Abstract
Purpose
Ultrasonography as the first choice for thyroid nodules is still difficult to distinguish between solid follicular thyroid neoplasm (FTN) and solid nodular goiter (NG). We tried to investigate the value of relative size (M/S, M: the maximum diameter of target nodule, S: the maximum diameter of the largest of the remaining nodules) that may help to differentiate FTN from NG.
Methods
T test and chi-square test were used to retrospectively analyze the differences of the clinical and ultrasonographic characteristics between FTN and NG in 422 cases in our hospital. T test was used to analyze the difference of M/S value in the two kinds of nodules. ROC was used to evaluate the accuracy of M/S value in distinguishing the two.
Results
There were statistically significant differences in age, echogenicity, calcification, peripheral halo and blood supply between the two. The M/S value is not only significantly different in the two kinds of nodules but also can be used as a quantitative indicator to guide ultrasound diagnosis. ROC analysis showed that the cutoff point and AUC of M/S value were 1.94 and 0.709, respectively.
Conclusion
In the ultrasound diagnosis of multiple thyroid nodules, the M/S value can better distinguish FTN and NG. We need to be aware of FTN when the M/S value of the nodule is greater than 2.
Introduction
Follicular thyroid neoplasm (FTN) mainly included follicular adenoma (FA), follicular thyroid carcinoma (FTC) and their subtypes. It is one of the special pathological types in thyroid tumors. Among them, widely invasive FTC is considered a more aggressive disease for its distant metastasis through hematogenous dissemination, which leads to a poorer prognosis. Even preoperative fine needle aspiration (FNA) is also difficult to distinguish FTC from FA, so early detection of FTN is the most important thing to improve the prognosis.Citation1–Citation3 Compared with papillary thyroid carcinoma (PTC), FTC only has some descriptive features, such as be larger, with rich blood supply, and with a peripheral halo of uneven thickness, etc, more often lack the suspicious ultrasound features which characterize PTC.Citation4,Citation5 Single follicular carcinoma is easy to arouse our vigilance, but still a large number of FTC often coexist with benign nodules such as nodular goiter (NG) and lead to misdiagnosis. Since FTN is usually large, it should be prominent in the background of NG. Based on this, we attempted to explore the value of M/S value in ultrasonic diagnosis between FTN and NG.
Materials and Methods
This study was approved by the Ethics Committee of the First Affiliated Hospital of Fujian Medical University ([2015] 084-1).
Patients and Data Collection
This retrospective cohort study included patients who were diagnosed as FTC, FA or nodular goiter after thyroidectomy from January 2012 to May 2020 at the First Affiliated Hospital of Fujian Medical University. A total of 422 nodules in 422 patients in our hospital were selected as the target nodules. Inclusion criteria for nodules: 1) Multiple nodules were included, with at least one nodule on both sides, and the number of nodules is no less than 3; 2) The ultrasonographic data of thyroid nodules was complete and clear, the location and size of the nodules corresponded to the pathological findings. Exclusion criteria: 1) The nodules were treated with drugs; 2) The nodules were poorly defined boundaries due to diffuse lesions such as Hashimoto’s thyroiditis, hyperthyroidism, and subacute thyroiditis.
Ultrasound Analysis
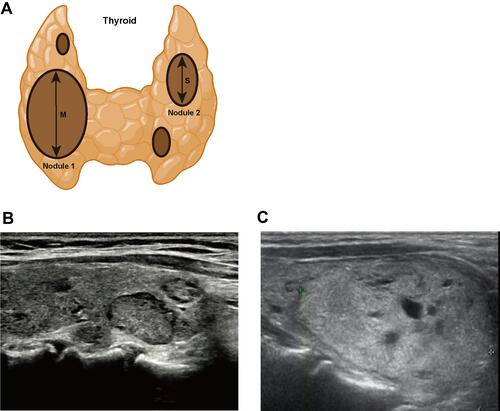
The preoperative ultrasonography was performed with a GE Logiq E9 or Toshiba Aplio 500 with a 10–14 MHz linear array probe. The number of the thyroid nodules, location, size, echogenicity, calcification, peripheral halo and blood supply were evaluated by ultrasonography. The M/S value (M: the maximum diameter of target nodule, S: the maximum diameter of the largest of the remaining nodules) was recorded (). Each ultrasonography was evaluated by physicians with no less than 5 years of experience, and at least two physicians participated in each evaluation.
