Abstract
Objective
The aim of this study was to investigate the effectiveness of booster vaccination of adults with measles-mumps-rubella in the COVID-19 infection rates.
Methods
In order to investigate this hypothesis, we tested COVID-19 positivity rate through PCR assay on the participants (n=245; male), who had to share the same student accommodation together with the same dining hall to provide governmental service. Participants were divided into two groups based on their booster vaccination status with measles-mumps-rubella: the non-vaccinated group (n=207) and the vaccinated group (n=38). The rate of COVID-19 seropositivity, age, body mass index (BMI), active smoking and presence of comorbidity were also measured and recorded.
Results
All of the participants were healthy, and age distribution, comorbidity rates, active smoking status and BMI did not vary significantly among the two groups (p=0.305, p=0.594, p=0.280, and p=0.922, respectively). About 36.7% (n=90) of the participants were found to be COVID-19 positive by PCR among which the non-vaccinated cases had higher rates of COVID-19 seropositivity than the vaccinated cases (40.6% vs 15.8%) (OR=3.6, 95%CI: 1.5–9.0, p=0.004).
Conclusion
Based on these results, we cautiously predict that immunity produced by MMR vaccination boosters may provide some degree of protection against COVID-19 in the adult population.
Introduction
Coronavirus disease 2019 (COVID-19), due to the new coronavirus (SARS-CoV-2) is an emerging pandemic disease, affecting millions of people worldwide.Citation1 SARS-CoV-2 is an enveloped, positive-sense, single-stranded RNA virus classified within the genus beta-corona virus in the Coronaviridae family, which has been shown to have 79% and 50% sequence similarity with SARS-CoV and MERS-CoV, respectively.Citation2 This novel virus has been reported to cause severe lower respiratory tract infection in humans which often leads to deadly respiratory insufficiency.Citation3,Citation4 According to the WHO report on July 7, 2020, the number of confirmed cases reached almost 11.5 million and more than half a million of them died all around the world so far.Citation5
The present pandemic of COVID-19 is increasing and expanding rapidly at an alarming rate around the world. However, the infection rate has varied greatly among countriesCitation6 and it has been speculated that this difference could be related with routine live vaccination policies carried out in each country.Citation6,Citation7 For instance, the findings of an epidemiological research conducted by Gold, indicated that the number of COVID-19 cases are lower in countries where live viral vaccines, such as measles, mumps, rubella (MMR), were applied routinely at every age group including the adult population.Citation8
Currently, enormous efforts are being devoted to develop a vaccine against this virus to control the pandemic. There has also been growing interest in the repurposing of existing vaccines owing to the possible difficulties in the development of a new vaccine targeting SARS-CoV-2.Citation9 Despite the existence of unproven hypotheses on the protectiveness of live attenuated vaccines as MMR on COVID-19 disease, there is no clinical study investigating the protectiveness of the booster dose of those kind of vaccines against COVID-19 on adults.Citation10–Citation12
Right after the first case of COVID-19 in Turkey, we had a chance to investigate whether the booster vaccination (including MMR and varicella) may provide a meaningful protective effect against COVID-19 using cohort study, for which we obtained data from well-organized military officers with the aim of launching some operational activities.
Methods
Study Subjects
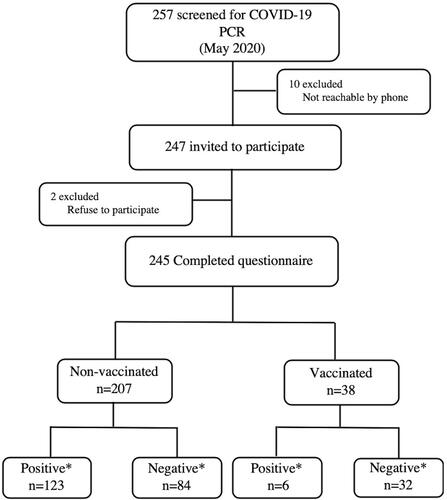
The study participants were taken from the cohort of the military officers (n=257) who were recruited by the Hatay Governorate. Subjects shared the same accommodation (military base) over the period March to June 2020. In March 2020, 38 soldiers were given booster live vaccinations including a booster dose of live attenuated vaccines (including MMR and varicella) and killed vaccines (including HAV, HBV, MenACWY and diphtheria-tetanus vaccine) for potential cross-border operations. These participants were selected as the vaccine cohort and rest of the soldiers (n=219) were selected as the non-vaccine cohort ().
