Abstract
Objective
In order to evaluate the effect of dyslipidemia on cellular or humoral immunity in patients, changes in the absolute number of lymphocyte subsets were detected.
Methods
Flow cytometry was applied to determine the absolute value of lymphocyte subsets: B cell, NK cell, CD4+ T cell including the functional subset (CD4+CD28+), native subset (CD4+CD45RA+CD62L+), memory T cell subset (CD4+CD45RA−), CD8+ T cell including the functional subset (CD8+CD28+) and activated subsets (CD8+CD38+ and CD8+DR+). The relationship between lymphocyte subsets and hypercholesterolemia and hypertriglyceridemia was analyzed.
Results
The absolute values of CD19+ B cell, CD3+ T cell, CD4+ Th cell, CD4+CD28+ cell, naive CD4+ T cell and memory CD4+ T cell in patients with dyslipidemia were markedly higher than those in healthy controls (P<0.05). There was no significant difference between healthy controls and dyslipidemia patients in other lymphocyte subsets (P>0.05). The absolute values of CD3+ T cell and naive CD4+ T cell were significantly positively correlated with hypercholesterolemia in peripheral blood (r=0.291 and 0.306, respectively, all P<0.05). There was no significant correlation between hypertriglyceridemia and lymphocyte subsets (P>0.05).
Conclusion
Dyslipidemia has potential effects on immune profiles in lymphocytes subsets, and changes in lymphocyte subsets in dyslipidemia patients may lead to immune dysfunction.
Introduction
Dyslipidemia, which comes with high levels of total cholesterol (TC), triglycerides (TG) and low-density lipoprotein (LDL) or low levels of high-density lipoprotein (HDL) in plasma for clinical feature, is a risk factor for atherosclerotic cardiovascular disease, cerebrovascular accident, peripheral arterial disease and type 2 diabetes.Citation1,Citation2 However, the pathogenesis of other diseases caused by dyslipidemia is complex and has not been fully elucidated so far. To overcome this gap, more and more researchers have studied the interaction between dyslipidemia and immunity. Studies on the mechanism of dyslipidemia related to cardiovascular diseases or immunity disorder have found that lipoprotein metabolism plays an important role in the development of coronary atherosclerosis and the activation of white blood cells, whereas the lowering of lipid interventions has been good for immunomodulatory and anti-inflammatory clinical studies.Citation3,Citation4 Evidence from clinical data and mechanism study suggested that hypercholesterolemia may induce an interaction between mast cell and CD4 + T cell in coronary atherosclerosis.Citation5 In addition, dyslipidemia induced an increase in the number of both neutrophil granulocyte and mononuclear leucocyte in animal models and clinical trials.Citation6 Moreover, an increased number of both types of cells have been shown to correlate with plaque load in cardiovascular regions.Citation7
Lymphocytes, produced by lymphatic organs, are a significant part of the Immunity function and the main performer of immune function. They are responsible for fighting external infection and monitoring cell variation, which can be divided into T lymphocytes, B lymphocytes and NK cells, and mature T cells can be divided into CD3+CD4+ cells and CD3+CD8+ cells. According to the different surface molecular markers and functions, CD4+ and CD8+ T lymphocytes can be further divided into functional subsets (CD4+/CD8+CD28+), activation subsets (CD4+/CD8+CD38+), innocence subsets (CD4+CD45RA+CD62L+) and memory subsets (CD4+CD45RA−). CD4+ T lymphocytes can assist the development and differentiation of other lymphocytes and secrete a large number of cytokines to regulate cellular immunity and humoral immunity. CD8+ T lymphocytes have inhibitory and lethal effects, among which CD8+ inhibitory T cells have the function of inhibiting humoral immunity and cellular immunity. B lymphocytes play an immunomodulatory role in humoral immunity mainly by producing antibodies. Natural killer (NK) cells participate in natural immunity and have the role of killing target cells. Peripheral blood lymphocyte subsets are important indicators to evaluate the immune function. In a recent study, CD4+ T cells subsets were found to be differentially expressed in patients with atherosclerosis.Citation8 Moreover, differential peripheral white blood cell counts may indicate the risk of dyslipidemia.Citation9 Some studies have also found that neutrophil subsets are expressed differently in patients with dyslipidemia.Citation10 Several studies have suggested that dyslipidemia is related to inflammation and immune disorder, but studies on the relationship between dyslipidemia and lymphocyte subsets are still lacking.Citation11,Citation12 In order to explore the influence of dyslipidemia on immune function, the absolute level of peripheral blood lymphocyte subsets in patients with dyslipidemia was detected, and the correlation between peripheral blood lymphocyte subsets and hypercholesterolemia and hypertriglyceridemia was analyzed in this study, which aim to provide a new idea for clinical diagnosis, therapy and prevention of dyslipidemia.
