Abstract
This review describes the epidemiology and various treatments in chronic rhinosinusitis (CRS) with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP). Evidence for short-term use of systemic corticosteroids has been shown to be favorable in CRSwNP, but still limited in CRSsNP. Topical corticosteroids improve symptom scores in both CRS subgroups. The role of microbes in CRS is still controversial. Culture-directed antibiotics are recommended for CRSsNP with exacerbation. Long-term use of low dosage antibiotics is recommended for CRSsNP for their anti-inflammatory effects. Other emerging treatment options are also discussed.
Clinical characteristics of chronic rhinosinusitis
Rhinosinusitis is an inflammatory disease of the nasal and paranasal sinus mucosa. It is defined as chronic when it lasts longer than 3 months without complete symptom resolution. Diagnostic criteria consist of the presence of symptoms including purulent nasal discharge, nasal obstruction, facial pain/pressure/fullness, and/or decreased sense of smell plus either endoscopic findings of inflammation, purulent discharge or edema of the middle meatus or ethmoid region, polyps in the nasal cavity or the middle meatus, and/or radiographic imaging showing inflammation of the paranasal sinuses.Citation1,Citation2 Chronic rhinosinusitis (CRS) is further divided into CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP). As for the use in epidemiologic studies, CRS is defined as the presence of two or more symptoms, one of which should be either nasal blockage/obstruction/congestion or nasal discharge (anterior/posterior nasal drip) and/or facial pain/pressure and/or reduction or loss of smell for more than 12 weeks with validation by telephone or interview.Citation1,Citation3
The pathogenesis of CRS remains controversial. Multifactorial factors altering the host-environment interaction such as bacteria, fungi, viruses, allergens, or environmental toxins may trigger the inflammatory process.
Epidemiology of chronic rhinosinusitis and associated complications
CRS is a common health problem which significantly affects quality of life. CRS has a significant impact on patients in seven of eight domains of the 36-item short form health survey (SF-36).Citation4 Patients have significantly higher bodily pain and decreased social function compared to other chronic diseases (congestive heart failure, angina, chronic obstructive pulmonary disease, and back pain) (P < 0.05).Citation5 According to a US national health interview survey of the prevalence of chronic conditions, CRS has been estimated to affect 12.5% to 15.5% of the total population, making it the second most common chronic condition in the United States.Citation6,Citation7 However, the prevalence of doctor-diagnosed CRS is much lower; a prevalence of 2% was found using International Statistical Classification of Diseases and Related Health Problems (ICD)-10 codes as an identifier.Citation8 The prevalence rate is substantially higher in females with a female:male ratio of 6:4Citation7 and increases with age, with a mean of 2.7% and 6.6% in the age groups of 20 to 29 years and 50 to 59 years, respectively, and leveling off at 4.7% after 60 years.Citation9
An epidemiology study in Europe was conducted by The Global Allergy and Asthma Network of Excellence (GA2LEN) by sending questionnaires on The European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS) criteria to a random sample of adults aged 15–75 years.Citation10 They found the overall prevalence of CRS was 10.9%, which confirmed the burden as a common chronic disease and pointed out the underestimation of this disease.
Pathogenesis
The etiology and pathogenesis of chronic rhinosinusitis are not clearly understood. Traditionally, it was believed that the chronic inflammatory process is the end stage of untreated or partially treated acute rhinosinusitis or severe atopy from nasal polyps. This hypothesis leads to the use of antibiotics and anti-inflammatory drugs, eg, corticosteroids for treating CRS patients. Alternative hypotheses include excessive host response to fungi,Citation11,Citation12 aspirin intolerance due to defects in the eicosanoid pathway,Citation13,Citation14 staphylococcal superantigen resulting in exotoxin effects including tissue damage,Citation15,Citation16 coordinated mechanical barrier and the innate immune response of the sinonasal mucosa,Citation17 defects in the immune barrier and biofilms formation.Citation18
There is a growing body of evidence supporting an emerging hypothesis that a dysfunctional host–environment interaction involving various exogenous agents results in the sinonasal inflammation. In concert with the definition of CRS as an inflammatory disorder, there has been movement away from pathogen-driven hypotheses. This overall concept is in agreement with the current understanding of the etiology and pathogenesis of chronic mucosal inflammatory disorders in general, which describes a balance of interactions between the host, commensal flora, potential pathogens, and exogenous stresses.
Diagnosis
CRS, with or without nasal polyps in adults is defined as:
inflammation of the nose and the paranasal sinuses characterized by two or more symptoms, one of which should be either nasal blockage/obstruction/congestion or nasal discharge (anterior/posterior nasal drip) ± facial pain/pressure ± reduction or loss of smell for ≥ 12 weeks.
This should be supported by demonstrable disease with endoscopic signs of:
nasal polyps, and/or mucopurulent discharge primarily from middle meatus and/or edema/mucosal obstruction primarily in middle meatus.
and/or
computed tomography (CT) changes: mucosal changes within the ostiomeatal complex and/or sinuses.
Current and emerging treatment options
The aims of treatment in CRS include elimination of the infection, reduced sinonasal inflammation, and maintained patent sinonasal passage drainage. In addition, CRS may be associated with precipitating factors including allergies, cystic fibrosis, gastroesophageal reflux, sinonasal anatomic obstruction in the ostiomeatal unit, and immunologic disorders. Therefore, the management of these risk factors should also be optimized.
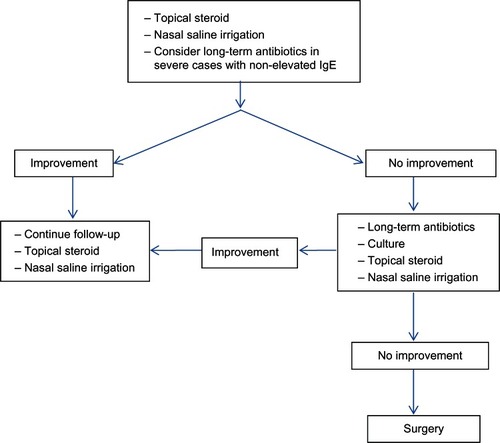
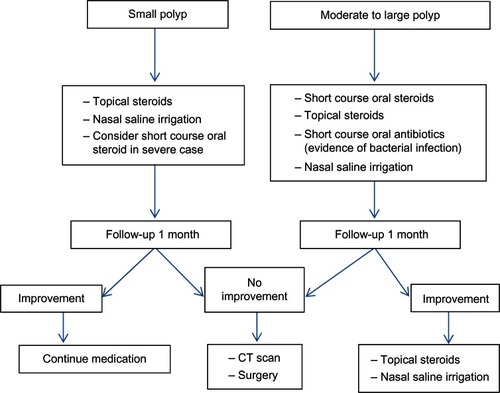
Treatment of CRS includes medical and surgical therapy. Medical therapy often requires combining multiple medications including antibiotics, nasal decongestants, topical nasal steroids and/or oral steroids, and saline irrigation. The rationale of this regimen is to control precipitating factors, treat the infection, reduce mucosal edema, and facilitate drainage. However, some patients do not respond with full medical treatment alone; in these cases treatment with endoscopic sinus surgery should be considered as an alternative. Management schemes for CRSsNP and CRSwNP are displayed in and , respectively.
