Abstract
Purpose
The guidelines recommend urgent biliary drainage (BD) for severe acute cholangitis, without a clear definition of “urgent”. To explore the optimal time, we identified the impact of timing of BD on clinical outcomes in severe acute cholangitis.
Patients and Methods
A retrospective study of patients with severe acute cholangitis was conducted based on the Multiparameter Intelligent Monitoring in Intensive Care III (MIMIC-III) database. Multivariable regressions were used to identified the effect of timing of BD on in-hospital mortality, 30-day mortality, and the length of stay (LOS) in hospital and the intensive care unit (ICU) with adjustment for confounding factors.
Results
A total of 106 severe acute cholangitis patients underwent BD with a median time of 14.14 hours (IQR: 7.60–32.59). Among them, 67.9% were performed within 24 hours and 80.2% within 48 hours. Median length of stay was 2.65 days (IQR: 1.70–5.12) in the ICU and 7.54 days (IQR: 4.49–17.17) in hospital. The in-hospital and 30-day mortality rates were 13.2% and 14.2%, respectively. On multivariate analysis, every 1-day delay of BD increased 1.49 days of stay in hospital (P<0.0001). Delayed BD (>48 hours) was linked with 5.56 days longer ICU LOS (P = 0.0096), while urgent BD (<24 hours) did not significantly shorten the ICU stay (P = 0.0997). No significant increase was observed on in-hospital mortality (OR = 1.03; 95% CI 0.93–1.13) nor 30-day mortality (OR=1.01; 95% CI 0.87–1.14) with BD delay in this population.
Conclusion
In severe acute cholangitis patients, delay in BD increased in-hospital LOS. BD after 48 hours was associated with longer ICU LOS. Yet, BD within 24 hours did not significantly reduce the mortality nor shortened the ICU LOS.
Introduction
Acute cholangitis (AC) is an ascending bacterial infection of the bile duct, presenting heterogeneously in clinical entity from mild to severe in a rapid progression pattern. The development of early recognition and timely treatment of AC, including biliary drainage (BD), fluid resuscitation and antibiotic administration, have improved the clinical outcome dramatically. However, the mortality remains 10–30% in the setting of severe acute cholangitis.Citation1–Citation3
The Tokyo Guidelines 2018 (TG18) recommend urgent biliary drainage for patients with severe acute cholangitis, lacking evidenced based data or limited to expert opinion.Citation4 Despite updates of the guidelines, the time frame of BD is not specified and the definition of “early” or “urgent” is unclear. A number of studies have reported that delayed biliary drainage (>48 hour or >72 hour) worsened the clinical outcome.Citation1,Citation5–Citation7 Still, it is in doubt whether patients with severe acute cholangitis can safely delay BD by 24 or 48 hours to allow adequate resuscitation, in order to lower the procedure complications, without increasing mortality. Experts’ opinions advocated an urgent biliary decompression, and even some proposed “as early as possible” or “less than 12 hours”.Citation8 On the other hand, researchers claimed that the “weekend effect” had no impact on clinical outcomes such as mortality and length of stay in hospital in acute cholangitis.Citation9–Citation11 Hakuta et al suggested no association of timing of endoscopic biliary drainage with clinical outcomes in patients with non‑severe acute cholangitis.Citation12 However, little investigation has been focused on the timing of BD in the severe acute cholangitis population. Given the lack of evidenced based data, we aimed to explore the effect of timing of biliary drainage on clinical outcomes defined as mortality and length of stay (LOS) in hospital and the intensive care unit (ICU) in patients with severe acute cholangitis.
Patients and Methods
Study Design
This was a retrospective cohort study, which extracted the data from MIMIC-III (the Medical Information Mart for Intensive Care III V1.4).Citation13 MIMIC-III is a single-center database that contains 53,432 stays for adult patients in critical care units at Beth Israel Deaconess Medical Center Boston, MA, USA between 2001 and 2012. The data was from version 1.4, updated on 2 September 2016.