Statistical Analysis
SPSS22 was used for statistical analysis. T test and chi-square test were used to retrospectively analyze the differences of the clinical and ultrasonographic characteristics between FTN and NG. T test was used to analyze the difference of M/S value in the two kinds of nodules. ROC was used to evaluate the accuracy of M/S value in distinguishing the two. All the p-values were two-sided, and the significance level was set at 0.05.
Results
Clinical and Ultrasonic Features of FTN and NG
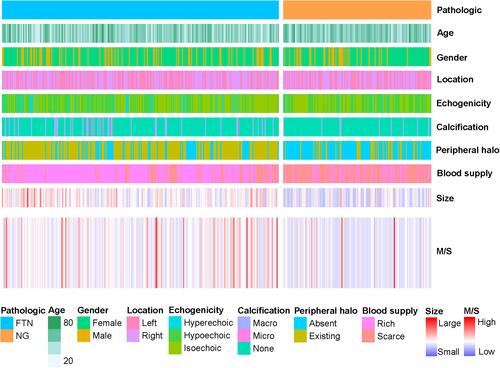
A total of 422 target nodules diagnosed as FTN or NG in our hospital were included in this study. We summarized the distribution of clinical and ultrasonic features in these patients (). These characteristics include the patient’s gender, age, location of the nodule, echogenicity, calcification, peripheral halo, blood supply and size. These features show different distribution trends in FTN and NG. We further compared the differences in these clinical and ultrasonic features between FTN and NG (). The results showed that there were statistically significant differences between FTN and NG in patients’ age, echogenicity, calcification, peripheral halo, blood supply and size. FTN was more likely to be larger than NG, and often had calcification, peripheral halo and rich blood supply.
Table 1 Differential Analysis of Clinical and Ultrasonographic Features Between FTN and NG
Figure 2 The clinical and ultrasonographic characteristics of FTN and NG.
FTN can be further subdivided into FTC and FA, so we further analyzed the differences in clinical and ultrasonic characteristics between FTC and FA (). The results showed that there were statistically significant differences in echogenicity, calcification, blood supply and size between FTC and FA.
Table 2 Differential Analysis of Clinical and Ultrasonographic Features Between FTC and FA
Differential Analysis of M/S Value Between FTN and NG
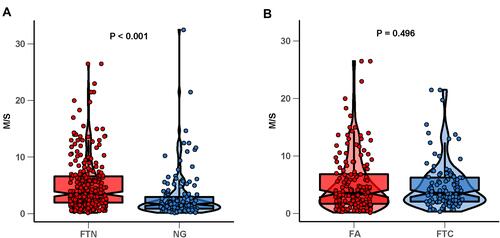
Through the observation of 422 target nodules, we found that the distribution of M/S values in FTN and NG showed different trends (). Therefore, we further analyzed the difference of M/S values between FTN and NG. Our results showed that the M/S values of FTN were higher than that of NG (, ), and the difference was statistically significant. FTN can be further divided into FTC and FA, so we analyzed it by stratification. However, our results showed that there was no significant difference in M/S value between FTCand FA (, ). Therefore, we speculated that M/S value may have a certain potential value in ultrasonic diagnosis of FTN and NG.
Table 3 Differential Analysis of M/S Values Between FTN (Including FTC and FA) and NG
The Predictive Value of M/S Values of FTN and NG in Ultrasonic Diagnosis and the Distribution of Them with M/S Values
In order to evaluate the potential value of M/S value in ultrasonic diagnosis of FTN and NG, we drew the ROC based on the distribution of M/S values in FTN and NG (). The optimal cutoff value was 1.94, the sensitivity was 0.756, the specificity was 0.612, and the AUC was 0.709. Therefore, the optimal cutoff value (1.94) could better distinguish FTN from NG.