Corticosteroid
The aim of corticosteroid therapy in CRS is to reduce inflammation via directly reducing eosinophil viability and activation.Citation19,Citation20 In addition, an indirect effect can be to reduce the secretion of chemotactic cytokines from the nasal mucosa and polyp’s epithelial cells.Citation21–Citation24
Systemic corticosteroid
Oral steroids have been introduced as a systemic form to control inflammation. They are administrated as part of a multidrug regimen. To date, no evidence advocates for their use alone.Citation25
CRSsNP
There is limited evidence to support the use of oral steroids in CRSsNP. Tosca et alCitation26 investigated the efficacy of oral steroids as part of a multidrug regimen in children with CRS and asthma. They demonstrated better outcomes and cytokine profiles after treatment including improved nasal endoscopic condition in allergic children (87.5%) and nonallergic children (85.7%), statistically significant reduction of inflammatory infiltration in all children (P < 0.05), significant decrease of interleukin (IL)-4 in allergic children (P = 0.0002) and nonallergic children (P = 0.0007), significant increase of interferon-gamma in allergic children (P = 0.03), and nonsignificant increase in nonallergic children. Additionally, two retrospective studies investigated the benefit of using oral steroids in a multidrug regimen. Subramanian et alCitation27 reported that 90% of patients had improved symptoms and/or CT at 6–8 weeks after treatment. Another study by Lal et alCitation28 reported the complete resolution in patients with CRSwNP or CRSsNP at 2 months after treatment (51.03%). According to this result, a subgroup of CRSsNP was analyzed with a success rate of 54.88%.
Although these results showed beneficial effects of oral steroids, randomized placebo-controlled trials are required to support the use of oral steroids in CRSsNP.
CRSwNP
According to a recent Cochrane review,Citation29 when data of 166 patients were pooled from three randomized controlled trials, the effects favored systemic corticosteroids. PrednisoloneCitation30–Citation33 and methylprednisoloneCitation34 are most commonly used. Due to the side-effects of corticosteroids, we do not recommend systemic form usage for long-term treatment.
Safety and tolerability
Adverse systemic effects of treatment with systemic steroids include Cushing’s syndrome, steroid induced diabetes, gastric ulcers, gastrointestinal bleeding, and avascular necrosis of the femoral head.Citation35 These side-effects are increased with dose and duration of treatment.
Topical corticosteroids
Topical corticosteroids are used as part of a multidrug regimen. There are numerous preparations that can be classified by systemic bioavailability as first generation intranasal corticosteroids including beclomethasone dipropionate, triamcinolone acetonide, flunisolide, and budesonide; and the newer generation includes fluticasone propionate, mometasone furoate, ciclesonide, and fluticasone furoate.Citation36
The delivery method of topical steroids is an imperative factor. Classification of delivery methods can be divided by site (nose or paranasal sinus), volume, and pressure. The delivery methods to the nasal site include drops, sprays, and nebulizers. Paranasal sinus delivery requires devices cannulated through the nose. Volume of delivery method can be divided into low volume, which is defined as a simple spray volume less than 1 mL, or large volume, which is defined as any significant volume more than 60 mL (eg, simple irrigation syringe, irrigation devices). Delivery method may also be classified as low pressure (eg, spray, nebulizers, instilled solution through a tube, and nonpressure irrigation) and high pressure methods (eg, positive pressure irrigation).
CRSsNP
Numerous clinical controlled trials have investigated the efficacy of inhaled intranasal corticosteroids. Several studies compared the first generation of inhaled intranasal corticosteroids with placebo. Lund et alCitation37 reported that the use of budesonide 128 μg twice a day significantly improved symptom scores (P < 0.05). Similarly, Qvarnberg et alCitation38 reported the beneficial effects of budesonide 400 μg daily. It reduced nasal symptoms to a greater extent than placebo together with a significantly greater reduction in facial pain. In addition, Lavigne et alCitation39 found a decrease in CD-3 (P = 0.02) and eosinophils (P = 0.002), and a decrease in the density of cells expressing IL-4 (P = 0.0001) and IL-5 messenger RNA (P = 0.006) after treatment.
Hansen et alCitation40 studied the efficacy of fluticasone 400 μg twice a day via an OptiNose device (Optinose US Inc; Yardley, PA, USA). When compared with placebo, it improved mucosal edema (P = 0.015), increased peak nasal inspiratory airflow at 4 and 8 weeks (P = 0.006 and P = 0.03, respectively), improved magnetic resonance imaging (MRI) scores after 12 weeks (P = 0.039), and improved nasal rhinosinusitis outcome measure-31 (RSOM-31) subscale scores at 4 and 8 weeks (P < 0.009 and P < 0.016, respectively). In addition, it significantly improved symptoms including sense of smell and nasal discomfort (P < 0.05). Conversely, Dijkstra et alCitation41 compared the efficacy of two regimes of fluticasone nasal spray (400 μg versus 800 μg twice a day) and placebo. The results showed no significant difference in total symptom score on the 0–100 scale. Similarly, Jorissen et alCitation42 reported no significant difference in endoscopic score when mometasone nasal spray was compared with placebo (P = 0.905).
Regarding the method of delivery, a meta-analysis showed significantly greater effects in sinus delivery methods (direct cannulation or irrigation post-surgery) than nasal delivery methods (drops, sprays, or nebulizer) (P = 0.04).Citation36
Although, the significant benefit of using intranasal corticosteroids is not shown by several studies,Citation41,Citation42 the evidence from a meta-analysisCitation36 showed benefits in symptom improvement.
CRSwNP
Thirty-eight randomized controlled trials were included for a meta-analysis.Citation3 The steroid agents used were fluticasone propionate,Citation43–Citation56 beclomethasone dipropionate,Citation46,Citation51,Citation57–Citation59 betamethasone sodium phosphate,Citation60 mometasone furoate,Citation64 flunisolide,Citation65,Citation66 and budesonide.Citation67–Citation75 When compared to placebo, the steroid group could decrease symptom scores by 0.46 (95% confidence interval [CI] 0.27–0.65) and decrease polyp size score by 0.48 (95% CI 0.21–0.75).
Nasal aerosols and turbuhalers were more effective than nasal sprays in symptom control but there was no difference in polyp size reduction.
