role of GSH in immunity might explain why in many diseases, not only AIDS, decreased GSH levels are associated with an increased susceptibility to infection. These include COPD,Citation46 cystic fibrosis,Citation47,Citation48 influenza infection,Citation49 and alcoholism,Citation50,Citation51 as ethanol impairs Th1/Th2 balance via GSH depletionCitation52 ().
Table 3 Glutathione (GSH) depletion is associated with increases susceptibility to infections
GSH and immune defense against infection: four examples
Example 1: viral infections – oxidative stress and the pathogenesis of influenza
The role of oxidative stress in the pulmonary damage by influenza virus is well characterized in the mouse model. Mice infected with influenza show pulmonary damage associated with a dramatic decrease in pulmonary GSH levels as well as an increase of oxidative stress markers such as oxidized glutathione (GSSG) and lipid peroxides.Citation53 In these mice, oxidative stress could be due to the induction of xanthine oxidase,Citation54 a well-known superoxide-generating enzyme.Citation55 Furthermore, influenza infection is associated with an induction of inflammatory cytokines,Citation56,Citation57 which might represent a likely GSH-sensitive step in its pathogenesis. Among the antioxidants shown to be protective in animal models of influenza are the xanthine oxidase inhibitor allopurinol,Citation54 quercetin,Citation58 superoxide dismutase,Citation59 thioredoxin,Citation60 NAC (aloneCitation61 or with ribavirinCitation62 or oseltamavirCitation63), and GSH itself.Citation64 A study reported that influenza virus M2 protein augments ROS production in human airway cells in vitro, resulting in toxic effects that are prevented by the addition of GSH ester.Citation65 Although these studies indicate a protective role of GSH at the level of the pathogenesis of pulmonary damage, there is a report that BSO increases viral replication,Citation66 implying a possible antiviral role of endogenous GSH. One clinical study has shown that NAC administration improves parameters of cell-mediated immunity in patients with influenza,Citation66 suggesting a possible clinical relevance of these observations.
Example 2: AIDS – importance of GSH in immunity
Historically, the entire field of the role of GSH in immunity and its effect on NF-κB received the strongest boost by studies on AIDS, particularly by a study from the group of Wulf Droge who showed that AIDS patients have a low concentration of plasma cysteine.Citation67 The reasons for this are not clear, but AIDS patients have a deficit in the enzyme gamma cystathionase, which synthesizes cysteine from the methionine.Citation10 Later research showed that AIDS causes a decrease in intracellular GSH in CD4 T cells, and that low GSH is associated with decreased survival.Citation68
The lower GSH levels in AIDS patients could have various consequences. GSH depletion, at least in vitro, augments HIV replication,Citation69 while its precursor NAC blocks the stimulatory effect of tumor necrosis factor on HIV replication. Citation70 Furthermore, the neurotoxic effect of HIV proteins Tat and gp120 is associated with oxidative stress and antagonized by NAC.Citation71,Citation72
Example 3: bacterial infections – tuberculosis
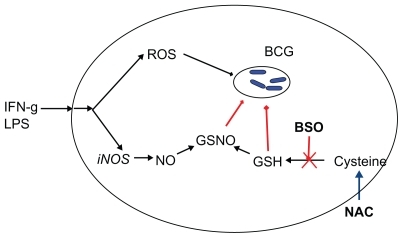
Mycobacterium tuberculosis is an intracellular pathogen that grows in the phagosomes, where it is protected from immune system effectors such as antibodies and T lymphocytes. Although the literature showing that GSH levels are lower in patients with tuberculosis dates back to the 1950s, it was not until the research of Venketaraman and colleagues that the effect of GSH on M. tuberculosis infection was studied in depth. Using a mouse macrophage cell line, the authors show that IFN-gamma and endotoxin increase both nitric oxide (NO) production and bactericidal activity; the paralleled decrease in GSH suggests that GSH reacts with NO to form S-nitrosoglutathione (GSNO). Under these experimental conditions, GSH depletion with BSO inhibited the microbicidal activity of macrophages while its precursor NAC increased intracellular killing of mycobacteria also from human macrophages, which are normally not very effective in killing mycobacteria.Citation73,Citation74 Other investigators have shown that the trans-sulfuration pathway, which converts methionine into cysteine and has a key role in maintaining cysteine, and hence GSH levels, is essential for mycobacterial killing.Citation75 In that study, it was found that not only are trans-sulfuration pathway enzymes increased in human monocytes as a response to mycobacteria, but their inhibitor propargylglycine lowered GSH levels and inhibited clearance of mycobacteria and phagolysosome fusion, while NAC increased them.Citation75 Similar results were obtained in whole human blood cultures.Citation76 In vitro, both GSH and GSNO have direct bactericidal activity against these pathogens.Citation77
This complex picture has been nicely reviewed by Connell and VenkataramanCitation78 and is summarized in . Depletion of GSH (by BSO or diethylmaleate) also inhibits macrophage leishmanicidal activity, as well as NO production, while the GSH precursor GSH-ethyl ester restores them,Citation79 suggesting that the requirement for the GSH/NO pathway might be a common feature of resistance to intracellular pathogens.
