Abstract
Introduction
The goal of insulin therapy in patients with either type 1 diabetes mellitus (T1DM) or type 2 diabetes mellitus (T2DM) is to match as closely as possible normal physiologic insulin secretion to control fasting and postprandial plasma glucose. Modifications of the insulin molecule have resulted in two long-acting insulin analogs (glargine and detemir) and three rapid-acting insulins (aspart, lispro, and glulisine) with improved pharmacokinetic/pharmacodynamic (PK/PD) profiles. These agents can be used together in basal-bolus therapy to more closely mimic physiologic insulin secretion patterns.
Methods
This study reviews effects of the multiple demographic and clinical parameters in the insulin analogs glargine, detemir, lispro, aspart, and glulisine in patients with T2DM. A search was conducted on PubMed for each major topic considered (effects of injection site, age, race/ethnicity, obesity, renal or hepatic dysfunction, pregnancy, exercise, drug interactions) using the topic words and name of each type of insulin analog. Information was also obtained from the prescribing information for each insulin analog.
Results
The PK/PD profiles for insulin analogs may be influenced by many variables including age, weight, and hepatic and renal function. However, these variables do not have equivalent effects on all long-acting or rapid-acting insulin analogs.
Conclusion
Rapid-acting and long-acting insulin analogs represent major advances in treatment for patients with T2DM who require insulin therapy. However, there are potentially important PK and PD differences between the two long-acting agents and among the three rapid-acting insulin analogs, which should be considered when designing treatment regimens for special patient groups.
Introduction
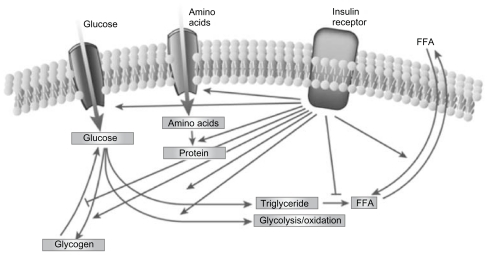
Type 2 diabetes mellitus (T2DM) is characterized by a marked and progressive disruption of normal physiologic insulin secretion. There are two key aspects of insulin secretion in healthy individuals: (1) basal insulin secretion by pancreatic β cells that occurs continuously to maintain steady glucose levels for extended periods between meals, and (2) prandial secretion in which insulin is rapidly released in an initial first-phase spike in response to a meal, followed by prolonged insulin secretion that returns to basal levels after 2–3 hours ().Citation1–Citation3 Insulin is a potent anabolic hormone that binds to insulin receptors to lower blood glucose by facilitating cellular uptake of glucose, amino acids, and fatty acids into skeletal muscle and fat and by inhibiting the output of glucose from the liver. Insulin increases the expression or activity of enzymes that catalyze glycogen, lipid, and protein synthesis, while inhibiting lipolysis and, proteolysis ().Citation4,Citation5 In normal healthy individuals, physiologic basal and prandial insulin secretions maintain euglycemia, which affects fasting plasma glucose (FPG) and postprandial plasma glucose (PPG) concentrations.
Figure 1 Mean 24-hour physiologic serum insulin and plasma glucose levels in nondiabetic subjects.Citation3
Reprinted from Am J Med, vol. 113, issue 4, Gerich, Novel insulins: expanding options in diabetes management, pp. 308–316, Copyright (2002), with permission from Elsevier.
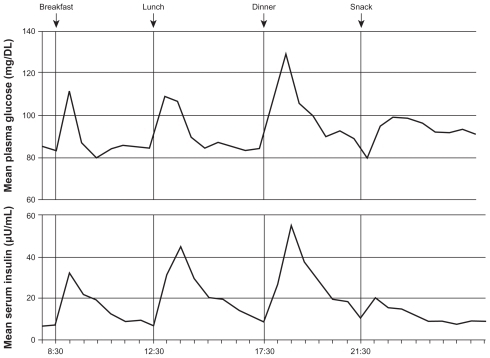
Figure 2 The regulation of metabolism by insulin.Citation5
Reprinted from Nature, vol. 414, issue 6865, Saltiel and Kahn, Insulin signalling and the regulation of glucose and lipid metabolism, pp. 799–806, Copyright (2001), with permission from Nature Publishing Group.