Study Population
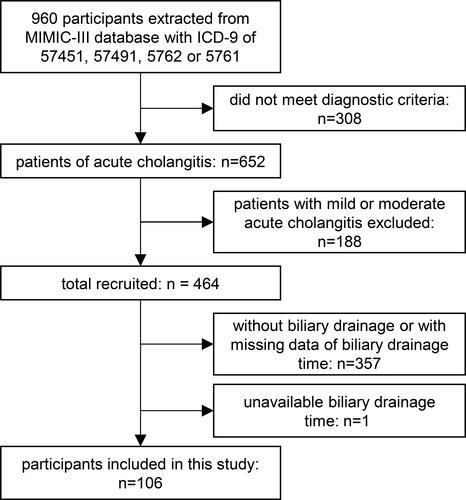
Adult patients (>18 years old) in their first ICU stay with ICD-9 diagnosis code of 57,451, 57,491, 5762 or 5761 were eligible for inclusion. According to the diagnoses criteria and severity criteria of Tokyo Guidelines 2018 (TG18) within the first 24 hours after admission, patients with severe acute cholangitis were selected. Then we excluded patients without biliary drainage or with missing data of BD timing.
Study Variables
We extracted from the MIMIC-III the demographic data and biochemistry data within the first 24 hours after admission, including age, sex, race, albumin, bicarbonate, bilirubin, creatinine, lactate, platelet count, blood urea nitrogen (BUN), white blood cell (WBC), international normalized ratio (INR), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP) and presence of bacteremia. The median value was taken when there were multiple measurements of biochemistry indexes. The Sequential Organ Failure Assessment (SOFA) was measured as severity grading and modified Elixhauser comorbidity index (ECI, Van Walraven version) was applied to assess the co-comorbidities.Citation14 Organ failure was defined according to the severity criteria of the Tokyo Guidelines 2018 (TG18), applying the GCS score, blood pressure and biochemistry data. ICD-9 diagnosis codes and ICD-9 procedure codes of each patient were extracted to record the etiology of cholangitis and type of drainage procedure, respectively. Anti-microbials prescription were obtained, which referred to the types of antibiotics within the first 24 hours.
Outcomes
The exposure was the timing of biliary drainage, which was defined as the duration from admission time to the first biliary drainage procedure (ERCP or PBD, percutaneous biliary drainage) time. The primary outcome of this study were in-hospital mortality and 30-day mortality which was defined as death occurring within 30 days from the date of admission to hospital. The secondary outcomes included the length of stay (LOS) in hospital and the ICU.
Statistical Analysis
Continuous variables including age, ECI, SOFA, number of organ failures, biochemistry profiles, timing of biliary drainage and LOS were expressed as mean ± standard deviation or median with interquartile range. Sex, race, organ failure, etiology of acute cholangitis, type of anti-microbials, procedures of biliary Drainage, in-hospital mortality and 30-day mortality were defined as categorical variables and reported as percentages. The comparison of BD timing between survivors and nonsurvivors were analyzed with the Mann–Whitney test. Length of stay difference among different BD timing groups (<24 hours, 24–48 hours, >48 hours) were analyzed with the Kruskal–Wallis test.
We applied multivariable linear regression models evaluating the association between timing of biliary drainage (days) and length of stay in the ICU and hospital. The BD timing was analyzed as a continuous variable and categorical variable with 24 hours or 48 hours as the cut-off, respectively. Multicollinearity in regression models was evaluated by calculating the variance inflating factor. Values of the variance inflating factor exceeding 10 were considered to indicate multicollinearity. Excluding collinearity variables, the clinical meaningful variables or ones that effected the linear regression coefficients estimate by more than 10%, were selected as confounders to adjust in the final model, which including cardiovascular dysfunction, neurological dysfunction, etiology of bile duct stone and ECI. Interaction and stratified analyses were conducted according to age grouping (above and below 75 years), bilirubin level (above and below 5 mg/dL), and ECI score (above and below 10) with adjusted confounders.