Safety and tolerability
Adverse effects reported were mostly mild or moderate, consisting of local effects at the site of application. Giger et alCitation76 presented side effects including epistaxis, dry nose, nasal burning, nasal itching, sinusitis, pharyngitis, otitis, change of taste, eczema, nausea/diarrheas, nasal irritation, and common cold. Using intranasal corticosteroid is generally safe. It does not provide increased incidence of infectionCitation76 or candidiasis,Citation37 or produce a change in morning serum cortisol level.Citation37
Antibiotics
The role of microbes in CRS as causative agents or for colonization is unclear. Pandak et alCitation77 attempted to prove this controversial issue. The presence of an insignificant number of leukocytes in each sinus and nasopharyngeal swab shown by this study indicated bacterial colonization of sinonasal mucosa, not infection. Although there is substantial evidence of bacterial colonization in CRS, antibiotics still play a major role for occurrences of acute exacerbation of CRS.Citation3,Citation78
Bacterial organisms of CRS differ from acute rhinosinusitis. The main organisms include Staphylococcus aureus, Enterobacteriaceae spp., and Pseudomonas spp., and less commonly Streptococcus pneumoniae, Haemophilus influenza, and beta hemolytic streptococci. In addition, anaerobes (eg, Peptostreptococcus, Prevotella, Porphyromonas, Bacteroides, Fusobacterium species) are possible organisms in CRS.
Systemic antibiotics
Systemic antibiotic treatment of CRSsNP can be administrated as either short- or long-term treatment. Short-term treatment is defined as the duration of usage less than 4 weeks in order to eradicate the organisms; conversely, long-term treatment is used for anti-inflammatory effects rather than antibacterial effects. Although, infection is not well established to be causative, the expert committee recommended using antibiotics as short-term treatment in CRSsNP with exacerbation with a positive culture.Citation3
CRSsNP
The appropriate antibiotic for short-term treatment is usually broad spectrum to control both aerobic and anaerobic organisms. In addition, beta-lactamase-producing organisms and methicillin-resistant S. aureus (MRSA) are possible pathogens, thus empiric antibiotics are frequently prescribed for coverage of these organisms.
Extended spectrum antibiotics (eg, amoxicillin/clavulanic acid, fluoroquinolone) are commonly used. Legent et alCitation79 compared amoxicillin/clavulanic acid with ciprofloxacin. The results showed no significant differences in clinical cure (51.2% versus 58.6%) and bacteriological eradication rate (90.5% versus 88.9%) for amoxicillin/clavulanic acid and ciprofloxacin. This result was similar to the result of Namyslowski et alCitation80 which showed no significant difference between amoxicillin/clavulanic acid and cefuroxime axetil in clinical response (95% versus 88%) and bacterial eradication (65% versus 68%). Ciprofloxacin and cefuroxime axetil may be a useful alternative choice of therapeutic treatment.
Regarding long-term antibiotic treatment, the anti-inflammatory effects of macrolides have been investigated. Numerous studies have demonstrated the efficacy of macrolides in reducing inflammatory markers and an increasing ciliary beat frequency, indicating less sticky secretions.Citation81–Citation85 Furthermore, Wallwork et alCitation86 showed a significant anti-inflammatory effect of roxithromycin on the sinonasal outcome test (SNOT)-20 score, nasal endoscopy, saccharin transit time, and IL-8 levels (P < 0.05) in a randomized placebo-controlled trial for CRSsNP. Conversely, the result of Videler et alCitation87 showed no significant anti-inflammatory effects on SNOT-22, patient response rating scale, visual analog score, and SF-36. These different outcomes between the two studies may be explained by using different inclusion criteria. Wallwork et alCitation86 included only patients with CRSsNP, whereas Videler et alCitation87 included both CRSwNP and CRSsNP. Subgroup analysis in the study of Wallwork et alCitation86 demonstrated that the subpopulation of patients with normal immunoglobulin E (IgE) levels had a higher response rate to the macrolide treatment than patients with elevated IgE. Therefore, serum IgE is a helpful indicator to identify responders to long-term macrolide treatment.
The recent Cochrane reviewCitation78 found that there was limited good quality evidence to compare using antibiotics versus placebo in CRS; thus, future well-designed studies should be conducted.
CRSwNP
There were two randomized placebo controlled trials for short-term antibiotics.Citation34,Citation88 Doxycycline 100 mg for 20 days could significantly reduce polyp size and post-nasal drip score,Citation34 while other antibiotics (quinolone, amoxicillin/clavulanate, or co-trimoxazole) had no significant effect but had a trend towards benefit.Citation88
There was some evidence of long-term antibiotics use for CRSwNP using macrolides which showed a decrease in polyp size and patient symptoms, but all were nonrandomized trials.Citation81,Citation83,Citation89
Safety and tolerability
Common adverse effects of antibiotics include gastrointestinal symptoms, skin rash, and reversible elevation of liver enzymes. Adverse events from antibiotic use in CRS were observed in an amoxicillin/clavulanic acid group (4.4%) and cefuroxime group (4.3%).Citation80 These events were minor complications; diarrhea was the most common event. However, one serious urticaria occurred in the cefuroxime group.Citation80
Resistant bacterial strains from long-term antibiotic treatment are of concern due to using the low dose form which does not reach the minimal inhibitory concentration. A controlled trial found that three of 50 cultures had positive macrolide resistant strains before treatment, and four of 43 cultures had resistant strains after treatment.Citation87 Although, there seems to be no significant difference of resistant strains between before and after treatment, increased macrolide-resistant bacterial strains have been reported.Citation90,Citation91 Therefore, development of resistant bacterial strains should be monitored by nasal swab culture every 3 months.Citation3
Topical antibiotics CRSsNP
Topical antibiotics have been administrated to treat CRS with the aim of providing higher concentrations of drug and acting directly on the site of infection; however, placebo controlled trials showed only minimal benefit.Citation94,Citation95 Desrosiers et alCitation92 reported significant improvements in quality of life, symptoms, and sinonasal endoscopic appearance in both the saline and tobramycin group (P < 0.05). Similarly, Videler et alCitation93 compared bacitracin/colimycin topical spray with placebo and reported improvements in both groups without significant differences in SF-36 or sinonasal endoscopic appearance. These studies showed no significant additive effects of topical antibiotics; therefore, topical antibiotics should not be used as first-line management but may be prescribed in patients refractory to traditional topical steroids and oral antibiotics.Citation94
CRSwNP
There was no evidence regarding the use of topical antibiotics in CRSwNP.
Safety and tolerability
The most common adverse effects included intranasal stinging or burning sensation, moderate pain, throat irritation, cough, and dry skin.Citation3 However, Desrosiers et alCitation92 reported no statistically significant difference of adverse events between topical tobramycin and placebo. No tobramycin resistant bacterial strains were reported from this study.
Other emerging options
Many adjunctive agents have been utilized to control CRS including antimycotics,Citation95–Citation98 anti-IgE,Citation99 anti-IL5,Citation100,Citation101 antihistamine,Citation102,Citation103 aspirin desensitization,Citation104 bacterial lysates,Citation105–Citation108 capsaicin,Citation109 complementary and alternative medicine,Citation3,Citation110–Citation114 decongestants,Citation115 furosemide,Citation116 immunosuppressants,Citation117,Citation118 leukotriene antagonists,Citation119,Citation120 nasal irrigation,Citation121–Citation127 mucolytic agents,Citation128 phototherapy,Citation129 probiotics,Citation130 and proton pump inhibitors (PPIs).Citation131 There was limited evidence on the effect of these options. We will focus this topic only on medications with positive effects.