Basal and prandial insulin secretion are impaired in T2DM and early post-meal response is absent.Citation6,Citation7 The loss or blunting of this first-phase insulin secretion is important in deterioration of glucose tolerance and elevation of PPG concentrations.Citation7 Reduction of basal insulin secretion in patients with T2DM causes unopposed glucagon secretion by pancreatic α-cells, which results in increased hepatic glucose output.Citation8,Citation9 These effects cause elevations in FPG. Although T2DM is characterized by alterations in insulin secretion, this is only one of many pathologic changes observed in patients. T2DM is also associated with insulin resistance (IR) in peripheral muscle and adipose tissues, which contributes to significantly elevated FPG.Citation7 Thus, altered insulin secretion, increased hepatic glucose output, and IR all contribute to loss of glucose control in patients with T2DM.
Treatment of patients with T2DM may include oral and injectable therapies. This paper focuses on insulin therapy for the pharmacotherapeutic treatment of T2DM.
Many patients with T2DM ultimately require insulin therapy to maintain glycemic control. A survey including information from 61,890 patients with T2DM indicated that at <1, 1–5, 6–10, 11–15, 16–20, and >20 years after diagnosis 11%, 15%, 36%, 57%, 71%, and 76% of patients, respectively, were receiving insulin monotherapy or insulin in combination with oral antidiabetes agents.Citation10,Citation11 The goal of insulin therapy in type 1 diabetes (T1DM) or T2DM is to match as closely as possible physiologic insulin secretion to control FPG and PPG.Citation9 Before the development of insulin analogs, achieving this goal was limited by the characteristics of insulin preparations.
Neutral protamine Hagedorn (NPH) insulin and regular human insulin (RHI) can replace or supplement basal and prandial insulin to control PPG and FPG, but they have important limitations,Citation3,Citation12–Citation14 and use of these agents does not result in a good approximation of physiologic insulin secretion. The pharmacokinetic/pharmacodynamic (PK/PD) profile for NPH has a distinct peak; it does not match peak-less physiologic basal insulin secretion and requires multiple daily injections ().Citation12 RHI is administered to control PPG concentrations, but its PK profile is not ideal for achieving this goal. It reaches peak activity relatively slowly (>60 minutes after dosing),Citation13 and must be administered at least 30 minutes before meals to control PPG.Citation15 Its duration of action is approximately 6–8 hours and, as a result, plasma insulin levels remain elevated for longer periods after meals, increasing risk for hypoglycemia and insulin stacking with more than twice-daily dosing ().Citation3,Citation13,Citation15–Citation17 One benefit of the longer action profile of RHI is that it may be used more effectively in patients with gastroparesis and slowed gastric emptying who have delayed carbohydrate absorption.Citation18 Overall, however, combination therapy using NPH and RHI does not closely approximate physiologic insulin secretion, may provide limited control over FPG and PPG, and may be associated with high risk for hypoglycemia.Citation3
Table 1 Insulin pharmacokinetic profilesTable Footnotea
Modifications of the insulin molecule have resulted in two long-acting insulin analogs (glargine and detemir) and three rapid-acting insulin analogs (aspart, lispro, and glulisine) with improved PK/PD profiles ().Citation4,Citation19–Citation24 These agents can be used together in basal-bolus therapy to more closely mimic physiologic insulin secretion patterns.Citation3,Citation15,Citation25 Although they may be used together, one difference with insulin analogs compared with human insulin, such as NPH and RHI, is that long-acting analogs may not be mixed in the same syringe with any other insulin preparations and this requires separate injections.
Glargine and high-dose detemir have more predictable flat, time-action, profiles that provide up to 24-hour glucose control and lower risk for hypoglycemia than NPH in patients with T2DM.Citation26–Citation29 Equivalent doses of glargine and detemir may provide 24-hour glycemic control in patients with T2DM, although occasionally, higher doses of detemir are required.Citation30,Citation31 Rapid-acting insulin analogs have a faster onset and shorter duration of action (DOA) than RHI and provide better control of PPG and may decrease risk for post-meal hypoglycemia.Citation28,Citation32–Citation34
The most recent consensus statement from the American Association of Clinical Endocrinologists and the American College of Endocrinology strongly recommends against NPH insulin in favor of longer-acting insulin analogs.Citation35 This guideline states that use of NPH as a basal insulin has been superseded by the synthetic analogs glargine and detemir, which both provide a relatively peakless profile for approximately 24 hours. Although NPH is less expensive, the analogs offer the opportunity for more physiologic action profiles. The guideline also notes that insulin analogs yield better reproducibility and consistency between and within patients, and a corresponding reduction in risk of hypoglycemia.Citation35 Variability in the efficacy and dosing of insulin analogs may occur in certain patient populations, thereby complicating diabetes treatment and increasing the risk of adverse effects. The purpose of this paper is to review the PK/PD characteristics of long- and rapid-acting insulin analogs and their use in special patient populations with T2DM.