Figure 5 Glutathione in the defense against mycobacterial infections.
Example 4: sepsis and its complications – GSH as a regulatory molecule
ARDS is one of the most serious complications in critically ill septic patients. Many studies have pointed out a role for oxidative stress in ARDS and shown a protective effect of GSH precursors.Citation80,Citation81
Most of the studies of ARDS in animal models, including those cited above, are based on the administration of lipopolysaccharide (LPS), a bacterial endotoxin. In mice, administration of LPS induces an acute lung injury similar to clinical ARDS, with production of inflammatory cytokines, leukocyte infiltration in the lung, and pulmonary edema. In this model, various thiol-based antioxidants are protective.Citation80–Citation82 However, LPS administration is in fact a model of endotoxic shock, involving no live bacteria, to induce a state similar to what was once called septic shock and is now defined as systemic inflammatory response syndrome.Citation83–Citation85
However, in septic patients, survival is affected by 2 opposite contributions of innate immunity: innate immunity is detrimental as systemic inflammation, which results in ARDS and shock, but on the other hand it is an essential component of the immune defense against infection. It is difficult to foresee how GSH affects this balance.
One more realistic animal model is that induced by cecal ligation and puncture (CLP), where puncturing the cecum causes the release of fecal material in the peritoneum that results in a polymicrobial peritoneal sepsis. This model allows studying the relevance of both arms of innate immunity, the detrimental and the protective one.
We have used this model to study the role of endogenous GSH in sepsis.Citation86 In this model, CLP induced PMN infiltration in the peritoneal cavity, the site of infection, as well as in the lung, ultimately resulting in lung injury and death, with a concomitant decrease in endogenous GSH.Citation86 Lowering GSH further with BSO worsened the clinical settings. Not only did BSO increase PMN infiltrate in the lung, but it also diminished PMN infiltration in the site of infection, thus increasing bacterial growth. As a result we had more inflammation and less immunity, and survival was dramatically decreased. Repleting GSH with NAC had the opposite effect of reducing PMN infiltration to the lung but increasing that to the site of infection, thus decreasing bacterial colonies.
The picture that emerges from these experiments is that endogenous GSH is not just an inhibitor of inflammation, but it is required to allow a proper response to infection, and “direct” the migration of inflammatory PMN away from the lung, where they cause ARDS, and towards the site of infection, where they kill bacteria ().
Figure 6 Glutathione regulates the balance between innate immunity or leukocyte infiltration at the site of infection to kill bacteria, and inflammation or leukocyte infiltration to the lung to cause organ failure.
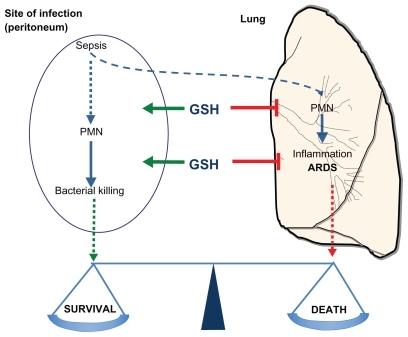
The idea, therefore, is that GSH is not just an inhibitor of inflammation but also a regulator of innate immunity in a direction favorable to the host.