Anti-IgE
Several investigators found that CRSwNP patients have higher IgE in polyps and serum than controls.Citation132–Citation134 One randomized controlled trial used omalizumab for 6 months compared with placebo in CRS patients.Citation99 They found improvement of sinus opacification in CT-scans and the SNOT-20, but there was not a significant difference.
Anti-IL-5
IL-5 is the key mediator in eosinophil activation. Sejima et al found that patients with CRSwNP had higher levels of IL-5 compared with patients with CRSsNP.Citation138 There were some small Phase II randomized controlled trials that found a positive effect of reslizumab and mepolizumab in decreasing polyp size.Citation100,Citation101 These drugs may have a possible role in treatment of CRSwNP in the future.
Bacterial lysates
The mechanisms of bacterial lysates are hypothesized to enhance the process of postnatal maturation of T helper (Th)1 function and dendritic cells.Citation105,Citation106 The efficacy of bacterial lysates (Broncho-Vaxom, OM Pharma, Geneva, Switzerland) was investigated compared with placebo.Citation107 They found a significant improvement in symptoms including headache, purulent discharge, cough, and expectoration in the bacterial lysates group.Citation107
Capsaicin
The calcitonin gene-related peptide (CGRP) is a vasodilator agent present in sensory nerves and may play a major role in the vascular component of neurogenic inflammation. Repeated intranasal applications of capsaicin induced a reduction in both concentration of CGRP-like immunoreactivity and rhinitis symptoms.Citation139 One randomized controlled trial found that patients treated with capsaicin showed a significant smaller staging of their nasal polyposis compared with the control group.Citation109
Complementary and alternative medicine
The complementary and alternative medicines used to treat CRS include herbal medicine, vitamins, homeopathy, acupuncture, massage, reflexology, yoga, and chiropractics.Citation110 Richstein and MannCitation111 compared the herbal preparation (European elder, common sorrel, cowslip, European vervain and gentian) with placebo, and found improvement of the overall clinical status and possible improvement on the radiological findings in the herbal preparation group (12/16 patients) and placebo group (6/15 patients). Another study reported a significant effect on nasal mucosa inflammation reduction and overall rating in the herbal preparation group, but no significant difference in other symptoms including nasal mucosa edema, nasal discharge, and breathing difficulties.Citation112
Furosemide
Furosemide could induce cell shrinkage by mediating the net influx of osmotically active ionsCitation140 and hypothetically have immunomodulatory and anti-inflammatory effects in hyperactive airway disease.Citation141,Citation142 One randomized controlled trial compared topical furosemide versus oral methylprednisolone for 7 days preoperatively.Citation116 Furosemide could significantly reduce the subjective and endoscopic score when compared to baseline but was not significant when compared to oral methylprednisolone.Citation116
Nasal irrigation
Nasal irrigation has been introduced as an adjunctive treatment. It facilitates mechanical removal of mucus, infective pathogens, and inflammatory mediators and promotes ciliary beat frequency. Freeman et alCitation121 studied the efficacy of saline irrigation post-endoscopic sinus surgery. At 3 weeks postoperatively, the outcomes showed a significant improvement of discharge in the saline douching group compared with no treatment (P = 0.046). However, at 3 months postoperatively, there was only a minimal difference with crusting (P = 0.18) and edema (P = 0.32), and no difference with adhesions, discharge, and polyps.Citation121 Khianey et al also found a small clinical benefit of the nasal saline irrigation with minimal side effects.Citation127
Mucolytic agents
Some studies used mucolytic agents as an adjunctive drug for treating patients with tenacious mucus. Majima et alCitation128 assessed the efficacy of S-carboxymethylcysteine in CRS patients without nasal polyps or with small nasal polyps. After 12 weeks of treatment, the nasal discharge and post-nasal discharge were significantly improved in the S-carboxymethylcysteine group (P = 0.008 and P = 0.002, respectively). However, the SNOT-20 and CT scores were not significantly different between groups.Citation128
PPIs
Esophageal reflux was considered a potential cause of CRS. Using PPIs to decrease acid reflux may reduce sinonasal mucosal damage. An uncontrolled trial evaluating PPIs in CRS patients reported improvement in sinus symptoms (nasal congestion, nasal drainage, sinus pressure, facial headache, malaise) and global satisfaction (25%–89% and 91%, respectively).Citation131
Phototherapy
Near-infrared laser illumination (NILI), with or without photo-activated (PA) agents, has bactericidal and wound healing promoting effects which may have a potential role in managing CRS. Krespi et alCitation129 conducted a prospective randomized study with 23 symptomatic post-surgical CRS patients comparing NILI versus NILI with PA. Both therapy arms demonstrated clinical efficacy. The SNOT-20 score change was 0.9 for the NILI group and 0.8 for the NILI with PA group (P < 0.05).Citation129
Surgery
CRSsNP
The aim of surgery includes clearing diseased mucosa, eliminating infection, relieving drainage obstruction, and restoring ventilation. Two randomized controlled trials compared the efficacy between surgery and medication in CRS. Hartog et alCitation140 showed no significant difference in overall cure rates between the medication group (sinus irrigation plus loracarbef) and surgical group (sinus irrigation plus loracarbef plus endoscopic sinus surgery). This result was similar to the result of Ragab et al,Citation141 which showed no difference in total symptom scores in the medication group (erythromycin plus nasal steroid plus nasal douche) and surgical group (endoscopic sinus surgery plus nasal steroid plus nasal douche). The Cochrane review suggested that functional endoscopic sinus surgery has not been demonstrated to confer additional benefits to those obtained by medical treatment.Citation142 We recommend surgical intervention only when there is no response to maximal medical treatment.
CRSwNP
Surgical intervention involves clearance of polyps and abnormal mucosa and opening of the sinus openings. There was limited evidence based on nonrandomized controlled trials which found that endoscopic sinus surgery was safe and effective.Citation143
Conclusion
Several therapies have been proven by studies with a high level of evidence to improve clinical symptoms and objective outcomes. Some therapies still need validation through well-conducted studies, in which randomized controlled trials may be a difficult task due to confounding factors and trial participation. Even though it remains a challenge to cure the root cause of CRS, an algorithm of multidrug regimen and endoscopic sinus surgery after fully implemented medication can help to decrease the disease burden and improve the quality of life of this group of patients.
Acknowledgment
We would like to thank Associate Professor Kornkiat Snidvongs for his advice and English language editing.
Disclosure
The authors report no conflicts of interest in this work.