Multivariable logistic regression models were applied to access for the 30-day mortality or in-hospital mortality with BD timing. Multicollinearity in regression models was evaluated by calculating the variance inflating factor. Values of the variance inflating factor exceeding 10 were considered to indicate multicollinearity. Excluding collinearity variables. The clinically meaningful variables and variables that showed a univariate relationship with the outcome (p < 0.1) were considered as candidate confounders. The top three effected the initial regression coefficients, were adjusted in the final model, included percutaneous drainage, cardiovascular dysfunction, bicarbonate and ECI. The relationship between BD timing and mortality might not be linear, thus adjusted smoothing spline plots of mortality by BD timing were investigated to explore the relationship of these two. Interaction and stratified analyses were conducted according to age, bilirubin, bacteremia and ECI with adjusted confounders.
The details of missing data were displayed in the supplementary materials Figure S1. In analysis, the missing data were acquired with 5 sets of data via the multiple imputation method and a chained equation approach method in the R MI procedure, to account for missing data. The distributions of all the variables of missing data were of similar values with the imputation data (Table S1). Multivariable regression models applying the original data were undertaken in the sensitivity analysis (Tables S2-S3).
All of the analyses were performed with the R package (version 3.6.0) and EmpowerStats (http://www.empowerstats.com, X&Y Solution, Inc., Boston, MA). All tests were 2-sided, and P < 0.05 was considered statistically significant.
Results
Demographic Data and Baseline Characteristics
A total of 106 severe acute cholangitis patients with timing of BD were enrolled in the analysis cohort. The flow diagram of the study population is shown in . The characteristics of the population are summarized in . During the study period, 106 patients (median age, 75 years; median SOFA score, 14; median comorbidity ECI score, 10) are of 84.9% benign etiology and 15.1% malignant etiology. In the cohort, cardiovascular dysfunction was detected in 67/106 (63.2%), neurological dysfunction in 46/106 (43.4%), respiratory dysfunction in 38/106 (86.4%), renal dysfunction in 31/106 (29.2%), hepatic dysfunction in 49/106 (46.2%) and hematological dysfunction in 29/106 (27.4%). The median of numbers of organ failures is 4. Patients underwent the biliary drainage with a median time of 14.14 hours (IQR: 7.60–32.59). Among them, 67.9% underwent biliary drainage within 24 hours, 80.2% within 48 hours and 82.1% within 72 hours, which included 70.8% stent/tube insertion, 31.1% stone extraction, 40.6% procedures on duodenal papilla and 20.8% percutaneous drainage. The bacteremia presented in 34.9% severe acute cholangitis patients and 61.3% patients were prescribed a combination of anti-microbial agents. Median length of stay was 2.65 days (IQR: 1.70–5.12) in the ICU and 7.54 days (IQR: 4.49–17.17) in hospital. The in-hospital and 30-day mortality rates were 13.2% and 14.2% respectively.
Table 1 Baseline Characteristics of 106 Patients with Severe Acute Cholangitis
The Association Between Timing of Biliary Drainage and Length of Stay
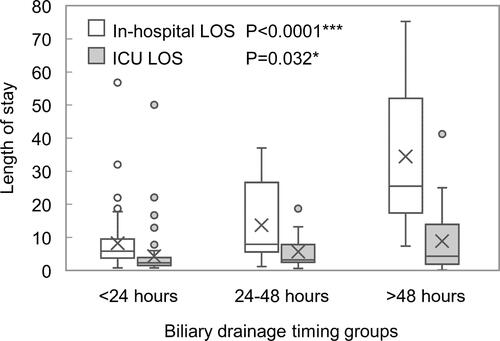
Median length of stay was 2.65 days (IQR: 1.70–5.12) in the ICU and 7.54 (IQR: 4.49–17.17) in hospital, with a significant increase across the <24 hours, 24–48 hours and ≥48 hours groups (in-hospital LOS, P < 0.0001; ICU LOS, P = 0.032) ().