On May 2, 2020 a subject tested positive for the COVID-19 and all subjects (257), who shared the same accommodation, were scanned for the COVID-19 using the real-time transcriptase polymerasechainreaction (RT-PCR) on nasal and pharyngeal swab in accordance with published guidelines.Citation13 All subjects, whose PCR results were negative, were quarantined and monitored for 14 days. After the follow-up, the subjects were re-tested for COVID-19. This retrospective cohort study was approved by the Hatay Mustafa Kemal University, Non-Interventional Clinical Research Ethics Committee (approval number: 08–32/2020) and by the Ministry of Health of Turkey.
Data Source
A total of 245 participants (38 from the vaccine cohort group and 207 from the non-vaccine cohort) agreed to respond to the self-administered questionnaire, and were therefore included in this study. Each person was informed about the study and assigned a unique identification number. We obtained data on vaccine status for each participant from the provincial health directorate. We also obtained information on demographic (age), clinical data, body mass index (BMI), comorbidities, smoking habits and vaccination status from interviewer-administrated questionnaires.
Statistics
The data were analyzed using SPSS v. 21.0 software. (IBM Corporation, Armonk, NY, USA). Continuous variables were tested for normality with onesample Kolmogorov–Smirnov test. Nominal variables were compared using chi-squared test and Fisher’s exact test between the groups, while Mann–Whitney U-test was used for continuous variables. For all statistical data, p<0.05 was considered significant. was plotted using GraphPad Prism v 7.0d for Mac (GrapPad Software, La Jolla, CA, USA).
Results
The median age was 26 (23–53) years for the vaccinated group and 27 (21–54) years for the non-vaccinated group (p=0.305).
The BMI of the vaccinated group and non-vaccinated group was 24.82 (20.20–28.41) and 25 (19.05–30.58), respectively (p=0.922). There was no past or present history of chronic disease in the vaccinated group, whereas six subjects (2.9%) within the non-vaccinated group had chronic disease (p=0.594). Regarding the smoking habits, 50% of the vaccinated subjects (n=19) and 59.4% (n=123) of non-vaccinated subjects were active smokers (p=0.280) ().
Table 1 The Comparison of Demographic Variables Between the Groups
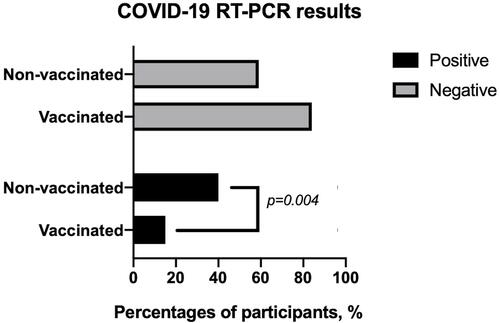
During the study period, a total of 90 participants (36.7%) were diagnosed with COVID-19 by RT-PCR. According to the PCR results, the significant difference for COVID-19 seropositivity was observed among vaccinated group (n=6; 15.8%) and non-vaccinated group (n=84; 40.6%) (OR=3.6, 95%CI: 1.5–9.0, p=0.004, ).
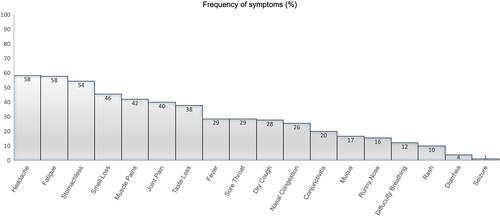
All COVID-19 positive patients had mild to moderate symptoms for which headache and fatigue (58.4–57.8%, respectively) were most frequently identified (). Although there were no severe cases, 11 subjects (10 from the non-vaccinated group; one from the vaccinated group) had shortness of breath; but it was mild and nonprogressive. Fever was reported more frequently in the non-vaccinated group compared to vaccinated group (29.7% vs 2.6%, respectively p<0.05).
Discussion
Epidemiologically, children and the elderly are two groups with a high risk of viral respiratory infections.Citation14 However, information from COVID-19 patients shows that children are less susceptible to the disease compared to adults and the elderly.Citation15,Citation16 This difference is explained by the trained immunity induced by frequent viral infections and routine live attenuated vaccines in children which led to a protective effect, not only on the targeted pathogen, but also on other unrelated pathogens for at least three months.Citation10,Citation11,Citation16–Citation18 However, it is not yet clear whether a live attenuated vaccine booster has a protective effect against the development of COVID-19 disease in adults. The results of the current study showed that non-vaccinated participants have a 3.6 times higher probability of developing COVID-19 disease than participants who recently (within the last three months) received a booster dose of live viral vaccines (including MMR and varicella).