References
- FokkensWLundVMullolJEuropean Position Paper on Rhinosinusitis and Nasal Polyps groupEuropean position paper on rhinosinusitis and nasal polyps 2007Rhinol Suppl200720113617844873
- RosenfeldRMAndesDBhattacharyyaNClinical practice guideline: adult sinusitisOtolaryngol Head Neck Surg2007137Suppl 3S1S3117761281
- FokkensWJLundVJMullolJEPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologistsRhinology201250111222469599
- WangPCTaiCJLinMSChuCCLiangSCQuality of life in Taiwanese adults with chronic rhino-sinusitisQual Life Res200312444344812797716
- GliklichREMetsonRThe health impact of chronic sinusitis in patients seeking otolaryngologic careOtolaryngol Head Neck Surg199511311041097603703
- AdamsPFHendershotGEMaranoMACenters for Disease Control and Prevention/National Center for Health StatisticsCurrent estimates from the National Health Interview Survey, 1996Vital Health Stat 101999200120315782448
- CollinsJGPrevalence of selected chronic conditions: United States, 1990–1992Vital Health Stat101997194189
- ShashyRGMooreEJWeaverAPrevalence of the chronic sinusitis diagnosis in Olmsted County, MinnesotaArch Otolaryngol Head Neck Surg2004130332032315023840
- BonfilsPHalimiPLe BihanCNorèsJMAvanPLandaisPCorrelation between nasosinusal symptoms and topographic diagnosis in chronic rhinosinusitisAnn Otol Rhinol Laryngol20051141 Pt 1748315697167
- HastanDFokkensWJBachertCChronic rhinosinusitis in Europe – an underestimated disease. A GA(2)LEN studyAllergy20116691216122321605125
- PonikauJUSherrisDAKernEBThe diagnosis and incidence of allergic fungal sinusitisMayo Clin Proc199974987788410488788
- SasamaJSherrisDAShinSHKephartGMKernEBPonikauJUNew paradigm for the roles of fungi and eosinophils in chronic rhinosinusitisCurr Opin Otolaryngol Head Neck Surg20051312815654207
- Van CrombruggenKZhangNGevaertPTomassenPBachertCPathogenesis of chronic rhinosinusitis: inflammationJ Allergy Clin Immunol2011128472873221868076
- Roca-FerrerJGarcia-GarciaFJPeredaJReduced expression of COXs and production of prostaglandin E(2) in patients with nasal polyps with or without aspirin-intolerant asthmaJ Allergy Clin Immunol201112816672 e121397936
- BachertCGevaertPHoltappelsGJohanssonSGvan CauwenbergePTotal and specific IgE in nasal polyps is related to local eosinophilic inflammationJ Allergy Clin Immunol2001107460761411295647
- BachertCZhangNPatouJvan ZeleTGevaertPRole of staphylococcal superantigens in upper airway diseaseCurr Opin Allergy Clin Immunol200881343818188015
- KernRCConleyDBWalshWPerspectives on the etiology of chronic rhinosinusitis: an immune barrier hypothesisAm J Rhinol200822654955918786300
- ForemanAHoltappelsGPsaltisAJAdaptive immune responses in Staphylococcus aureus biofilm-associated chronic rhinosinusitisAllergy201166111449145621834937
- XaubetAMullolJLópezEComparison of the role of nasal polyp and normal nasal mucosal epithelial cells on in vitro eosinophil survival. Mediation by GM-CSF and inhibition by dexamethasoneClin Exp Allergy19942443073178039016
- MullolJXaubetALópezERoca-FerrerJPicadoCComparative study of the effects of different glucocorticosteroids on eosinophil survival primed by cultured epithelial cell supernatants obtained from nasal mucosa and nasal polypsThorax19955032702747660341
- MullolJLópezERoca-FerrerJEffects of topical anti-inflammatory drugs on eosinophil survival primed by epithelial cells. Additive effect of glucocorticoids and nedocromil sodiumClin Exp Allergy19972712143214419433939
- XaubetAMullolJRoca-FerrerJEffect of budesonide and nedocromil sodium on IL-6 and IL-8 release from human nasal mucosa and polyp epithelial cellsRespir Med200195540841411392584
- MullolJRoca-FerrerJXaubetARaserraJPicadoCInhibition of GM-CSF secretion by topical corticosteroids and nedocromil sodium. A comparison study using nasal polyp epithelial cellsRespir Med200094542843110868704
- MullolJXaubetAGayaACytokine gene expression and release from epithelial cells. A comparison study between healthy nasal mucosa and nasal polypsClin Exp Allergy19952576076158521179
- LalDHwangPHOral corticosteroid therapy in chronic rhinosinusitis without polyposis: a systematic reviewInt Forum Allergy Rhinol20111213614322287332
- ToscaMACosentinoCPallestriniEMedical treatment reverses cytokine pattern in allergic and nonallergic chronic rhinosinusitis in asthmatic childrenPediatr Allergy Immunol200314323824112787306
- SubramanianHNSchechtmanKBHamilosDLA retrospective analysis of treatment outcomes and time to relapse after intensive medical treatment for chronic sinusitisAm J Rhinol200216630331212512904
- LalDSciannaJMStankiewiczJAEfficacy of targeted medical therapy in chronic rhinosinusitis, and predictors of failureAm J Rhinol Allergy200923439640019671254
- Martinez-DevesaPPatiarSOral steroids for nasal polypsCochrane Database Syst Rev20117CD00523221735400
- VaidyanathanSBarnesMWilliamsonPHopkinsonPDonnanPTLipworthBTreatment of chronic rhinosinusitis with nasal polyposis with oral steroids followed by topical steroids: a randomized trialAnn Intern Med2011154529330221357906
- BenitezPAlobidIde HaroJA short course of oral prednisone followed by intranasal budesonide is an effective treatment of severe nasal polypsLaryngoscope2006116577077516652085
- AlobidIBenitezPPujolsLSevere nasal polyposis and its impact on quality of life. The effect of a short course of oral steroids followed by long-term intranasal steroid treatmentRhinology200644181316550943
- HissariaPSmithWWormaldPJShort course of systemic corticosteroids in sinonasal polyposis: a double-blind, randomized, placebo-controlled trial with evaluation of outcome measuresJ Allergy Clin Immunol2006118112813316815148
- Van ZeleTGevaertPHoltappelsGOral steroids and doxycycline: two different approaches to treat nasal polypsJ Allergy Clin Immunol2010125510691076 e420451040
- RupaVJacobMMathewsMSSeshadriMSA prospective, randomised, placebo-controlled trial of postoperative oral steroid in allergic fungal sinusitisEur Arch Otorhinolaryngol2010267223323819714349
- SnidvongsKKalishLSacksRCraigJCHarveyRJTopical steroid for chronic rhinosinusitis without polypsCochrane Database Syst Rev20118CD00927421833974