Figure 2 Length of stay of in-hospital and ICU among different biliary drainage timing groups (<24 hours, 24–48 hours, and >48 hours). *P < 0.05 and *** P < 0.001.
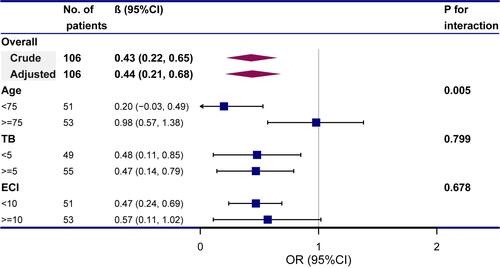
The multivariate logistic regression suggested that every delayed day of BD increased LOS by 1.49 days (95% CI 1.09–1.89; P < 0.0001) (). The patients who underwent BD after 24 hours had 11.86 days (95% CI 5.14–18.59; P=0.0008) longer in-hospital LOS, compared with BD within 24 hours. When performed after 48 hours, it increased to 20.91 days (95% CI 13.82–28.00; P<0.0001) (). The sensitivity analyses involving the original data was consistent with it (Table S2). The interaction analysis indicated that bilirubin level (P for interaction = 0.005) and ECI score (P for interaction = 0.002) might play an interactive role in the association between BD timing and in-hospital LOS ().
Table 2 Multivariable Linear Regression Models Evaluating the Association Between Timing of Biliary Drainage and in-Hospital Length of Stay (LOS)
Figure 3 Subgroup analyses of the association between timing of biliary drainage (days) and length of stay of in-hospital.
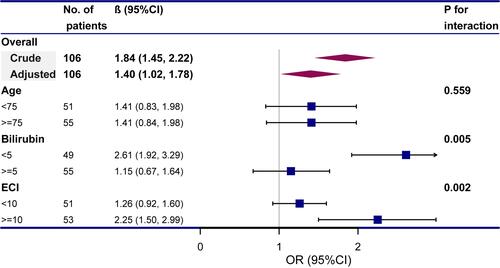
The ICU LOS increased by 0.47 days (95% CI 0.23–0.71; P = 0.0002) with every delayed day of biliary drainage in multivariable linear regression models (). And different effects of BD timing on the ICU LOS were detected in different aging group (P = 0.005), but was stable in subgroup analyses in different bilirubin or ECI scored patients (). When using 24 hours as a cut-off, there was no difference in the ICU LOS (P = 0.0997); while delayed BD (≥48 hours) related to a 5.56 (95% CI 1.43–9.68; P=0.0096) days prolonged ICU LOS (). The sensitivity analyses involving the original data was consistent with it (Table S2).
Table 3 Multivariable Linear Regression Models Evaluating the Association Between Timing of Biliary Drainage and ICU LOS
The Association Between Timing of Biliary Drainage and Mortality
The total in-hospital and 30-day mortality rates were 13.2% and 14.2% respectively, with 11.1% and 13.9% in <24 hours group, with 15.4% and 7.7% in 24–48 hours group, and with 19.0% and 19.0% in >48 hours (). There was no significant difference in timing of BD between survivors and nonsurvivors (in-hospital mortality, P = 0.52; 30-day mortality, P = 0.78) ().
Figure 5 In-hospital mortality and 30-day mortality among different biliary drainage timing groups (<24 hours, 24–48hours, and >48 hours).