Generally, vaccines provide elicit a specific immune response against targeted pathogen. However, live attenuated vaccines (MMR, rotavirus, smallpox, varicella, BCG) were capable of inducing trained immunity, which mediates protection to unrelated pathogens, too.Citation19 Consequently, it is thought that at least one of those two live viral vaccines (varicella, MMR) might have had a protective effect against the development of COVID-19 disease in our study.
While the SARS-CoV-2 virus is an RNA virus, varicella vaccine consists of a human DNA virus (varicella-zoster virus). In contrast, based on the current literature there has been no evidence as to whether the varicella vaccine could provide protection against COVID-19.Citation20 However, the MMR vaccine consists of weakened enveloped RNA viruses. The spike (S) glycoprotein of SARS-CoV-2 virus plays a key role in the viral entry into the cells, which makes it an important target for developing new vaccines.Citation9 Due to the close similarities between the spike glycoprotein sequences of SARS-CoV-2 and viruses in MMR vaccine (the measles, mumps, and rubella spike glycoprotein sequences with a rate of 32%, 31% and 33%, respectively), MMR vaccine-induced antibodies might provide cross protection against COVID-19.Citation11,Citation21 Furthermore considering that the measles virus has similar cell entry and replication mechanisms, similar transmission level and clinical findings, in addition to the structural similarity with the SARS-CoV-2 virus containing in the MMR vaccine, this live vaccine appears to be more likely to be MMR.Citation21
A previous experimental study by Escriou et al provide concrete evidence that receiving a booster MMR vaccine may offer protection against SARS-CoV infection. In the same study the researchers evaluated the effectiveness of a viral vector vaccine containing the attenuated measles virus against SARS-CoV infection. In this study it has been presented that the neutralizing antibody titers formed after two successfully applied immunizations have reached a level of complete protection against experimental intranasal SARS-CoV infection on rats. In this regard, they suggested that the recombinant measles virus vaccine can be used for the immunization of both adults and children in new SARS-CoV outbreaks that may occur in future.Citation22 Even though the presence of a great similarity between SARS-CoV-2 and SARS-CoV was proven before, there is no immunologic study showing whether the same effect on SARS-CoV-2 could be provided with MMR vaccine or not.
The possible role of killed vaccines (including hepatitis B, hepatitis A, Men ACWY and tetanus-diphtheria (Td) vaccines) administered at the same time as live vaccines (including MMR and varicella vaccines) in mediating this possible protection from COVID-19 disease cannot be ruled out in the current study. However, the split inactivated vaccines tend to be less immunogenic compared to whole viral particles in live vaccines.Citation23 This makes it harder to claim that these killed vaccines may contribute to partial protection, but still possible.
Epidemiological data from several studies suggest a correlation between receiving a booster dose of MMR vaccine may be associated with a lower severity of COVID-19 disease.Citation8 But the present study failed to show this association. All COVID-19 positive patients had mild to moderate disease. This inconsistency may be due to the fact that participants from both groups were in the same race, gender, with a similar comorbidity rate and both groups consisted of younger adults. Another possible explanation for this is the small number of vaccinated COVID-19 positive cases, which limits our ability to draw conclusions about how vaccination affects the clinical course.
Due to the possible difficulties in the development of a new vaccine targeting SARS-COV-2, it is thought that clinical studies on live attenuated vaccines will continue or even gain momentum in the near future.Citation9,Citation24
Limitations
It is certain that this research has some limitations. One of the most important limitations is that, all subjects are young adults. Therefore, this result cannot be generalized to other age groups. Another important limitation in this study is that it is not clear exactly which vaccine may have been effective in this partial protection. Prospective randomized controlled trials are needed to understand which vaccine is the main protective one. Also the antibody status not only to SARS-CoV-2 (which is not mentioned at all), but also to the pathogens in the formula of vaccines that should be tested.
Conclusion
Based on these results, we cautiously predict that immunity produced by MMR booster vaccination may make adults less susceptible to COVID-19.
Declaration
Ethical statement: Local Research Ethics Committee (2020/08; 32) in accordance with the Declaration of Helsinki.
Acknowledgment
The authors would like to thank HMKU for providing English editing service.
Disclosure
The authors report no conflicts of interest in this work.