- LundVJBlackJHSzabóLZSchreweliusCAkerlundAEfficacy and tolerability of budesonide aqueous nasal spray in chronic rhinosinusitis patientsRhinology2004422576215224630
- QvarnbergYKantolaOSaloJToivanenMValtonenHVuoriEInfluence of topical steroid treatment on maxillary sinusitisRhinology19923021031121411095
- LavigneFCameronLRenziPMIntrasinus administration of topical budesonide to allergic patients with chronic rhinosinusitis following surgeryLaryngoscope2002112585886412150618
- HansenFSDjupeslandPGFokkensWJPreliminary efficacy of fluticasone delivered by a novel device in recalcitrant chronic rhinosinusitisRhinology201048329229921038019
- DijkstraMDEbbensFAPoublonRMFokkensWJFluticasone propionate aqueous nasal spray does not influence the recurrence rate of chronic rhinosinusitis and nasal polyps 1 year after functional endoscopic sinus surgeryClin Exp Allergy20043491395140015347372
- JorissenMBachertCEffect of corticosteroids on wound healing after endoscopic sinus surgeryRhinology200947328028619839251
- AukemaAAMulderPGFokkensWJTreatment of nasal polyposis and chronic rhinosinusitis with fluticasone propionate nasal drops reduces need for sinus surgeryJ Allergy Clin Immunol200511551017102315867860
- Bross-SorianoDArrieta-GómezJRPrado-CallerosHInfections after endoscopic polypectomy using nasal steroidsOtolaryngol Head Neck Surg2004130331932215054373
- EhnhageAOlssonPKölbeckKGNAFS Study GroupFunctional endoscopic sinus surgery improved asthma symptoms as well as PEFR and olfaction in patients with nasal polyposisAllergy200964576276919191775
- HolmbergKJuliussonSBalderBSmithDLRichardsDHKarlssonGFluticasone propionate aqueous nasal spray in the treatment of nasal polyposisAnn Allergy Asthma Immunol19977832702769087151
- HolmströmMClinical performance of fluticasone propionate nasal dropsAllergy199954Suppl 53212510442547
- JankowskiRKlossekJMAttaliVCosteASerranoELong-term study of fluticasone propionate aqueous nasal spray in acute and maintenance therapy of nasal polyposisAllergy200964694495019298572
- JurkiewiczDZielnik-JurkiewiczBAWEffectiveness of fluticasone propionate in nasal polyps treatmentInternational Review of Allergology and Clinical Immunology20041012224
- KeithPNieminenJHollingworthKDolovichJEfficacy and tolerability of fluticasone propionate nasal drops 400 microgram once daily compared with placebo for the treatment of bilateral polyposis in adultsClin Exp Allergy200030101460146810998024
- LundVJFloodJSykesA PRichardsDHEffect of fluticasone in severe polyposisArch Otolaryngol Head Neck Surg199812455135189604976
- MastalerzLMilewskiMDuplagaMNizankowskaESzczeklikAIntranasal fluticasone propionate for chronic eosinophilic rhinitis in patients with aspirin-induced asthmaAllergy19975298959009298173
- OlssonPEhnhageANordinSStjarnePNAF2S2 Study GroupQuality of life is improved by endoscopic surgery and fluticasone in nasal polyposis with asthmaRhinology201048332533021038024
- PenttiläMPoulsenPHollingworthKHolmströmMDose-related efficacy and tolerability of fluticasone propionate nasal drops 400 microg once daily and twice daily in the treatment of bilateral nasal polyposis: a placebo-controlled randomized study in adult patientsClin Exp Allergy20003019410210606936
- Rowe-JonesJMMedcalfMDurhamSRRichardsDHMackayISFunctional endoscopic sinus surgery: 5 year follow up and results of a prospective, randomised, stratified, double-blind, placebo controlled study of postoperative fluticasone propionate aqueous nasal sprayRhinology200543121015844495
- VlckovaINavrátilPKanaRPavlicekPChrbolkaPDjupeslandPGEffective treatment of mild-to-moderate nasal polyposis with fluticasone delivered by a novel deviceRhinology200947441942619936370
- el NaggarMKaleSAldrenCMartinFEffect of Beconase nasal spray on olfactory function in post-nasal polypectomy patients: a prospective controlled trialJ Laryngol Otol1995109109419447499945
- KarlssonGRundcrantzHA randomized trial of intranasal beclomethasone dipropionate after polypectomyRhinology19822031441486753091
- MygindNPedersenCBPrytzSSørensenHTreatment of nasal polyps with intranasal beclomethasone dipropionate aerosolClin Allergy1975521591641095246
- ChaltonRMackayIWilsonRColePDouble blind, placebo controlled trial of betamethasone nasal drops for nasal polyposisBr Med J (Clin Res Ed)19852916498788
- PassàliDBernsteinJMPassaliFMDamianiVPassàliGCBellussiLTreatment of recurrent chronic hyperplastic sinusitis with nasal polyposisArch Otolaryngol Head Neck Surg2003129665665912810472
- SmallCBHernandezJReyesAEfficacy and safety of mometasone furoate nasal spray in nasal polyposisJ Allergy Clin Immunol200511661275128116337459
- StjärnePMosgesRJorissenMA randomized controlled trial of mometasone furoate nasal spray for the treatment of nasal polyposisArch Otolaryngol Head Neck Surg2006132217918516490876
- StjärnePBlomgrenKCayé-ThomasenPSaloSSøderstrømTThe efficacy and safety of once-daily mometasone furoate nasal spray in nasal polyposis: a randomized, double-blind, placebo-controlled studyActa Otolaryngol2006126660661216720445
- DingsørGKramerJOlsholtRSøderstrømTFlunisolide nasal spray 0.025% in the prophylactic treatment of nasal polyposis after polypectomy. A randomized, double blind, parallel, placebo controlled studyRhinology198523149584001759
- DrettnerBEbbesenANilssonMProphylactive treatment with flunisolide after polypectomyRhinology19822031491586753092
- FiliaciFPassaliDPuxedduRSchreweliusCA randomized controlled trial showing efficacy of once daily intranasal budesonide in nasal polyposisRhinology200038418519011190754
- HartwigSLindénMLaurentCVargöAKLindqvistNBudesonide nasal spray as prophylactic treatment after polypectomy (a double blind clinical trial)J Laryngol Otol198810221481513279143
- HolopainenEGrahneBMalmbergHMäkinienJLindqvistNBudesonide in the treatment of nasal polyposisEur J Respir Dis Suppl19821222212286958488
- JankowskiRSchreweliusCBonfilsPEfficacy and tolerability of budesonide aqueous nasal spray treatment in patients with nasal polypsArch Otolaryngol Head Neck Surg2001127444745211296057
- Vendelo JohansenLIllumPKristensenSWintherLVang PetersenSSynnerstadBThe effect of budesonide (Rhinocort) in the treatment of small and medium-sized nasal polypsClin Otolaryngol Allied Sci19931865245278877234