Conclusions: GSH from a simple free-radical scavenger to a regulatory molecule
In parallel to the studies of GSH in immunity, more complex molecular and biochemical studies have pointed out a regulatory role of GSH. This was a result of an evolution from the concept of oxidative stress outlined above to that of redox regulation. The concept of redox regulation implies that some oxidative changes (such as changes in the redox state of protein cysteines) are not necessarily damaging but can have regulatory properties. While this idea shows a more complex picture from the popular one where free radicals and oxidants are bad and antioxidants are good, it implies that GSH is an essential molecule not only in acting as an antioxidant but also in the absence of oxidative stress, as an endogenous signaling molecule. The molecular mechanisms of GSH-mediated redox regulation are being actively investigated and have in part been identified.Citation87–Citation89
Although the present review focused on the immuno pathogenesis of pulmonary diseases, the key concepts outlined here may help in interpreting the role of redox in other pathological conditions.
Disclosure
The author discloses no conflicts of interest.
References
- DormandyTLBiological rancidificationLancet1969276226846884185423
- TappelAZalkinHInhibition of lipid peroxidation in microsomes by vitamin ENature19601853513836891
- LyonsJRauh-PfeifferAMing-YuYCysteine metabolism and whole blood glutathione synthesis in septic pediatric patientsCrit Care Med200129487087711373484
- BioloGAntonioneRDe CiccoMGlutathione metabolism in sepsisCrit Care Med2007359 SupplS591S59517713414
- RistoffELarssonAInborn errors in the metabolism of glutathioneOrphanet J Rare Dis200721617397529
- RistoffEMayatepekELarssonALong-term clinical outcome in patients with glutathione synthetase deficiencyJ Pediatr20011391798411445798
- SpielbergSPBoxerLAOliverJMAllenJMSchulmanJDOxidative damage to neutrophils in glutathione synthetase deficiencyBr J Haematol1979422215223465367
- BoxerLAOliverJMSpielbergSPAllenJMSchulmanJDProtection of granulocytes by vitamin E in glutathione synthetase deficiencyN Engl J Med197930117901905481537
- JacksonAAGibsonNRLuYJahoorFSynthesis of erythrocyte glutathione in healthy adults consuming the safe amount of dietary proteinAm J Clin Nutr200480110110715213035
- MartinJASastreJde la AsuncionJGPallardoFVVinaJHepatic gamma-cystathionase deficiency in patients with AIDSJAMA2001285111444144511255419
- MalmezatTBreuilleDCapitanPMirandPPObledCGlutathione turnover is increased during the acute phase of sepsis in ratsJ Nutr200013051239124610801925
- MalmezatTBreuilleDPouyetCMethionine transsulfuration is increased during sepsis in ratsAm J Physiol Endocrinol Metab20002796E1391E139711093928
- HunterEAGrimbleRFCysteine and methionine supplementation modulate the effect of tumor necrosis factor alpha on protein synthesis, glutathione and zinc concentration of liver and lung in rats fed a low protein dietJ Nutr1994124122319232816856311
- HunterEAGrimbleRFDietary sulphur amino acid adequacy influences glutathione synthesis and glutathione-dependent enzymes during the inflammatory response to endotoxin and tumour necrosis factor-alpha in ratsClin Sci (Lond)19979232973059093011
- SchreckRRieberPBaeuerlePAReactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1Embo J1991108224722582065663
- BaeuerlePAHenkelTFunction and activation of NF-kappa B in the immune systemAnnu Rev Immunol1994121411798011280
- BlackwellTSBlackwellTRHoldenEPChristmanBWChristmanJWIn vivo antioxidant treatment suppresses nuclear factor-kappa B activation and neutrophilic lung inflammationJ Immunol19961574163016378759749
- KloekJVan ArkIDe ClerckFBloksmaNNijkampFPFolkertsGModulation of airway hyperresponsiveness by thiols in a murine in vivo model of allergic asthmaInflamm Res200352312613112755377