Materials and Methods
Patient Enrollment and Blood Specimen Collection
Under the diagnostic standard of dyslipidemia from “The 2017 guidelines for prevention and treatment of dyslipidemia in Chinese adults”, 51 adult patients with 32 male and 19 female diagnosed as having dyslipidemia, which manifested without any other physical disorders that may lead dyslipidemia, as well as 51 adult healthy volunteers (control) with 32 male and 19 female, whose presented normal blood lipids and no concurrent diseases or drug therapy at the moment of blood drawing, were selected into our research. The healthy people were matched with dyslipidemia patients of similar ages and genders. 10 mL whole blood sample from each subject was allowed to collect into a tube with heparin. The blood samples were stored at ambient temperature and moved within 2 hours from the clinical wards to the analytical laboratory, where they were immediately processed.
Criteria for determining dyslipidemia: “The 2017 guidelines for prevention and treatment of dyslipidemia in Chinese adults”: One or more abnormalities in TC≥ 6.22 mmol/L, TG≥ 2.3 mmol/L, and LDL≥ 4.14 mmol/L were defined as dyslipidemia. The reference range of dyslipidemia in the biochemical room of our hospital was TC≥ 5.71 mmol/L, TG≥ 1.70 mmol/L, and LDL≥ 3.12 mmol/L, respectively. Exclusion criteria: (1) patients with various acute and chronic infections; (2) Patients with diabetes and complications; (3) Patients with diseases of the blood system; (4) Patients with autoimmune diseases; (5) Patients using hormones; (6) Patients with hyperuricemia; (7) Patients with malignant tumor; (8) Patients with coronary heart disease; (9) Patients with hypertension; (10) Patients with endocrine-related diseases, such as thyroid dysfunction.
Detection of Lymphocyte Subsets in Peripheral Blood
One BD Trucount absolute counter tube was selected and labeled as No. 1 tube, and two tubes for flow cytometry were labeled as No. 2 and No. 3 tubes, respectively. 20 ul of MultitestTM 6-Color TBNK Reagent and 50 ul of heparin anticoagulant peripheral blood were allowed to be added into the No.1 tube. After mixing evenly, it was incubated for 20 mins in dark at room temperature. Afterward, 450 ul diluted Red Blood Cell Lysis Buffer was added into the absolute counter tube, mixed evenly and kept in the dark at room temperature for 20 mins, and then the flow cytometer (Becton Dickinson) was applied to detect and analyze the lymphocyte subsets. 20 ul fluorescent antibodies HLA-DR-FITC, CD28-PE, CD4-PerCP-Cy5.5, CD8-PE-Cy7, CD38-APC, and CD3-APC-Cy7 were, respectively, added into No. 2 tube. In addition, 20 ul of fluorescent antibodies CD45RA-FITC, CD62L-PE, CD4-PerCP-Cy5.5 were, respectively, added into No. 3 tube. 50 ul heparin anticoagulant peripheral blood was added into the No.2 and No.3 tubes, respectively, and then, the tubes were mixed by eddy oscillation and incubated for 20 mins in the dark at room temperature. 2 mL hemolysin was added, and tubes were incubated for 20 mins in the dark at room temperature, followed by centrifugation at 500 g for 5 mins. After completion of centrifugation, the supernatant was poured out and the precipitation was left. 1 mL PBS buffer was added to resuspend the cell, and then, the cell was mixed by vortex and ready to be tested.