Figure 4 (A) The predictive value of M/S value in thyroid ultrasonography. (B) Corresponding relationship between two kinds of thyroid nodules with M/S value. (C) The distribution of FTN and NG with M/S values in 422 thyroid target nodules.
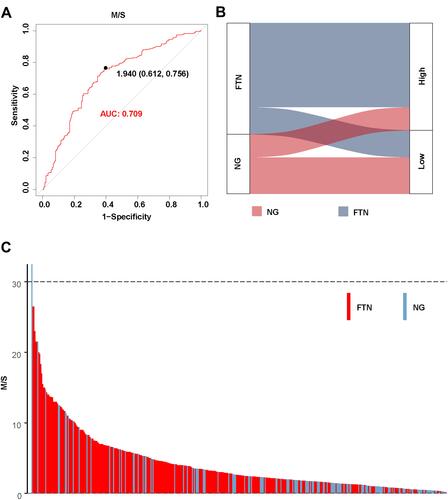
Here, the M/S values of FTN and NG were divided into High group (greater than 1.94) and Low group (less than 1.94) based on the optimal cutoff value of M/S (1.94). We found that FTN was mostly distributed in the High group, while NG was mostly distributed in the Low group (). Notably, we found that with the increase of M/S value, the probability of thyroid nodules being FTN was higher. On the contrary, with the decrease of M/S value, the possibility of thyroid nodules being NG was higher (). Therefore, the optimal cutoff value (1.94) could better distinguish FTN from NG.
Discussion
FTC originates from the follicular epithelium of thyroid. Though with rare lymph node metastasis,Citation6 FTC has a high risk of distant metastasis. The risk of distant metastasis in FTC ranges from a low of 3.0% to almost 30%.Citation7 Distant metastasis is closely associated with increased vascular invasion, which also occurred in some patients with capsular invasion alone, leading to a worse prognosis.Citation8–Citation11 It is difficult to distinguish between FTC and FA before surgery, and the reliability in detecting FTN by several current mainstream ultrasound risk stratification systems were also considered to be in need of further improvement.Citation12,Citation13 The risk of malignancy with a FNAB reading of FTN (Bethesda IV) is 10–40% and 6–28% with AUS/FLUS (Bethesda III).Citation14,Citation15 The distinction between benign and malignant FTN still requires complete surgical pathology. FTN lacks the suspicious ultrasound features which characterize PTC, and is very similar to the common non-neoplastic adenomatous hyperplastic nodule. Therefore, it is challenging and important to screen out FTN among the numerous thyroid nodules.
Ultrasound is the most convenient and effective tool for diagnosing thyroid nodules currently. The FTN and the NG groups showed significant differences in echogenicity, calcification, etc. But those differences were descriptive characteristics, which were not enough to make a diagnosis. Moreover, signs such as calcification as a sonographic indicator of FTN remain under debate.Citation5 Some studies have reported that nodules with irregular margin and unclear border tend to be malignant,Citation16,Citation17 but these descriptive features were subject to a certain degree. In our study, the more prominent differences between the FTN group and the NG group were the presence of peripheral halo and the blood supply status of the nodules. Most studies also supported that a peripheral halo of uneven thickness and abundant blood supply were better indicators for distinguishing FTN from NG.Citation18
In NG, the formation and growth of nodules are mainly caused by the heterogeneity among follicular cells, which show different growth potential when stimulated by TSH. So the natural history of these benign nodules resulted in gradually increasing size. FTN is a kind of neoplasm with the ability of colony-formation, accompanied by molecular genetic changes. Oncogenic changes in FTC are primarily RAS point mutations and PAX8/PPARγ rearrangements.Citation7,Citation19,Citation20 It is precisely because of the different growth mechanisms leading to the fact that FTN grows faster than NG, and makes FTN appeared more prominent and eye-catching in the background of NG. Based on this principle, we had observed that the most intuitive expression was that FTN tended to appear as “outstanding” nodules, that was, the nodules were significantly larger than the surrounding nodules. In our study, the FTN and the NG groups showed significant difference in M/S value, that was, the M/S values of FTN were higher than that of NG. The FTC and FA groups showed no significant difference in M/S value was considered as the similar pathogenesis of the two. According to the ROC we drew, the optimal cutoff value of M/S was 1.94, and the sensitivity and specificity of diagnosis at this time were 75.6% and 61.2%, respectively.