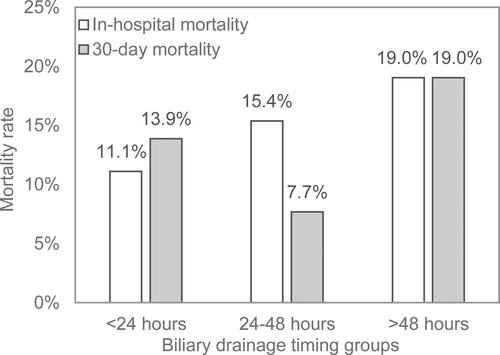
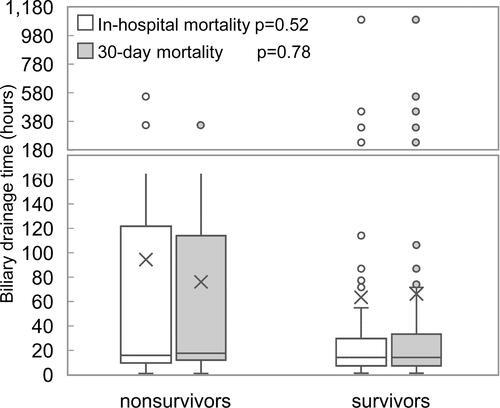
Figure 6 Distribution of biliary drainage timing in survivors and nonsurvivors by in-hospital mortality and 30-day mortality.
After adjusting for potential confounders, a linear relationship between BD timing and mortality was observed according to smoothing spline plots (Figure S2). However, there was no significant increase effect on both in-hospital (OR = 1.03; 95% CI 0.93–1.13; P = 0.9327) and 30-day mortality (OR = 1.01; 95% CI 0.87–1.14; P = 0.9461) with BD delay in adjusted multivariable logistic regression models (). The sensitivity analyses applying the original data was consistent with it (Table S3). According to subgroup analyses, the results of in-hospital mortality were stable for different ages, bilirubin levels, ECI scores and bacteremia conditions of patients (Figure S3a). The interaction analysis indicated that the presence of bacteremia (P for interaction = 0.010) might play an interactive role in the association between BD timing and 30-day mortality (Figure S3b).
Table 4 Multivariable Logistic Regression Models Evaluating the Association Between Timing of Biliary Drainage and Mortality
Discussion
In this retrospective study, we investigated the timing of biliary drainage (BD) effect on clinical outcomes in severe acute cholangitis patients, taking competing clinical risk factors into account. Overall, every 1-day delay of BD timing increased LOS in hospital by 1.49 (95% CI 1.09–1.89; P<0.0001) days. Bilirubin level (P for interaction = 0.005) and ECI score (P for interaction = 0.002) might play an interactive role in it. Regarding the ICU LOS, delayed BD (>48 hours) was linked with 5.56 (95% CI 1.43–9.68; P=0.0096) days longer stay, while urgent BD patients (<24 hours) had no significant difference in ICU LOS, compared with those who underwent BD after 24 hours (P = 0.0997). There was no significant increase effect with BD delay on in-hospital mortality (OR = 1.03; 95% CI 0.93–1.13; P = 0.9327) and 30-day mortality (OR = 1.01; 95% CI 0.87–1.14; P = 0.9461) in our severe acute cholangitis population.
Acute cholangitis is considered an emergent condition, especially severe acute cholangitis, which advances in a rapid way. The optimal treatment strategy, namely early recognition, biliary drainage procedures, fluid resuscitation, and prompt antibiotics introduction, has decreased the mortality from 50~60% to approximately 20%.Citation15,Citation16 Our results have testified that an earlier BD timing benefits patients with shorter in-hospital LOS, in accordance with a number of studies and we further proved that the benefit maintained in the severe acute cholangitis.Citation5–Citation7,Citation17,Citation18 Bilirubin level as well as ECI score might impact the effects.