Statistical Analysis
Data are expressed as mean ± SD. A Student’s t-test was used to determine the difference between the two groups. An X2 test was used for comparison of enumeration data. Statistical analyses were performed by using the Statistical Program for Social Sciences (SPSS) 20.0 software (SPSS Inc. Chicago, IL, USA). A P-value <0.05 was considered statistically significant. The two groups of data conformed to the normal distribution and the correlation analysis was performed by Pearson test.
Results
Basic Information of Peripheral Blood in Two Groups
The level of TC, TG and LDL in dyslipidemia patients was significantly higher than those in healthy controls (P<0.05). In addition, there were no markedly differences in age, gender, fasting plasma glucose (FPG) and uric acid (UA) between healthy controls and dyslipidemia patients (P>0.05) ().
Table 1 Comparison of Peripheral Blood Features Between Healthy Controls and Dyslipidemia Patients in Peripheral Blood
The Absolute Value of Lymphocyte Subsets in Peripheral Blood of Each Group
The absolute values of CD19+ B cell, CD3+ T cell, CD4+ Th cell, CD4+CD28+ cell, naive CD4+ T cell and memory CD4+ T cell in patients with dyslipidemia were significantly higher than those in healthy controls (P<0.05). There was no significant difference between healthy controls and dyslipidemia patients in other lymphocyte subsets (P>0.05) ( and and ).
Table 2 Comparison of Lymphocyte Subsets Between Healthy Controls and Dyslipidemia Patients in Peripheral Blood (Cells/uL)
Figure 1 Comparison of lymphocyte subsets between healthy controls and dyslipidemia patients. *Comparison with the group of healthy controls (P<0.05).
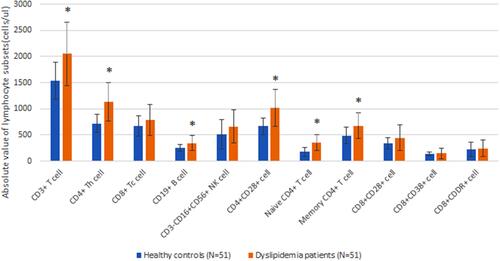
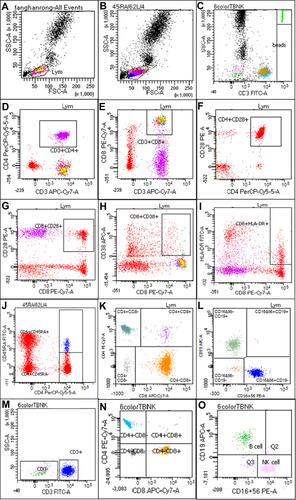
Figure 2 Flow cytometry of lymphocyte subsets (A–O). T lymphocytes (CD3+), B lymphocytes (CD19+), Natural killer (NK) lymphocytes (CD3−CD16+ and/or CD56+), Helper inducer T lymphocytes (CD3+CD4+), Functional subset of CD4+ T cell (CD4+CD28+), Native subset of CD4+ T cell (CD4+CD45RA+CD62L+), memory T cell subset (CD4+CD45RA−), Suppressor cytotoxic T lymphocytes (CD3+CD8+), Functional subset of CD8+ T cell (CD8+CD28+), Activated subsets of CD8+ T cell (CD8+CD38+ and CD8+DR+).
Correlation Analysis of Lymphocyte Subsets with Total Cholesterol and Triacylglycerol in Dyslipidemia Patients
The absolute values of CD3+ T cell and naive CD4+ T cell were markedly positively correlated with hypercholesterolemia in peripheral blood (r=0.291 and 0.306, respectively, all P<0.05). There was no significant correlation between hypertriglyceridemia and lymphocyte subsets (P>0.05) ().