The incidence of FTC is relatively lower than that of PTC, FA and minimally invasive FTC occupy the majority of FTN.Citation21 To avoid overdiagnosis and overtreatment of thyroid nodules, we considered “the M/S value >2” to be more suitable, as the specificity can be appropriately increased to reduce overdiagnosis. In addition, the value of M/S >2 is simple and convenient for clinical application. Nodules which were predominantly cystic were excluded in our study. Both FTN and NG can have cystic change, which leads to nodules growth in a short time, rather than substantial growth. The M/S value cannot be generalized, of course. For example, if the nodules are spongy or with multiple separated cystic regions accompanied by spots with comet tail sign inside,Citation22 no matter how high the M/S value is, NG is the first consideration. In the same way, even if the nodule does not appear “outstanding”, it is accompanied by some typical features such as a peripheral halo with uneven thickness, FTN is the first consideration.
Conclusion
During the ultrasonography of thyroid nodules, if one of the multiple nodules is significantly larger than other surrounding nodules and with rich blood supply, especially when the M/S is higher than 2, we should beware of the possibility of FTN.
Abbreviations
FA, follicular adenoma; FTN, follicular thyroid neoplasm; FTC, follicular thyroid carcinoma; NG, nodular goiter; PTC, papillary thyroid carcinoma.
Data Sharing Statement
All data used in this study are included within the article.
Ethics Approval and Informed Consent
This study was approved by the Ethics Committee of the First Affiliated Hospital of Fujian Medical University ([2015] 084-1). Due to the study was a retrospective analysis, patient consent to review their medical records was not required by the Ethics Committee of the First Affiliated Hospital of Fujian Medical University. All data were anonymized to comply with the provisions of personal data protection legislation. This study adhered to the tenets of the Declaration of Helsinki.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that there is no conflict of interest regarding the publication of this paper.
Additional information
Funding
References
- Suster S. Controversies regarding the interpretation of follicular thyroid nodules. Arch Pathol Lab Med. 2019;143(12):1472–1476. doi:10.5858/arpa.2019-0301-RA
- Cracolici V, Ritterhouse LL, Segal JP, et al. Follicular thyroid neoplasms: comparison of clinicopathologic and molecular features of atypical adenomas and follicular thyroid carcinomas. Am J Surg Pathol. 2020;44(7):881–892. doi:10.1097/PAS.0000000000001489
- Sabra MM, Ghossein R, Tuttle RM. Time course and predictors of structural disease progression in pulmonary metastases arising from follicular cell-derived thyroid cancer. Thyroid. 2016;26(4):518–524. doi:10.1089/thy.2015.0395
- Liu BJ, Zhang YF, Zhao CK, Wang HX, Li MX, Xu HX. Conventional ultrasound characteristics, TI-RADS category and shear wave speed measurement between follicular adenoma and follicular thyroid carcinoma. Clin Hemorheol Microcirc. 2020;75(3):291–301. doi:10.3233/CH-190750
- Kuo TC, Wu MH, Chen KY, Hsieh MS, Chen A, Chen CN. Ultrasonographic features for differentiating follicular thyroid carcinoma and follicular adenoma. Asian J Surg. 2020;43(1):339–346. doi:10.1016/j.asjsur.2019.04.016