- JohanssonLHolmbergKMelenIStiernaPBendeMSensitivity of a new grading system for studying nasal polyps with the potential to detect early changes in polyp size after treatment with a topical corticosteroid (budesonide)Acta Otolaryngol20021221495311876598
- LildholdtTRundcrantzHLindqvistNEfficacy of topical corticosteroid powder for nasal polyps: a double-blind, placebo-controlled study of budesonideClin Otolaryngol Allied Sci199520126307788929
- RuhnoJAnderssonBDenburgJA double-blind comparison of intranasal budesonide with placebo for nasal polyposisJ Allergy Clin Immunol1990866 Pt 19469532262649
- TosMSvendstrupFArndalHEfficacy of an aqueous and a powder formulation of nasal budesonide compared in patients with nasal polypsAm J Rhinol19981231831899653476
- GigerRPaschePCheseauxCComparison of once- versus twice-daily use of beclomethasone dipropionate aqueous nasal spray in the treatment of allergic and non-allergic chronic rhinosinusitisEur Arch Otorhinolaryngol2003260313514012687385
- PandakNPajić-PenavićISekeljATomić-ParadžikMCabrajaIMiklaušićBBacterial colonization or infection in chronic sinusitisWien Klin Wochenschr201112323–2471071322127467
- PiromchaiPThanaviratananichSLaopaiboonMSystemic antibiotics for chronic rhinosinusitis without nasal polyps in adultsCochrane Database Syst Rev20115CD00823321563166
- LegentFBordurePBeauvillainCBerchePA double-blind comparison of ciprofloxacin and amoxycillin/clavulanic acid in the treatment of chronic sinusitisChemotherapy199440Suppl 18157805431
- NamyslowskiGMisiolekMCzeciorEComparison of the efficacy and tolerability of amoxycillin/clavulanic acid 875 mg b.i.d. with cefuroxime 500 mg b.i.d. in the treatment of chronic and acute exacerbation of chronic sinusitis in adultsJ Chemother200214550851712462431
- SuzukiHShimomuraAIkedaKOshimaTTakasakaTEffects of long-term low-dose macrolide administration on neutrophil recruitment and IL-8 in the nasal discharge of chronic sinusitis patientsTohoku J Exp Med199718221151249261930
- ScaddingGKLundVJDarbyYCThe effect of long-term antibiotic therapy upon ciliary beat frequency in chronic rhinosinusitisJ Laryngol Otol1995109124267876731
- IchimuraKShimazakiYIshibashiTHigoREffect of new macrolide roxithromycin upon nasal polyps associated with chronic sinusitisAuris, nasus, larynx19962348568809323
- NishiKMizuguchiMTachibanaH[Effect of clarithromycin on symptoms and mucociliary transport in patients with sino-bronchial syndrome.]Nihon Kyōbu Shikkan Gakkai Zasshi1995331213921400 Japanese8821993
- RubinBKDruceHRamirezOEPalmerREffect of clarithromycin on nasal mucus properties in healthy subjects and in patients with purulent rhinitisAm J Respir Crit Care Med19971556201820239196110
- WallworkBComanWMackay-SimAGreiffLCervinAA doubleblind, randomized, placebo-controlled trial of macrolide in the treatment of chronic rhinosinusitisLaryngoscope2006116218919316467702
- VidelerWJBadiaLHarveyRJLack of efficacy of long-term, low-dose azithromycin in chronic rhinosinusitis: a randomized controlled trialAllergy201166111457146821884529
- SchalekPPetrásPKlementVHahnAShort-term antibiotics treatment in patients with nasal polyps and enterotoxins producing Staphylococcus aureus strainsEur Arch Otorhinolaryngol2009266121909191319626332
- YamadaTFujiedaSMoriSYamamotoHSaitoHMacrolide treatment decreased the size of nasal polyps and IL-8 levels in nasal lavageAm J Rhinol200014314314810887619
- MaruyamaSYoshiokaHFujitaKTakimotoMSatakeYSensitivity of group A streptococci to antibiotics. Prevalence of resistance to erythromycin in JapanAm J Dis Child19791331111431145389035
- SeppäläHKlaukkaTVuopio-VarkilaJThe effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. Finnish Study Group for Antimicrobial ResistanceN Engl J Med199733774414469250845
- DesrosiersMYSalas-PratoMTreatment of chronic rhinosinusitis refractory to other treatments with topical antibiotic therapy delivered by means of a large-particle nebulizer: results of a controlled trialOtolaryngol Head Neck Surg2001125326526911555764
- VidelerWJvan DrunenCMReitsmaJBFokkensWJNebulized bacitracin/colimycin: a treatment option in recalcitrant chronic rhinosinusitis with Staphylococcus aureus? A double-blind, randomized, placebo-controlled, cross-over pilot studyRhinology2008462929818575008
- LimMCitardiMJLeongJLTopical antimicrobials in the management of chronic rhinosinusitis: a systematic reviewAm J Rhinol200822438138918702902
- PonikauJUSherrisDAKernEBThe diagnosis and incidence of allergic fungal sinusitisMayo Clinic proceedings. Mayo Clin Proc1999749877884
- LiangKLSuMCShiaoJYAmphotericin B irrigation for the treatment of chronic rhinosinusitis without nasal polyps: a randomized, placebo-controlled, double-blind studyAm J Rhinol2008221525818284860
- PonikauJUSherrisDAKitaHKernEBIntranasal antifungal treatment in 51 patients with chronic rhinosinusitisJ Allergy Clin Immunol2002110686286612464951
- KennedyDWKuhnFAHamilosDLTreatment of chronic rhinosinusitis with high-dose oral terbinafine: a double blind, placebo-controlled studyLaryngoscope2005115101793179916222197
- PintoJMMehtaNDiTineoMWangJBaroodyFMNaclerioRMA randomized, double-blind, placebo-controlled trial of anti-IgE for chronic rhinosinusitisRhinology201048331832421038023
- GevaertPLang-LoidoltDLacknerANasal IL-5 levels determine the response to anti-IL-5 treatment in patients with nasal polypsJ Allergy Clin Immunol200611851133114117088140
- GevaertPVan BruaeneNCattaertTMepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposisJ Allergy Clin Immunol20111285989995 e1–e821958585
- HayeRAanesenJPBurtinBDonnellyFDubyCThe effect of cetirizine on symptoms and signs of nasal polyposisJ Laryngol Otol1998112111042104610197141
- BraunJJAlabertJPMichelFBAdjunct effect of loratadine in the treatment of acute sinusitis in patients with allergic rhinitisAllergy19975266506559226059
- ParikhAAScaddingGKIntranasal lysine-aspirin in aspirin-sensitive nasal polyposis: a controlled trialLaryngoscope200511581385139016094110
- BowmanLMHoltPGSelective enhancement of systemic Th1 immunity in immunologically immature rats with an orally administered bacterial extractInfect Immun20016963719372711349036
- SpisekRBrazovaJRozkovaDZapletalovaKSedivaABartunkovaJMaturation of dendritic cells by bacterial immunomodulatorsVaccine20042221–222761276815246609