Table 3 Correlation Analysis of Lymphocyte Subsets with Hypercholesterolemia and Hypertriglyceridemia in Dyslipidemia Patients
Discussion
Clinical and Laboratory researchers have suggested that lipid metabolism acts as a pivotal part of immunity, which may have significant implications for cardiovascular disease, infectious disease, autoimmune disease and metabolic dysfunction.Citation4,Citation13–Citation15 In our study of 51 dyslipidemia patients and healthy controls who underwent physical examination in our hospital from January 2020 to October 2020, we have demonstrated that some lymphocyte subsets were significantly overexpressed in patients and there are significant relationships between CD3+/naive CD4+ T cell and hypercholesterolemia in peripheral blood. Our research found that dyslipidemia may have potential effects on immunity in both humoral and cellular immunity, whereas hypertriglyceridemia seems to have little correlation with lymphocyte subsets. Further, hypercholesterolemia majorly affects immune profiles in cellular immunity, leading to variable immune outcomes. Studies have shown that lipoprotein can directly affect the structure and activity of monocyte by regulating cholesterin flux of cell, modulating lipid rafts tissue, isolating pathogenic stimulus and providing immunoregulatory effect.Citation4,Citation16 Lymphocyte and monocyte are both a kind of leukocyte, but there are a limited number of reports about lipoprotein and lymphocyte subsets. As a matter of fact, our research is the first to uncover the interaction between lymphocyte subsets and dyslipidemia.
The relationship between lymphocyte subsets and lipids, as well as the excitatory or inhibitory regulation in these lymphocyte cells have been the research hotspots of cardiovascular disorders in recent research. In our study, the level of CD19+ B cell, CD3+ T cell, CD4+ Th cell, CD4+CD28+ cell, naive CD4+ T cell and memory CD4+ T cell were in a state of markedly increased expression in patients diagnosed as having dyslipidemia compared to healthy controls. B cells have three main effects: the production of antibody, the presentation of antigen or T cell interaction, and the release of cell factor. It was reported that all of the effect can regulate atherosclerosis and were associated with the activation status of the B cells,Citation17 which was consistent with the enhanced expression of B cells in patients with dyslipidemia observed in our experiment. Other experimental data indicated that changes in cellular lipid metabolism may have critical effects on humoral and cellular immunity, especially T cells and T cell subsets proliferation and cell fate decisions. Most adaptive immune responses require the activation of specific T cells via the T cell antigen receptor-CD3 complex. Researchers used a natural analogue of cholesterol that inhibited the phosphorylation reaction of CD3 ITAM, leading to a decrease in the activation of T cell.Citation18 Bagley et al found that hyperlipidemia promoted expedited repulsion of vascularized heart allografts in mouse by the inductive effect of anti-donor CD4+ Th cells and hyperlipidemia also promoted an increase in effector T cells.Citation19 These studies suggested that lipid metabolism may also play a critical role in activation-induced proliferation and differentiation of T lymphocyte. Together, we speculate that Lipid is not only a structural molecule required for cell membrane formation and proliferation but also a central switch regulating T cell fate determination. In addition, dyslipidemia profoundly affects T cell subsets and alterations in T cell subsets may also seem to cause the change in immune effect. Therefore, lipid-lowering therapy may be a potential treatment path for autoimmune diseases or immune rejection.