However, for the severe acute cholangitis patient, it is highly debatable what the optimal strategy is: performing biliary drainage procedures as early as possible or delaying the procedures until stabilization after adequate resuscitation? Our data concluded that delayed BD (≥48 hours) was linked to a 5.56 day increase of ICU stay. However, urgent BD (<24 hours) patients had no significant difference on ICU LOS. In patients older than 75, the prolonged ICU LOS effects of BD delaying might be more obvious. However, with BD timing delay, no significant increase effect on in-hospital and 30-day mortality rate was observed in this severe acute cholangitis population. A few studies also suggested no decrease on odds of mortality with urgent (defined as within 24 hours) or early (within 48 hours) biliary drainage. Aboelsoud et al enrolled the acute cholangitis patients from MIMIC-III. They found that using 24 hours or 48 hours as a cut-off, there was no difference on in-hospital mortality (OR = 0.47; 95% CI 0.17–1.29; p = 0.146) or 28-day mortality (OR = 0.61; 95% CI 0.24–1.53; p = 0.297). Though they reported a shorter ICU LOS in patients who underwent BD within 24 hours, the associated confounders as comorbidity and severity of the disease were not adjusted on the result.Citation17 One retrospective multicenter cohort analysis had a conclusion that the rate of ERCP and the timing from admission to ERCP were not associated with adverse outcomes, even when stratified by 24-hour intervals.Citation18 Interestingly, another retrospective cohort of 203 patients, taking persistent organ failure as outcome, revealed that delayed (>48h) ERCP were associated with the outcome, while procedures within 24 hourswere not (AOR = 2.1; 95% CI = 0.8–5.7).Citation1 Navaneethan compared the persistent OF and/or 30-d mortality among patients in every 24-hour interval, and there were no significant associations with the outcome in the 24–48 hours group and 48–72 hours group according to multivariate analysis.Citation6 Young ji et al observed no decrease in mortality among those who had ERCP on the day of admission in both Tokyo grade (TG) I, TG II and TG III cohorts in a national database analysis of 49,722 cholangitis patients.Citation9 Another population-based evidence with 107,253 patients included, stated that there was no difference in mortality between urgent ERCP (within 24 hours) and early ERCP (24~48 hours).Citation19 Also three large studies demonstrated no differences in mortality and LOS, comparing weekday and weekend admissions.Citation9–Citation11 These findings are congruent with our own, further supporting our hypothesis that urgent biliary drainage may not reduce the mortality and LOS in ICU, meaning a limited benefit on the severe acute cholangitis population. However, we may not confirm the conclusion with patients of BD timing after 24 hours, because relatively few patients fell into the category and reached the outcome of mortality. Interestingly, in the interaction analysis, the effects of delayed BD timing on 30-day mortality varied between different bacteremia conditions (P for interaction = 0.01). For patients without bacteremia, delayed BD timing seemed more related to 30-day mortality as a risk factor (OR = 1.26; 95% CI 0.24–1.53). Further investigation should be performed on this.
There were some studies that revealed early biliary drainage improved outcomes. A recent systematic review, including 9 observational studies and 2 database studies indicated decreased odds of mortality when ERCP was performed within 48 hours of admission, but comparison of within 24 hours and 24–48 hours was not conducted, because of inconsistent mortality data for those among 24–48 hours.Citation7 Another 166 acute cholangitis study suggested that early ERCP (within 24 hours) lowers 30-day mortality (OR = 0.23; 95% CI 0.05–0.95; p = 0.04), but in the 43% of the population were malignant etiology, which was different from ours and most studies and might potentially impact the 30-day mortality.Citation3 Further study about interaction between etiology and BD timing on outcomes should be conducted. Karvellas et al analyzed 260 patients with cholangitis-associated septic shock and proved that delayed biliary decompression >12 h was associated with increased mortality (OR = 3.40; 95% CI 1.12–10.31), however their timing of biliary decompression was defined as from the time of development of shock (mean arterial pressure <65 mmHg) to the time of receipt of therapy and further comparison among patients with different intervals would need to be made to decide the optimal time and subgroup.Citation2
To our knowledge, ours is a relatively large study on the association of biliary drainage timing and outcomes in the severe acute cholangitis population. Our results indicated that BD timing delay within 24 hours did not prolong the ICU LOS, nor increase the mortality significantly in the severe acute cholangitis population. A hasty intervention might increase the anesthetic risk and induce transient bacteremia that may lead to deterioration. Thus, in clinical, biliary decompression “as early as possible” strategy maybe not of the first importance for severe acute cholangitis patients, and could safely follow after initiation of antibiotics, adequate resuscitation and stabilization of organ function within 24 hours.