- LeeYCLeeKSParkSJBlockade of airway hyperresponsiveness and inflammation in a murine model of asthma by a prodrug of cysteine, L-2-oxothiazolidine-4-carboxylic acidFASEB J200418151917191915385436
- CuzzocreaSMazzonEDugoLProtective effects of N-acetylcysteine on lung injury and red blood cell modification induced by carrageenan in the ratFASEB J20011571187120011344087
- LaileyAFHillLLawstonIWStantonDUpshallDGProtection by cysteine esters against chemically induced pulmonary oedemaBiochem Pharmacol199142 SupplS47S541768285
- DemedtsMBehrJBuhlRHigh-dose acetylcysteine in idiopathic pulmonary fibrosisN Engl J Med2005353212229224216306520
- HagiwaraSIIshiiYKitamuraSAerosolized administration of N-acetylcysteine attenuates lung fibrosis induced by bleomycin in miceAm J Respir Crit Care Med2000162122523110903246
- AfaqFAbidiPRahmanQN-acetyl L-cysteine attenuates oxidant-mediated toxicity induced by chrysotile fibersToxicol Lett20001171–2536011033233
- RhodenCRLawrenceJGodleskiJJGonzalez-FlechaBN-acetylcysteine prevents lung inflammation after short-term inhalation exposure to concentrated ambient particlesToxicol Sci200479229630315056806
- LangleySCKellyFJN-acetylcysteine ameliorates hyperoxic lung injury in the preterm guinea pigBiochem Pharmacol19934548418468452559
- LevyMASikorskiBBrayTMSelective elevation of glutathione levels in target tissues with L-2-oxothiazolidine-4-carboxylate (OTC) protects against hyperoxia-induced lung damage in protein-energy malnourished rats: implications for a new treatment strategyJ Nutr199812846716769521626
- LucasMCLudenaMDBarberoEASanchez-GasconFEffects of N-acetylcysteine on hyperoxic lung in the ratRespir Med19958943117597274
- TirouvanziamRConradCKBottiglieriTHerzenbergLAMossRBHigh-dose oral N-acetylcysteine, a glutathione prodrug, modulates inflammation in cystic fibrosisProc Natl Acad Sci U S A2006103124628463316537378
- HodgeSMatthewsGMukaroVCigarette smoke-induced changes to alveolar macrophage phenotype and function is improved by treatment with procysteineAm J Respir Cell Mol Biol71 [Epub ahead of print]
- ParkEMParkYMGwakYSOxidative damage in tissues of rats exposed to cigarette smokeFree Radic Biol Med199825179869655525
- CuzzocreaSCostantinoGZingarelliBMazzonEMicaliACaputiAPThe protective role of endogenous glutathione in carrageenan-induced pleurisy in the ratEur J Pharmacol1999372218719710395099
- HaddadJJL-Buthionine-(S,R)-sulfoximine, an irreversible inhibitor of gamma-glutamylcysteine synthetase, augments LPS-mediated pro-inflammatory cytokine biosynthesis: evidence for the implication of an IkappaB-alpha/NF-kappaB insensitive pathwayEur Cytokine Netw200112461462411781188
- ThimmulappaRKLeeHRangasamyTNrf2 is a critical regulator of the innate immune response and survival during experimental sepsisJ Clin Invest2006116498499516585964
- MoinovaHRMulcahyRTUp-regulation of the human gamma-glutamylcysteine synthetase regulatory subunit gene involves binding of Nrf-2 to an electrophile responsive elementBiochem Biophys Res Commun1999261366166810441483
- CantinAMHubbardRCCrystalRGGlutathione deficiency in the epithelial lining fluid of the lower respiratory tract in idiopathic pulmonary fibrosisAm Rev Respir Dis198913923703722913886
- MeyerABuhlRMagnussenHThe effect of oral N-acetylcysteine on lung glutathione levels in idiopathic pulmonary fibrosisEur Respir J1994734314368013597
- SidoBBraunsteinJBreitkreutzRHerfarthCMeuerSCThiol-mediated redox regulation of intestinal lamina propria T lymphocytesJ Exp Med2000192690791210993921
- HadzicTLiLChengNWalshSASpitzDRKnudsonCMThe role of low molecular weight thiols in T lymphocyte proliferation and IL-2 secretionJ Immunol2005175127965797216339532
- OliverJMAlbertiniDFBerlinRDEffects of glutathione-oxidizing agents on microtubule assembly and microtubule-dependent surface properties of human neutrophilsJ Cell Biol1976713921932993272