- HeintzBSchlenterWWKirstenRNelsonKClinical efficacy of Broncho-Vaxom in adult patients with chronic purulent sinusitis – a multi-centric, placebo-controlled, double-blind studyInt J Clin Pharmacol Ther Toxicol198927115305342693373
- SchaadUBMütterleinRGoffinHBV-Child Study GroupImmunostimulation with OM-85 in children with recurrent infections of the upper respiratory tract: a double-blind, placebo-controlled multicenter studyChest200212262042204912475845
- ZhengCWangZLacroixJSEffect of intranasal treatment with capsaicin on the recurrence of polyps after polypectomy and ethmoidectomyActa Otolaryngol20001201626610779188
- YakirevitchABedrinLMigirovLWolfMTalmiYPUse of alternative medicine in Israeli chronic rhinosinusitis patientsJ Otolaryngol Head Neck Surg200938451752019755095
- RichsteinAMannWZur Behandlung der chronischen Sinusitis mit Sinupret [Treatment of chronic sinusitis with Sinupret.]Ther Ggw1980119910551060 German7466702
- TaubSJThe use of bromelains in sinusitis: a double-blind clinical evaluationEye Ear Nose Throat Mon19674633613625342723
- GuoRCanteryPHErnstEHerbal Medicines for the Treatment of Rhinosinusitis: A Systematic ReviewOtolaryngol Head Neck Surg2006135449650617011407
- ZimmerMGezielte konservative Therapie der akuten Sinusitis in der HNO-Praxis [Therapy of acute sinusitis]Therapiewoche19853540244028 German
- JohanssonLObergDMelénIBendeMDo topical nasal decongestants affect polyps?Acta Otolaryngol2006126328829016618656
- KroflicBCoerABaudoinTKalogjeraLTopical furosemide versus oral steroid in preoperative management of nasal polyposisEur Arch Otorhinolaryngol2006263876777116685542
- AsplundMSHagbergHHolmströmMChemotherapy in severe nasal polyposis – a possible beneficial effect? A report of three casesRhinology201048337437621038033
- BuyukozturkSGelincikAAslanIAydinSColakogluBDalMMethotrexate: can it be a choice for nasal polyposis in aspirin exacerbated respiratory disease?J Asthma200946101037104119995143
- StewartRARamBHamiltonGWeinerJKaneKJMontelukast as an adjunct to oral and inhaled steroid therapy in chronic nasal polyposisOtolaryngol Head Neck Surg2008139568268718984264
- PauliCFintelmannRKlemensCPolyposis nasi--Besserung der Lebensqualitat durch Leukotrien-Rezeptorantagonisten [Polyposis nasi – improvement in quality of life by the influence of leukotrien receptor antagonists]Laryngorhinootologie2007864282286 German17286243
- FreemanSRSivayohamESJepsonKde CarpentierJA preliminary randomised controlled trial evaluating the efficacy of saline douching following endoscopic sinus surgeryClin Otolaryngol200833546246518983380
- PynnonenMAMukerjiSSKimHMAdamsMETerrellJENasal saline for chronic sinonasal symptoms: a randomized controlled trialArch Otolaryngol Head Neck Surg2007133111115112018025315
- RazaTElsherifHSZulianelloLPlouin-GaudonILandisBNLacroixJSNasal lavage with sodium hypochlorite solution in Staphylococcus aureus persistent rhinosinusitisRhinology2008461152218444487
- WeissmanJDFernandezFHwangPHXylitol nasal irrigation in the management of chronic rhinosinusitis: A pilot studyThe Laryngoscope2011121112468247221994147
- ChiuAGPalmerJNWoodworthBABaby shampoo nasal irrigations for the symptomatic post-functional endoscopic sinus surgery patientAm J Rhinol2008221343718284857
- HarveyRHannanSABadiaLScaddingGNasal saline irrigations for the symptoms of chronic rhinosinusitisCochrane Database Syst Rev20073CD00639417636843
- KhianeyROppenheimerJIs nasal saline irrigation all it is cracked up to be?Ann Allergy Asthma Immunol20121091202822727153
- MajimaYKuronoYHirakawaKEfficacy of combined treatment with S-carboxymethylcysteine (carbocisteine) and clarithromycin in chronic rhinosinusitis patients without nasal polyp or with small nasal polypAuris Nasus Larynx2012391384721636230
- KrespiYPKizhnerVPhototherapy for chronic rhinosinusitisLasers Surg Med201143318719121290392
- MukerjiSSPynnonenMAKimHMSingerATaborMTerrellJEProbiotics as adjunctive treatment for chronic rhinosinusitis: a randomized controlled trialOtolaryngol Head Neck Surg2009140220220819201289
- DiBaiseJKOlusolaBFHuerterJVQuigleyEMRole of GERD in chronic resistant sinusitis: a prospective, open label, pilot trialAm J Gastroenterol200297484385012003417
- IkedaKShiozawaAOnoNSubclassification of chronic rhinosinusitis with nasal polyp based on eosinophil and neutrophilLaryngoscope2013 [Epub ahead of print]
- XuMZhaoFShenAZhouH[Serum allergen-specific IgE in patients with eosinophilic nasal polyps]Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi201226177723 Chinese23214310
- SejimaTHoltappelsGKikuchiHCytokine profiles in Japanese patients with chronic rhinosinusitisAllergol Int20126111152222377524
- SejimaTHoltappelsGKikuchiHImayoshiSIchimuraKBachertCCytokine profiles in Japanese patients with chronic rhinosinusitisAllergol Int201261111512222377524
- LacroixJSBuvelotJMPollaBSLundbergJMImprovement of symptoms of non-allergic chronic rhinitis by local treatment with capsaicinClin Exp Allergy19912155956001742652
- IwamotoLMFujiwaraNNakamuraKTWadaRKNa-K-2Cl cotransporter inhibition impairs human lung cellular proliferationAm J Physiol Lung Cell Mol Physiol20042873L510L51415155267
- YuengsrigulAChinTWNussbaumEImmunosuppressive and cytotoxic effects of furosemide on human peripheral blood mononuclear cellsAnn Allergy Asthma Immunol1999836 Pt 155956610619350
- PrandotaJFurosemide: progress in understanding its diuretic, anti-inflammatory, and bronchodilating mechanism of action, and use in the treatment of respiratory tract diseasesAm J Ther20029431732812115021
- HartogBvan BenthemPPPrinsLCHordijkGJEfficacy of sinus irrigation versus sinus irrigation followed by functional endoscopic sinus surgeryAnn Otol Rhinol Laryngol199710697597669302908
- RagabSMLundVJScaddingGEvaluation of the medical and surgical treatment of chronic rhinosinusitis: a prospective, randomised, controlled trialLaryngoscope2004114592393015126758
- KhalilHSNunezDAFunctional endoscopic sinus surgery for chronic rhinosinusitisCochrane Database Syst Rev20063CD00445816856048
- DalzielKSteinKRoundAGarsideRRoylePSystematic review of endoscopic sinus surgery for nasal polypsHealth Technol Assess2003717iii115912969541