There were several important limitations of our work. The data from MIMIC-III, which was a single centered retrospective cohort database, might lead to selection biases of treatment and patients. Nonetheless, we applied rigorous inclusion and exclusion criteria and adjusted for important confounders to obtain reliable results. The sample size was small, but for the timing of BD in severe acute cholangitis population study, it was relatively large. Besides, most patients in this cohort having procedures within 24 hours, limits our ability to describe and analyze the outcomes of patients receiving intervention after 24 hours.
Conclusion
In the severe acute cholangitis population, 1-day delay of biliary drainage timing increased hospital LOS by 1.49 days. Delayed BD (>48 hours) was associated with longer ICU LOS. However, the urgent BD (within 24 hours) was not associated with a decrease in mortality, nor significantly shorter ICU stay. Based on these, urgent biliary decompression may have limited benefit for severe acute cholangitis patients, and might be of secondary importance to adequate medical support care in the first day without increasing mortality. Thus, delaying BD by 24 hours, allowing adequate resuscitation, could possibly be one choice in managing severe acute cholangitis.
Abbreviations
BD, biliary drainage; MIMIC-III, Multiparameter Intelligent Monitoring in Intensive Care III; LOS, length of stay; ICU, intensive care unit; AC, acute cholangitis; TG18, Tokyo Guidelines 2018; BUN, blood urea nitrogen; WBC, white blood cell; INR, international normalized ratio; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; SOFA, Sequential Organ Failure Assessment; ECI, (vanWalRaven) Elixhauser comorbidity index; ERCP, endoscopic retrograde cholangiopancreatography; PBD, percutaneous biliary drainage.
Data Sharing Statement
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Statement
The studies involving human participants were reviewed and approved by The Massachusetts Institute of Technology and Beth Israel Deaconess Medical Center. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Acknowledgments
I would extend my sincere gratitude to professor Jiyong Jing for his constant guidance, valuable suggestions and inspiring advice of the thesis. I am also deeply indebted to all group members of our research team for their patience and serious practical discussion for every reading through of the draft. Last but not least, special thanks should go to my beloved husband for his continuous support and encouragement.
Disclosure
The authors declare that they have no conflicts of interest.
Additional information
Funding
References
- Lee F, Ohanian E, Rheem J, Laine L, Che K, Kim JJ. Delayed endoscopic retrograde cholangiopancreatography is associated with persistent organ failure in hospitalised patients with acute cholangitis. Aliment Pharmacol Ther. 2015;42(2):212–220. doi:10.1111/apt.13253
- Karvellas CJ, Abraldes JG, Zepeda-Gomez S, et al. The impact of delayed biliary decompression and anti-microbial therapy in 260 patients with cholangitis-associated septic shock. Aliment Pharmacol Ther. 2016;44(7):755–766. doi:10.1111/apt.13764
- Tan M, Schaffalitzky de Muckadell OB, Laursen SB. Association between early ERCP and mortality in patients with acute cholangitis. Gastrointest Endosc. 2018;87(1):185–192. doi:10.1016/j.gie.2017.04.009