- KuppnerMCScharnerAMilaniVIfosfamide impairs the allostimulatory capacity of human dendritic cells by intracellular glutathione depletionBlood2003102103668367412855564
- ArunachalamBPhanUTGeuzeHJCresswellPEnzymatic reduction of disulf ide bonds in lysosomes: characterization of a gamma-interferon-inducible lysosomal thiol reductase (GILT)Proc Natl Acad Sci U S A200097274575010639150
- ShortSMerkelBJCaffreyRMcCoyKLDefective antigen processing correlates with a low level of intracellular glutathioneEur J Immunol19962612301530208977298
- MurataYOhtekiTKoyasuSHamuroJIFN-gamma and pro-inflammatory cytokine production by antigen-presenting cells is dictated by intracellular thiol redox status regulated by oxygen tensionEur J Immunol200232102866287312355439
- JanewayCATraversPWalportMShlomchikMJImmunobiology6th Edition New York – LondonGarland Science2005
- ParameswaranGIMurphyTFInfections in chronic lung diseasesInfect Dis Clin North Am2007213673695viii17826618
- SorensenRUWallerRLKlingerJDCystic fibrosis. Infection and immunity to PseudomonasClin Rev Allergy199191–247741884328
- SchroederTHReinigerNMeluleniGGroutMColemanFTPierGBTransgenic cystic fibrosis mice exhibit reduced early clearance of Pseudomonas aeruginosa from the respiratory tractJ Immunol2001166127410741811390493
- McNameeLAHarmsenAGBoth influenza-induced neutrophil dysfunction and neutrophil-independent mechanisms contribute to increased susceptibility to a secondary Streptococcus pneumoniae infectionInfect Immun200674126707672116982840
- BrownLAHarrisFLPingXDGauthierTWChronic ethanol ingestion and the risk of acute lung injury: a role for glutathione availability?Alcohol200433319119715596087
- NelsonSKollsJKAlcohol, host defence and societyNat Rev Immunol20022320520911913071
- PetersonJDHerzenbergLAVasquezKWaltenbaughCGlutathione levels in antigen-presenting cells modulate Th1 versus Th2 response patternsProc Natl Acad Sci U S A1998956307130769501217
- SulimanHBRyanLKBishopLFolzRJPrevention of influenza-induced lung injury in mice overexpressing extracellular superoxide dismutaseAm J Physiol Lung Cell Mol Physiol20012801L69L7811133496
- AkaikeTAndoMOdaTDependence on O2- generation by xanthine oxidase of pathogenesis of influenza virus infection in miceJ Clin Invest19908537397452155924
- McCordJMFridovichIThe reduction of cytochromec by milk xanthine oxidaseJ Biol Chem196824321575357604972775
- WangJPBowenGNPaddenCToll-like receptor-mediated activation of neutrophils by influenza A virusBlood200811252028203418544685
- de JongMDSimmonsCPThanhTTFatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemiaNat Med200612101203120716964257
- KumarPSharmaSKhannaMRajHGEffect of Quercetin on lipid peroxidation and changes in lung morphology in experimental influenza virus infectionInt J Exp Pathol200384312713312974942
- OdaTAkaikeTHamamotoTSuzukiFHiranoTMaedaHOxygen radicals in influenza-induced pathogenesis and treatment with pyran polymer-conjugated SODScience198924449079749762543070
- NakamuraHTamuraSWatanabeIIwasakiTYodoiJEnhanced resistancy of thioredoxin-transgenic mice against influenza virus-induced pneumoniaImmunol Lett2002821–216517012008049
- UngheriDPisaniCSansonGProtective effect of N-acetylcysteine in a model of influenza infection in miceInt J Immunopathol Pharmacol200013312312812657201
- GhezziPUngheriDSynergistic combination of N-acetylcysteine and ribavirin to protect from lethal influenza viral infection in a mouse modelInt J Immunopathol Pharmacol20041719910215000873
- GarozzoATemperaGUngheriDTimpanaroRCastroAN-acetylcysteine synergizes with oseltamivir in protecting mice from lethal influenza infectionInt J Immunopathol Pharmacol200720234935417624247
- CaiJChenYSethSFurukawaSCompansRWJonesDPInhibition of influenza infection by glutathioneFree Radic Biol Med200334792893612654482