References
- Jin Y, Yang H, Ji W, et al. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. 2020;12(4). doi:10.3390/v12040372.
- Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi:10.1016/S0140-6736(20)30251-8
- Perlman S. Another decade, another coronavirus. N Engl J Med. 2020;382(8):760–762. doi:10.1056/NEJMe2001126
- Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, Evaluation, and Treatment of Coronavirus. Treasure Island (FL): StatPearls; 2020.
- World Health Organization. Novel Coronavirus (2019-nCoV) Situation Report-170; 2020.
- Team CC-R. Geographic differences in COVID-19 cases, deaths, and incidence - United States, February 12-April 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):465–471. doi:10.15585/mmwr.mm6915e4
- Anbarasu A, Ramaiah S, Livingstone P. Vaccine repurposing approach for preventing COVID 19: can MMR vaccines reduce morbidity and mortality?. Human Vaccines immunotherapeutics. 2020;16(9):2217–2218.
- Gold JE. MMR vaccine appears to confer strong protection from COVID-19: few deaths from SARS-CoV-2 in highly vaccinated populations. 2020. doi:10.13140/RG.2.2.32128.25607
- Caddy S. Developing a vaccine for covid-19. BMJ. 2020;369:m1790. doi:10.1136/bmj.m1790
- Lyu J, Miao T, Dong J, Cao R, Li Y, Chen Q. Reflection on lower rates of COVID-19 in children: does childhood immunizations offer unexpected protection? Med Hypotheses. 2020;143:109842. doi:10.1016/j.mehy.2020.109842
- Dhochak N, Singhal T, Kabra SK, Lodha R. Pathophysiology of COVID-19: why children fare better than adults?. Indian J Pediatr. 2020;87(7):537–546.
- Sidiq KR, Sabir DK, Ali SM, Kodzius R. Does early childhood vaccination protect against COVID-19? Front Mol Biosci. 2020;7:120. doi:10.3389/fmolb.2020.00120
- World Health Organization. Clinical management of COVID-19: interim guidance. (No. WHO/2019-nCoV/clinical/2020.5).
- Rogier van Doorn HYH. Viral Respiratory Infections. Hunter’s Trop Med Emerg Infect Dis. 2020;284–288.
- Dong YMX, Hu Y. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145(6). doi:10.1542/peds.2020-0702.
- Moorlag S, Arts RJW, van Crevel R, Netea MG. Non-specific effects of BCG vaccine on viral infections. Clin Microbiol Infect. 2019;25(12):1473–1478. doi:10.1016/j.cmi.2019.04.020
- Kleinnijenhuis J, Quintin J, Preijers F, et al. BCG-induced trained immunity in NK cells: role for non-specific protection to infection. Clin Immunol. 2014;155(2):213–219. doi:10.1016/j.clim.2014.10.005
- Fidel PL Jr Noverr MC. Could an unrelated live attenuated vaccine serve as a preventive measure to dampen septic inflammation associated with COVID-19 infection? mBio. 2020;11(3). doi:10.1128/mBio.00907-20
- Covian C, Retamal-Diaz A, Bueno SM, Kalergis AM. Could BCG vaccination induce protective trained immunity for SARS-CoV-2? Front Immunol. 2020;11:970. doi:10.3389/fimmu.2020.00970
- Blok BA, Arts RJ, van Crevel R, Benn CS, Netea MG. Trained innate immunity as underlying mechanism for the long-term, nonspecific effects of vaccines. J Leukoc Biol. 2015;98(3):347–356. doi:10.1189/jlb.5RI0315-096R
- Young A, Neumann B, Mendez RF, et al. Homologous protein domains in SARS-CoV-2 and measles, mumps and rubella viruses: preliminary evidence that MMR vaccine might provide protection against COVID-19. medRxiv. 2020. doi:10.1101/2020.10.28.20221747
- Escriou N, Callendret B, Lorin V, et al. Protection from SARS coronavirus conferred by live measles vaccine expressing the spike glycoprotein. Virology. 2014;452–453:32–41. doi:10.1016/j.virol.2014.01.002
- Ortbals DW, Liebhaber H. Comparison of immunogenicity of a whole virion and a subunit influenza vaccine in adults. J Clin Microbiol. 1978;8(4):431–434.
- Amanat F, Krammer F. SARS-CoV-2 Vaccines: status Report. Immunity. 2020;52(4):583–589. doi:10.1016/j.immuni.2020.03.007