Methods
Several methods were employed in order to identify the most relevant articles to include. A PubMed literature search was conducted to identify peer-reviewed articles published in English from 2000 to 2011. Search terms included “insulin analogs,” “insulin lispro,” “insulin aspart,” “insulin glulisine,” “insulin glargine,” “insulin detemir,” “regular human insulin,” “neutral protamine Hagedorn,” and “NPH.” Each of these terms was individually paired with the following terms: “pharmacokinetics,” “pharmacodynamics,” “type 2 diabetes,” “Caucasian,” “African American,” “ Japanese,” “Latino,” “Hispanic,” “Asian,” “obese,” “renal,” “kidney,” “nephropathy,” “hepatic,” “liver,” “pregnant,” “gestational diabetes mellitus,” “exercise,” “physical,” and “comorbidity.” The US prescribing information for each insulin was also searched for information related to each topic. Publications that addressed only oral antidiabetes drugs were not included, nor were letters, commentaries, or case studies.
Once the abstracts were reviewed, complete versions of articles that met the criteria noted above were obtained. The contributing authors and the impact factors of the journals in which articles were published were noted, and the study design, methodology, and clinical relevance were assessed. Once publications were identified as relevant, their bibliographies were reviewed and key references were obtained and assessed for inclusion.
The intent of this article is to address the clinical benefits of insulin for specific populations. As a result, a limitation of this analysis is that if an abstract of an article identified during literature searches did not provide information pertaining to such patient groups, the article was not included. Additionally, relevant publications may not have been identified in the PubMed search because they were not indexed in a manner that met the search criteria employed. Another potential limitation to consider is that the identification of relevant publications was performed by the author, and therefore has the potential for subjectivity.
Results
A total of 118 articles were obtained. After excluding publications that did not meet the pre-identified criteria or failed to provide information relevant to this manuscript, the results and clinical implications provided in 38 references were included.
Factors affecting pharmacokinetic/pharmacodynamic profiles for insulin analogs
Several factors may influence the PK/PD of insulin analogs in patients with T2DM. Two of the most important are insulin dose and injection site. The area under the curve (AUC) and DOA for long- and rapid-acting insulin analogs generally increase with dose elevation.Citation24,Citation34,Citation36,Citation37 For example, results from a study assessing PK of detemir of 0.15, 0.3, and 0.6 units/kg indicated dose-proportional effects on reduction in endogenous glucose production and increased glucose uptake across this dose range.Citation37 Results from a second study indicated that the DOA of detemir also increased proportionally with dose and was 5.7, 12.1, 19.9, 22.7, and 23.2 hours for doses of 0.1, 0.2, 0.4, 0.8, and 1.6 units/kg, respectively.Citation24 Glargine doses of 0.5, 1.0, 1.5, and 2.0 units/kg also produced dose-proportional increases in DOA as reflected by rate of glucose disappearance in a euglycemic clamp study.Citation38
The injection site of insulin analogs may also affect their PK/PD profiles. Generally, deeper subcutaneous (SC) injections cause more rapid insulin diffusion and absorption.Citation39 High SC fat may slow absorption, altering or delaying the time-action profile.Citation32 Severely obese patients may require elevated daily insulin doses and multiple split insulin injections at each dosing interval to improve insulin action and predictability (eg, glargine or detemir 140 units, delivered in two injections of 70 units once daily, with both injection sites separated by 2 inches). Moreover, obese patients with significant central obesity may also require longer needles for effective insulin delivery to prevent leakage of large doses in this dense SC area.