- LazrakAIlesKELiuGNoahDLNoahJWMatalonSInfluenza virus M2 protein inhibits epithelial sodium channels by increasing reactive oxygen speciesFASEB J200923113829384219596899
- NencioniLIuvaraAAquilanoKInfluenza A virus replication is dependent on an antioxidant pathway that involves GSH and Bcl-2FASEB J200317675876012594179
- EckHPGmunderHHartmannMPetzoldtDDanielVDrogeWLow concentrations of acid-soluble thiol (cysteine) in the blood plasma of HIV-1-infected patientsBiol Chem Hoppe Seyler198937021011082784973
- HerzenbergLADe RosaSCDubsJGGlutathione deficiency is associated with impaired survival in HIV diseaseProc Natl Acad Sci U S A1997945196719729050888
- GaraciEPalamaraATCirioloMRIntracellular GSH content and HIV replication in human macrophagesJ Leukoc Biol199762154599225993
- RoedererMStaalFJRajuPAElaSWHerzenbergLACytokine-stimulated human immunodeficiency virus replication is inhibited by N-acetyl-L-cysteineProc Natl Acad Sci U S A19908712488448882112750
- PuHTianJAndrasIEHIV-1 Tat protein-induced alterations of ZO-1 expression are mediated by redox-regulated ERK 1/2 activationJ Cereb Blood Flow Metab200525101325133515829913
- VisalliVMuscoliCSaccoIN-acetylcysteine prevents HIV gp 120-related damage of human cultured astrocytes: correlation with glutamine synthase dysfunctionBMC Neurosci2007810618062818
- VenketaramanVDayaramYKAminAGRole of glutathione in macrophage control of mycobacteriaInfect Immun20037141864187112654802
- VenketaramanVDayaramYKTalaueMTConnellNDGlutathione and nitrosoglutathione in macrophage defense against Mycobacterium tuberculosisInfect Immun20057331886188915731094
- GargSVitvitskyVGendelmanHEBanerjeeRMonocyte differentiation, activation, and mycobacterial killing are linked to transsulfuration-dependent redox metabolismJ Biol Chem200628150387123872017046819
- VenketaramanVMillmanASalmanMGlutathione levels and immune responses in tuberculosis patientsMicrob Pathog200844325526117959342
- GreenRMSethAConnellNDA peptide permease mutant of Mycobacterium bovis BCG resistant to the toxic peptides glutathione and S-nitrosoglutathioneInfect Immun200068242943610639400
- ConnellNDVenketaramanVControl of mycobacterium tuberculosis infection by glutathioneRecent Pat Antiinfect Drug Discov20094321422619832692
- Buchmuller-RouillerYCorrandinSBSmithJRole of glutathione in macrophage activation: effect of cellular glutathione depletion on nitrite production and leishmanicidal activityCell Immunol1995164173807543373
- DavreuxCJSoricINathensABN-acetyl cysteine attenuates acute lung injury in the ratShock1997864324389421857
- GattiSFaggioniREchtenacherBGhezziPRole of tumour necrosis factor and reactive oxygen intermediates in lipopolysaccharide-induced pulmonary oedema and lethalityClin Exp Immunol19939134564618443968
- BernardGRLuchtWDNiedermeyerMESnapperJROgletreeMLBrighamKLEffect of N-acetylcysteine on the pulmonary response to endotoxin in the awake sheep and upon in vitro granulocyte functionJ Clin Invest1984736177217846725559
- ACCPAmerican College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsisCrit Care Med19922068648741597042
- BoneRCSepsis and its complications: the clinical problemCrit Care Med1994227S8S118026190
- RedlHSchlagGBahramiSYaoYMAnimal models as the basis of pharmacologic intervention in trauma and sepsis patientsWorld J Surg19962044874928662140
- VillaPSaccaniASicaAGhezziPGlutathione protects mice from lethal sepsis by limiting inflammation and potentiating host defenseJ Infect Dis200218581115112011930321
- GhezziPDi SimplicioPGlutathionylation pathways in drug responseCurr Opin Pharmacol20077439840317611156
- GhezziPOxidoreduction of protein thiols in redox regulationBiochem Soc Trans200533Pt 61378138116246123
- GhezziPRegulation of protein function by glutathionylationFree Radic Res200539657358016036334