- Kiriyama S, Kozaka K, Takada T, et al. Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholangitis (with videos). J Hepatobiliary Pancreat Sci. 2018;25(1):17–30. doi:10.1002/jhbp.512
- Khashab MA, Tariq A, Tariq U, et al. Delayed and unsuccessful endoscopic retrograde cholangiopancreatography are associated with worse outcomes in patients with acute cholangitis. Clin Gastroenterol Hepatol. 2012;10(10):1157–1161. doi:10.1016/j.cgh.2012.03.029
- Navaneethan U, Gutierrez NG, Jegadeesan R, et al. Factors predicting adverse short-term outcomes in patients with acute cholangitis undergoing ERCP: a single center experience. World J Gastrointest Endosc. 2014;6(3):74–81. doi:10.4253/wjge.v6.i3.74
- Iqbal U, Khara HS, Hu Y, et al. Emergent versus urgent ERCP in acute cholangitis: a systematic review and meta-analysis. Gastrointest Endosc. 2020;91(4):753–760. doi:10.1016/j.gie.2019.09.040
- Khamaysi I, Taha R. ERCP for severe acute cholangitis: the earlier, the better. The Turkish Journal of Gastroenterology: The Official Journal of Turkish Society of Gastroenterology. 2020;31(1):78–79. doi:10.5152/tjg.2020.19103
- Seo YJ, Hadaya J, Sareh S, Aguayo E, de Virgilio CM, Benharash P. National trends and outcomes in timing of ERCP in patients with cholangitis. Surgery. 2020;168(3):426–433. doi:10.1016/j.surg.2020.04.047
- Inamdar S, Sejpal D, Ullah M, Trindade A. Weekend vs. Weekday Admissions for Cholangitis Requiring an ERCP: comparison of Outcomes in a National Cohort. Am J Gastroenterol. 2016;111(3):405–410. doi:10.1038/ajg.2015.425
- Tabibian J, Yang J, Baron T, Kane S, Enders F, Gostout C. Weekend Admission for Acute Cholangitis Does Not Adversely Impact Clinical or Endoscopic Outcomes. Dig Dis Sci. 2016;61(1):53–61. doi:10.1007/s10620-015-3853-z
- Hakuta R, Hamada T, Nakai Y, et al. No Association of Timing of Endoscopic Biliary Drainage with Clinical Outcomes in Patients with Non-severe Acute Cholangitis. Dig Dis Sci. 2018;63(7):1937–1945. doi:10.1007/s10620-018-5058-8
- Johnson A, Pollard T, Shen L, et al. MIMIC-III, a freely accessible critical care database. Scientific Data. 2016;3(1):160035. doi:10.1038/sdata.2016.35
- van Walraven C, Austin P, Jennings A, Quan H, Forster A. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. J Medical Care. 2009;47(6):626–633. doi:10.1097/MLR.0b013e31819432e5
- Kimura Y, Takada T, Kawarada Y, et al. Definitions, pathophysiology, and epidemiology of acute cholangitis and cholecystitis: tokyo Guidelines. J Hepatobiliary Pancreat Surg. 2007;14(1):15–26. doi:10.1007/s00534-006-1152-y
- Andrew D, Johnson S. Acute suppurative cholangitis, a medical and surgical emergency. A review of ten years experience emphasizing early recognition. Am J Gastroenterol. 1970;54(2):141–154.
- Aboelsoud M, Siddique O, Morales A, Seol Y, Al-Qadi M. Early biliary drainage is associated with favourable outcomes in critically-ill patients with acute cholangitis. Przeglad Gastroenterologiczny. 2018;13(1):16–21. doi:10.5114/pg.2018.74557
- Schwed A, Boggs M, Pham X, et al. Association of Admission Laboratory Values and the Timing of Endoscopic Retrograde Cholangiopancreatography With Clinical Outcomes in Acute Cholangitis. JAMA Surg. 2016;151(11):1039–1045. doi:10.1001/jamasurg.2016.2329
- Parikh MP, Wadhwa V, Thota PN, Lopez R, Sanaka MR. Outcomes Associated With Timing of ERCP in Acute Cholangitis Secondary to Choledocholithiasis. Clin Gastroenterol. 2018;52(10):e97–e102. doi:10.1097/MCG.0000000000000982