Insulin is most often injected into the SC fat in the abdomen, thigh, or deltoid. Studies exploring long-acting insulin analogs indicate differing effects on PK/PD profiles. Results for glargine suggest no clinically significant differences in DOA whether injected into the abdomen, thigh, or deltoid.Citation23 For detemir, absolute bioavailability is 64%, 59%, and 65% following SC injection in the abdomen, thigh, and deltoid, respectively. The detemir AUC and maximum plasma concentration (Cmax) are significantly higher (approximately 10% and 20%, respectively) after SC injection in the abdomen or deltoid, compared with the thigh.Citation40 As with any insulin preparations, heat or muscle activity can increase the absorption rate. Abdominal SC tissue provides a predictable absorption rate not as dependent on heat, exercise, or activity compared with muscle and is a preferred site for patients with T2DM.Citation41
Studies evaluating rapid-acting insulin analogs suggest that injection site has only a moderate effect on their PK/PD profiles. Results for aspart indicate short time to Cmax and time to maximal concentration (Tmax) and more rapid onset of PD effect with thigh injection versus deltoid or abdominal injection.Citation42 Studies with lispro have shown an earlier Tmax and higher Cmax with abdominal versus deltoid or thigh injection.Citation43 The time-concentration profile for glulisine is not significantly influenced by injection site, and bioavailability is 73% after SC injection into the abdomen, 71% after injection into the deltoid, and 68% after injection into the thigh.Citation21
Overall, having patients rotate insulin injections within the same location each week (eg, in the abdomen as the preferred site or thigh or deltoid) will decrease variability and improve predictability of insulin response.
Changes in insulin analog PK/PD profiles in special patient populations
Understanding how PK/PD profiles for specific insulin analogs may be altered in special patient populations may permit proactive dosing adjustments to achieve or maintain glycemic control and decrease the risk of hypoglycemia. Information about the issues addressed in this section is not available for all insulin analogs. For each topic, both published papers and US prescribing information were reviewed.
Elderly patients
Variability in PK related to declines in renal and/or hepatic function may complicate dosing of insulin and other medications in elderly patients.Citation44 Clearance (removal from the bloodstream) of detemir is decreased in the elderly (≥68 years of age) and AUC is increased, predisposing them to hypoglycemia. More frequent self-monitoring of blood glucose (BG) is required, especially before operating a vehicle or heavy machinery, and insulin dose adjustments may be needed.Citation4 Alterations in PK in elderly patients have not been studied for glargine. Among the rapid-acting insulin analogs, alterations in PK/PD have been studied only for aspart. Its PK/PD profile is the same in young and elderly (≥65 years old) patients.Citation45 Overall, if clearance is reduced and/or AUC increased, the risk for hypoglycemia is elevated, and matching carbohydrate meal content to insulin dose is a necessity to prevent low BG.
Specific racial and ethnic groups
The PK/PD profile for detemir is consistent in Caucasian, African American, Japanese, and Hispanic groups.Citation4,Citation46 Results from a study of 16 African American, 16 Hispanics/Latinos, and 16 Caucasian patients evaluated with 16-hour isoglycemic glucose clamps and detemir doses of 0.3, 0.6, and 1.2 units/kg indicated linear PK and no differences among racial or ethnic groups.Citation46 Results for glargine indicate more rapid absorption in Japanese than Caucasian subjects with T2DM.Citation47 PK studies with glargine have not been conducted in other racial or ethnic groups.
Assessment of the effects of race or ethnicity on the PK/PD of rapid-acting insulin analogs has been limited. Results for aspart indicate that its profile in Japanese patients does not differ significantly from that in Caucasians,Citation48 and those for glulisine have shown that Japanese subjects have higher initial exposure (33% higher by AUC over a period of 1 hour) versus Caucasians,Citation21 potentially allowing for smaller glulisine doses in Japanese patients with T2DM. The effects of race or ethnicity on the PK/PD of lispro have not been studied.
Obesity
The prevalence of overweight and obesity among patients with T2DM are both high. The Study to Help Improve Early evaluation and management of risk factors Leading to Diabetes (SHIELD) showed that 28% of individuals with T2DM are overweight (body mass index [BMI]: 25–29.99 kg/m2) and 59% are obese (BMI ≥ 30 kg/m2).Citation49 These associations are consistent for men and women and across racial and ethnic groups.Citation50 Obesity and associated SC fat may slow insulin absorption and reduce exposure, altering the PK profile. Obese patients may also require higher starting insulin doses due to obesity-associated IR,Citation51 but results suggest that these effects vary across currently available insulin analogs. The effects of obesity on the PK/PD of glargine and detemir have not been studied, but have been assessed for the rapid-acting insulin analogs. It has been reported that there is a significant correlation (r = 0.42, 95% CI: 0.02–0.82) between BMI and time to maximum activity for lispro.