- Zaydfudim V, Feurer ID, Griffin MR, Phay JE. The impact of lymph node involvement on survival in patients with papillary and follicular thyroid carcinoma. Surgery. 2008;144(6):1070–1077;discussion 1077–1078. doi:10.1016/j.surg.2008.08.034
- Daniels GH. Follicular Thyroid Carcinoma: a Perspective. Thyroid. 2018;28(10):1229–1242. doi:10.1089/thy.2018.0306
- Nguyen XV, Roy Choudhury K, Tessler FN, Hoang JK. Effect of tumor size on risk of metastatic disease and survival for thyroid cancer: implications for biopsy guidelines. Thyroid. 2018;28(3):295–300. doi:10.1089/thy.2017.0526
- O’Neill CJ, Vaughan L, Learoyd DL, Sidhu SB, Delbridge LW, Sywak MS. Management of follicular thyroid carcinoma should be individualised based on degree of capsular and vascular invasion. Eur J Surg Oncol. 2011;37(2):181–185. doi:10.1016/j.ejso.2010.11.005
- Sabra MM, Dominguez JM, Grewal RK, et al. Clinical outcomes and molecular profile of differentiated thyroid cancers with radioiodine-avid distant metastases. J Clin Endocrinol Metab. 2013;98(5):E829–836. doi:10.1210/jc.2012-3933
- Vogrin A, Besic H, Besic N, Music MM. Recurrence rate in regional lymph nodes in 737 patients with follicular or Hurthle cell neoplasms. Radiol Oncol. 2016;50(3):269–273. doi:10.1515/raon-2016-0025
- Castellana M, Piccardo A, Virili C, et al. Can ultrasound systems for risk stratification of thyroid nodules identify follicular carcinoma? Cancer Cytopathol. 2020;128(4):250–259. doi:10.1002/cncy.22235
- Trimboli P, Castellana M, Piccardo A, et al. The ultrasound risk stratification systems for thyroid nodule have been evaluated against papillary carcinoma. A meta-analysis. Rev Endocr Metab Disord. 2021;22(2):453–460. doi:10.1007/s11154-020-09592-3
- Cibas ES, Ali SZ. The 2017 Bethesda system for reporting thyroid cytopathology. Thyroid. 2017;27(11):1341–1346. doi:10.1089/thy.2017.0500
- Inabnet WB 3rd, Palazzo F, Sosa JA, et al. Correlating the Bethesda system for reporting thyroid cytopathology with histology and extent of surgery: a review of 21,746 patients from four endocrine surgery registries across two continents. World J Surg. 2020;44(2):426–435. doi:10.1007/s00268-019-05258-7
- Lai X, Jiang Y, Zhang B, et al. Preoperative sonographic features of follicular thyroid carcinoma predict biological behavior: a retrospective study. Medicine (Baltimore). 2018;97(41):e12814. doi:10.1097/MD.0000000000012814
- Grani G, Lamartina L, Durante C, Filetti S, Cooper DS. Follicular thyroid cancer and Hurthle cell carcinoma: challenges in diagnosis, treatment, and clinical management. Lancet Diabetes Endocrinol. 2018;6(6):500–514. doi:10.1016/S2213-8587(17)30325-X
- Kaliszewski K, Diakowska D, Wojtczak B, Forkasiewicz Z. Evaluation of selected ultrasound features of thyroid nodules with atypia of undetermined significance/follicular lesion of undetermined significance for the Bethesda reporting system for thyroid cytology. Cancer Manag Res. 2018;10:2223–2229. doi:10.2147/CMAR.S168409
- Yakushina VD, Lerner LV, Lavrov AV. Gene fusions in thyroid cancer. Thyroid. 2018;28(2):158–167. doi:10.1089/thy.2017.0318
- Nikiforov YE. Molecular diagnostics of thyroid tumors. Arch Pathol Lab Med. 2011;135(5):569–577. doi:10.5858/2010-0664-RAIR.1
- Segkos K, Porter K, Senter L, Ringel MD, Nabhan FA. Neck ultrasound in patients with follicular thyroid carcinoma. Horm Cancer. 2018;9(6):433–439. doi:10.1007/s12672-018-0345-6
- Rago T, Vitti P. Diagnostic role of ultrasound and elastosonography in nodular goiter. Best Pract Res Clin Endocrinol Metab. 2014;28(4):519–529. doi:10.1016/j.beem.2014.02.003