A study in subjects using aspart showed that increasing obesity was significantly correlated with decreased apparent clearance per kg body weight (P = 0.002), increased elimination half-life (P = 0.044), and elevated AUC (P = 0.006). However, it was also noted that these changes were not as great as within-subject variability for these PK parameters.Citation52 The prescribing information for aspart also notes that clearance is reduced by 28% in patients with BMI >32 kg/m2 versus those with BMI <23 kg/m2.Citation20 Published results have indicated no significant correlation between BMI and time to maximum activity for glulisine (r = 0.13, 95% CI: –0.33–0.59),Citation13 but the PI for glulisine does state that obesity increases Tmax from 60 minutes to 85 minutes.Citation21
Renal dysfunction
Approximately 20% of patients with diabetes have renal dysfunction,Citation53 and understanding the effects of this common complication on the PK and actions of insulin in these patients is important. Insulin levels are increased in patients with diabetes and overt nephropathy because 30%–80% of circulating insulin is removed by renal excretion. However, the metabolic response to insulin (ie, its effect on PG) is decreased in this population.Citation54 It has also been suggested that insulin dosing be decreased in patients with diabetes who have end-stage renal disease, and who are undergoing hemodialysis because they are at increased hypoglycemia risk.Citation55 Insulin requirements in these patients may change due to metabolic alterations influencing PK and PD. The prescribing information for detemir indicates no change in PK or PD in patients with renal impairment (RI), although frequent self-monitoring of blood glucose is recommended and dose adjustments may be necessary.Citation4
The effects of RI on the PK and actions of glargine have not been studied.Citation23 For the rapid-acting insulin analogs, there is an increased PD effect of lispro (ie, elevated glucose-lowering response) as renal function declines,Citation19 and renal dysfunction does not alter the PK of aspart.Citation20,Citation52 Results for glulisine indicate that subjects with moderate and severe RI have 29% and 40% increases in insulin exposure and 20% and 25% reductions in clearance, respectively, versus those with normal renal function.Citation21 Therefore, glulisine should be empirically reduced in patients with renal insufficiency to prevent hypoglycemia.
Hepatic dysfunction
The liver plays a central role in maintaining normal glucose concentrations in individuals without diabetes and mediates several processes, including hepatic glucose output and uptake. Changes in hepatic function may also affect the glucose-lowering effects of insulin.Citation56 Results for detemir indicate that patients with severe hepatic dysfunction and without diabetes have lower AUC values compared with healthy controls.Citation4 The effects of hepatic dysfunction on the PK/PD of glargine have not been studied.Citation23 Available information indicates no effects of hepatic impairment on the PK of either lispro or aspart,Citation19,Citation20,Citation52 while the effects of liver dysfunction on the PK of glulisine have not been studied.Citation21
Pregnancy
Results from older PK studies indicate that the PK of insulin are not altered during pregnancy.Citation57 However, IR is increased during pregnancy, and this may require a higher insulin dose.Citation57,Citation58 At present, there is no information about the effects of pregnancy on the PK/PD of any of the insulin analogs.
Exercise
Any factor that alters blood flow is also likely to alter insulin absorption, with greater blood flow being associated with more rapid insulin absorption. Conditions that modify local blood flow include exercise, massage, bathing in warm water, and the use of vasodilating or vasoconstricting drugs.Citation59 However, significant effects of these activities have not been invariably observed with all insulin analogs. Results from a study of 13 patients with T1DM who exercised for 30 minutes, then had radiolabeled glargine injected into the thigh, indicated no increase in the decay of radioactivity versus results obtained after injection without exercise.Citation60
Results from a study of eight patients with T1DM who were treated with a standard dose of lispro before a meal of 600 kcal (75 g carbohydrate) and who, 90 minutes later, undertook postprandial exercise at 25% maximum oxygen consumption (VO2 max) for 60 minutes, 50% VO2 max for 30 and 60 minutes, and 75% VO2 max for 30 minutes, indicated increased risk for hypoglycemia at all exercise intensities.Citation61 A reduced lispro dose or a pre-exercise snack should be considered before exercise to prevent low-BG events. It is reasonable to suggest that this recommendation should also be applied for patients taking other rapid-acting insulin analogs.
Drug interactions with insulin analogs
Patients with T2DM are likely to have comorbid conditions (eg, hypertension, dyslipidemia), which require chronic pharmacologic treatment.Citation62 Insulin treatment in these patients requires attention to drug interactions that may alter insulin PK/PD. Information about interactions between insulin and other medications is scarce; specific drug interactions are not listed in the PIs for the insulin analogs. This advice has been provided, however: drugs that potentially increase the BG-lowering effect of insulins and susceptibility to hypoglycemia include oral antidiabetic products, pramlintide, angiotensin-converting enzyme inhibitors, disopyramide, fibrates, fluoxetine, monoamine oxidase inhibitors, propoxyphene, pentoxifylline, salicylates, somatostatin analogs, and sulfonamide antibiotics. Drugs that potentially reduce the BG-lowering effect of insulins include corticosteroids, niacin, danazol, diuretics, sympathomimetic agents (eg, epinephrine, albuterol, terbutaline), glucagon, isoniazid, phenothiazine derivatives, somatropin, thyroid hormones, estrogens, progestogens (eg, in oral contraceptives), protease inhibitors, and atypical antipsychotic medications (eg, olanzapine and clozapine). Increased self-monitoring of BG and closer follow-up are required in patients taking these medications, and insulin doses should be adjusted based on pattern management of 1–2 weeks of patient glucose logbook records.Citation19–Citation21,Citation23
Conclusion
Many patients with T2DM will require insulin therapy to maintain glycemic control. Management of these patients has been facilitated by development of rapid-acting and long-acting insulin analogs, which can be combined to more closely mimic physiologic insulin secretion. PK/PD for insulin analogs may be influenced by age, weight, ethnicity or race, pregnancy, activity level, and hepatic and renal function ().Citation4,Citation13,Citation19–Citation21,Citation23,Citation24,Citation29,Citation32,Citation37,Citation38,Citation40–Citation61 However, these variables do not have equivalent effects on all rapid-acting or long-acting insulin analogs. While some effects on PK/PD of insulin analogs may seem subtle, they may account for the difficulties encountered by providers when establishing glycemic control. Patients with characteristics potentially affecting insulin PK/PD may require dosing alterations and closer monitoring than others with T2DM receiving insulin therapy. Because all these factors may affect PK/PD parameters, individualized monitoring is imperative to reach glucose goals effectively and safely and decrease the risks of long-term hyperglycemia.
Table 2 Variables and potential effects on insulin analog dosing
Acknowledgments
The author would like to thank Robert W Rhoades, PhD of MedVal Scientific Information Services, LLC, for providing medical writing and editorial assistance. The author verifies that she meets all International Committee of Medical Journal Editors (ICMJE) requirements for authorship. This manuscript was prepared according to the International Society for Medical Publication Professionals’ Good Publication Practice for Communicating Company-Sponsored Medical Research: The GPP2 Guidelines. Funding to support the preparation of this manuscript was provided by Novo Nordisk Inc.
Disclosure
The author reports no conflicts of interest in this work. The author did not receive an honorarium for this work.
References
- BogardusCTataranniPAReduced early insulin secretion in the etiology of type 2 diabetes mellitus in Pima IndiansDiabetes200251Suppl 1S262S26411815490
- KingABArmstrongDUBasal bolus dosing: a clinical experienceCurr Diabetes Rev2005121522018220597
- GerichJENovel insulins: expanding options in diabetes managementAm J Med200211330831612361817
- Levemir® (insulin detemir [rDNA origin] injection) [prescribing information]Princeton, NJNovo Nordisk Inc2010
- SaltielARKahnCRInsulin signalling and the regulation of glucose and lipid metabolismNature200141479980611742412
- KahnSEMontgomeryBHowellWImportance of early phase insulin secretion to intravenous glucose tolerance in subjects with type 2 diabetes mellitusJ Clin Endocrinol Metab2001865824582911739446
- GuillausseauPJMeasTVirallyMLaloi-MichelinMMedeauVKevorkianJPAbnormalities in insulin secretion in type 2 diabetes mellitusDiabetes Metab200834Suppl 2S43S4818640585
- BurcelinRKnaufCCaniPDPancreatic alpha-cell dysfunction in diabetesDiabetes Metabolism200834Suppl 2S49S5518640586
- CampbellRKWhiteJRJrInsulin therapy in type 2 diabetesJ Am Pharm Assoc (Wash)20024260261112150359
- EliassonBEeg-OlofssonKCederholmJNilssonPMGudbjornsdottirSAntihyperglycaemic treatment of type 2 diabetes: results from a national diabetes registerDiabetes Metab20073326927617499541
- OwensDRInsulin preparations with prolonged effectDiabetes Technol Ther201113Suppl 1S5S1421668337
- SchmidHNew options in insulin therapyJ Pediatria (Rio J)200783Suppl 5S146S155
- BeckerRHAFrickADBurgerFPotgieterJHScholtzHInsulin glulisine, a new rapid-acting insulin analogue, displays a rapid time-action profile in obese non-diabetic subjectsExp Clin Endocrinol Diabetes200511343544316151977
- OsterbergOErichsenLIngwersenSHPlumAPoulsenHEViciniPPharmacokinetic and pharmacodynamic properties of insulin aspart and human insulinJ Pharmacokinet Pharmacodyn20033022123514571692
- LevyPInsulin analogs or premixed insulin analogs in combination with oral agents for treatment of type 2 diabetesMed Gen Med2007912
- Novolin® R [prescribing information]Princeton, NJNovo Nordisk Inc5142010
- Novolin® N [prescribing information]Princeton, NJNovo Nordisk Inc5142010
- WalshJARobertsRBaileyTVarmaCBUsing Insulin: Everything You Need for Success With InsulinSan Diego, CATorrey Pines Press2003
- Humalog® (insulin lispro [rDNA origin] injection) [prescribing information]Indianapolis, INEli Lilly and Company5182011
- NovoLog® (insulin aspart [rDNA origin] injection) [prescribing information]Princeton, NJNovo Nordisk Inc72011
- Apidra® (insulin glulisine) [prescribing information]Bridgewater, NJSanofi-Aventis2009
- HelmsKLKelleyKWInsulin glulisine: an evaluation of its pharmacodynamic properties and clinical applicationAnn Pharmacother20094365866819336657
- Lantus® (insulin glargine [rDNA origin] injection) [prescribing information]Bridgewater, NJSanofi-Aventis322007
- PlankJBodenlenzMSinnerFA double-blind, randomized, dose-response study investigating the pharmacodynamic and pharmacokinetic properties of the long-acting insulin analog detemirDiabetes Care2005281107111215855574
- RaccahDBretzelRGOwensDRiddleMWhen basal insulin therapy in type 2 diabetes mellitus is not enough – what next?Diabetes Metab Res Rev20072325726417315242
- GarberAJClausonPPedersenCBKolendorfKLower risk of hypoglycemia with insulin detemir than with neutral protamine hagedorn insulin in older persons with type 2 diabetes: a pooled analysis of phase III trialsJ Am Geriatr Soc2007551735174017979896
- RiddleMCRosenstockJGerichJThe Treat-to-Target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patientsDiabetes Care2003263080308614578243
- HermansenKFontainePKukoljaKKPeterkovaVLethGGallMAInsulin analogues (insulin detemir and insulin aspart) versus traditional human insulins (NPH insulin and regular human insulin) in basal-bolus therapy for patients with type 1 diabetesDiabetologia20044762262915298338
- HompeschMOcheltreeSMWondmagegnehuETPharmacokinetics and pharmacodynamics of insulin lispro protamine suspension compared with insulin glargine and insulin detemir in type 2 diabetesCurr Med Res Opin2009252679268719761358
- KingABOnce-daily insulin detemir is comparable to once-daily insulin glargine in providing glycaemic control over 24 h in patients with type 2 diabetes: a double-blind, randomized, crossover studyDiabetes Obes Metab200911697119120433
- PschererSDietrichESDippelFWNeilsonARComparison of one- year costs of type 2 diabetes treatment with insulin glargine or insulin detemir in a basal supported oral therapy (BOT) in GermanyInt J Clin Pharmacol Ther20104812913720137765
- BarnettAHHow well do rapid-acting insulins work in obese individuals?Diabetes Obes Metab2006838839516776745
- RoachPNew insulin analogues and routes of delivery: pharmacodynamic and clinical considerationsClin Pharmacokinet20084759561018698880
- BeckerRHFrickADClinical pharmacokinetics and pharmacodynamics of insulin glulisineClin Pharmacokinet20084772018076215
- RodbardHWJellingerPSDavidsonJAStatement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic controlEndocr Pract20091554055919858063
- LindholmAJacobsenLVClinical pharmacokinetics and pharmacodynamics of insulin aspartClin Pharmacokinet20014064165911605714
- WutteAPlankJBodenlenzMProportional dose-response relationship and lower within-patient variability of insulin detemir and NPH insulin in subjects with type 1 diabetes mellitusExp Clin Endocrinol Diabetes200711546146717647145
- WangZHedringtonMSGogitidzeJNDose response effects of insulin glargine in type 2 diabetesDiabetes Care2010331555156020357371
- GinHHanaire-BroutinHReproducibility and variability in the action of injected insulinDiabetes Metab20053171315803107
- EMEAScientific discussion of insulin detemir2004 Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000528/WC500036658.pdfAccessed November 16, 2011
- Injection site selection Available at: http://www.bd.com/us/diabetes/page.aspx?cat=7001&id=7261Accessed November 10, 2011
- MudaliarSRLindbergFAJoyceMInsulin aspart (B28 aspinsulin): a fast-acting analog of human insulin: absorption kinetics and action profile compared with regular human insulin in healthy nondiabetic subjectsDiabetes Care1999221501150610480516
- ter BraakEWWoodworthJRBianchiRInjection site effects on the pharmacokinetics and glucodynamics of insulin lispro and regular insulinDiabetes Care199619143714408941480
- CorsonelloAPedoneCIncalziRAAge-related pharmacokinetic and pharmacodynamic changes and related risk of adverse drug reactionsCurr Med Chem20101757158420015034
- KronesRSchutteCHeiseTThe rapid-acting properties of insulin aspart are preserved in elderly people with type 2 diabetesDiabetes Obes Metab200911414419120432
- HompeschMTroupinBHeiseTTime-action profile of insulin detemir and NPH insulin in patients with type 2 diabetes from different ethnic groupsDiabetes Obes Metab2006856857316918592
- RaveKNosekLHeinemannLFrickABeckerRTime-action profile of the long-acting insulin analogue insulin glargine in comparison to NPH insulin in Japanese volunteersDiabetes Metab20032943043114526272
- KakuKMatsudaMUraeAIrieSPharmacokinetics and pharmacodynamics of insulin aspart, a rapid-acting analog of human insulin, in healthy Japanese volunteersDiabetes Res Clin Pract20004911912610963823
- BaysHEChapmanRHGrandySThe relationship of body mass index to diabetes mellitus, hypertension and dyslipidaemia: comparison of data from two national surveysInt J Clin Pract20076173774717493087
- CrawfordAGCoteCCoutoJPrevalence of obesity, type II diabetes mellitus, hyperlipidemia, and hypertension in the United States: findings from the GE centricity electronic medical record databasePopul Health Manag20101315116120521902
- Fernandez-VeledoSNieto-VazquezIVila-BedmarRGarcia- GuerraLAlonso-ChamorroMLorenzoMMolecular mechanisms involved in obesity-associated insulin resistance: therapeutical approachArch Physiol Biochem200911522723919673658
- HolmesGGalitzLHuPLynessWPharmacokinetics of insulin aspart in obesity, renal impairment, or hepatic impairmentBr J Clin Pharmacol20056046947616236036
- CraigKJDonovanKMunneryMOwensDRWilliamsJDPhillipsAOIdentification and management of diabetic nephropathy in the diabetes clinicDiabetes Care2003261806181112766114
- RaveKHeiseTPfutznerAHeinemannLSawickiPTImpact of diabetic nephropathy on pharmacodynamic and pharmacokinetic properties of insulin in type 1 diabetic patientsDiabetes Care20012488689011347749
- ShrishrimalKHartPMichotaFManaging diabetes in hemodialysis patients: observations and recommendationsCleve Clin J Med20097664965519884294
- HomePDPaciniGHepatic dysfunction and insulin insensitivity in type 2 diabetes mellitus: a critical target for insulin-sensitizing agentsDiabetes Obes Metab20081069971817825080
- GrayRSCowanPSteelJMJohnstoneFDClarkeBFDuncanLJInsulin action and pharmacokinetics in insulin treated diabetics during the third trimester of pregnancyDiabetes Metab19841273278
- KliegerCPollexEKazminAKorenGHypoglycemics: pharmacokinetic considerations during pregnancyTher Drug Monit20093153354119730277
- GuerciBSauvanetJPSubcutaneous insulin: pharmacokinetic variability and glycemic variabilityDiabetes Metab2005314S74S2416389894
- PeterRLuzioSDDunseathGEffects of exercise on the absorption of insulin glargine in patients with type 1 diabetesDiabetes Care20052856056515735188
- Rabasa-LhoretRBourqueJDucrosFChiassonJLGuidelines for premeal insulin dose reduction for postprandial exercise of different intensities and durations in type 1 diabetic subjects treated intensively with a basal-bolus insulin regimen (ultralente-lispro)Diabetes Care20012462563011315820
- EDIC Research GroupEpidemiology of Diabetes Interventions and Complications (EDIC): design, implementation, and preliminary results of a long-term follow-up of the diabetes control and complications trial cohortDiabetes Care1999229911110333910