Based on a further study of the relationships between dyslipidemia and lymphocyte subsets, we found that CD3+ T cell and naive CD4+ T cell were markedly positively associated with hypercholesterolemia in peripheral blood. Mailer et al suggested that hypercholesterolemia enhanced T cell antigen receptor stimulation and T cell development, as well as T cell proliferation.Citation20 However, Maganto-García et al found that prolonged hypercholesterolemia can impair T cell but not the accumulation of lymphocyte, and the lipid-lowering can prevent the decrease of T lymphocyte in lesion.Citation21 Additionally, Baardman et al suggested that the low level of T cells and Impaired inhibition effect were related to the progress of atherosclerosis.Citation22 Together, atherosclerosis is a chronic inflammatory disease of the arterial wall and a major cause of cardiovascular disease. Its progression is closely related to the number and function of lymphocyte subsets, especially T cell subsets. Therefore, we speculate that therapeutic strategy to improve the level or function of T lymphocyte could be beneficial to preventing the development of dyslipidemia-related diseases.
Conclusion
In summary, dyslipidemia has potential effects on immune profiles in lymphocytes subsets, and changes in the absolute number of lymphocyte subsets in dyslipidemia patients may lead to immune dysfunction.
Ethics Approval
The study protocols were conducted according to the principles of the Declaration of Helsinki and were approved by the Scientific and Medical Ethical Committee of the Second Affiliated Hospital of Shantou University Medical College. All the subjects gave their written informed consent before their inclusion in the study.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval for the version to be published; and agreed to be accountable for all aspects of the work.
Disclosure
Da-Ming Xu, Qian Li, and Jing-Xing Yi are co-first authors for this study. The authors declare that they have no competing interests.
Additional information
Funding
References
- Kopin L, Lowenstein C. Dyslipidemia. Ann Intern Med. 2017;167(11):ITC81–ITC96. doi:10.7326/AITC201712050
- Mooradian AD. Dyslipidemia in type 2 diabetes mellitus. Nat Clin Pract Endocrinol Metab. 2009;5(3):150–159. doi:10.1038/ncpendmet1066
- Yoon SS, Dillon CF, Carroll M, Illoh K, Ostchega Y. Effects of statins on serum inflammatory markers: the U.S. national health and nutrition examination survey 1999–2004. J Atheroscler Thromb. 2010;17(11):1176–1182. doi:10.5551/jat.5652
- Andersen CJ. Impact of dietary cholesterol on the pathophysiology of infectious and autoimmune disease. Nutrients. 2018;10(6):764. doi:10.3390/nu10060764
- Kritikou E, van der Heijden T, Swart M, et al. Hypercholesterolemia induces a mast cell-CD4+ T cell interaction in atherosclerosis. J Immunol. 2019;202(5):1531–1539. doi:10.4049/jimmunol.1800648
- Giugliano G, Brevetti G, Lanero S, Schiano V, Laurenzano E, Chiariello M. Leukocyte count in peripheral arterial disease: a simple, reliable, inexpensive approach to cardiovascular risk prediction. Atherosclerosis. 2010;210(1):288–293. doi:10.1016/j.atherosclerosis.2009.11.009
- Drechsler M, Megens RT, van Zandvoort M, Weber C, Soehnlein O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation. 2010;122(18):1837–1845. doi:10.1161/CIRCULATIONAHA.110.961714
- Tay MHD, Lim SYJ, Leong YFI, et al. Halted lymphocyte egress via efferent lymph contributes to lymph node hypertrophy during hypercholesterolemia. Front Immunol. 2019;10:575. doi:10.3389/fimmu.2019.00575
- Corbi SCT, de Vasconcellos JF, Bastos AS, et al. Circulating lymphocytes and monocytes transcriptomic analysis of patients with type 2 diabetes mellitus, dyslipidemia and periodontitis. Sci Rep. 2020;10(1):8145. doi:10.1038/s41598-020-65042-9
- Guasti L, Maresca AM, Schembri L, et al. Relationship between regulatory T cells subsets and lipid profile in dyslipidemic patients: a longitudinal study during atorvastatin treatment. BMC Cardiovasc Disord. 2016;16:26. doi:10.1186/s12872-016-0201-y
- Liu Y, Kong X, Wang W, et al. Association of peripheral differential leukocyte counts with dyslipidemia risk in Chinese patients with hypertension: insight from the China stroke primary prevention trial. J Lipid Res. 2017;58(1):256–266. doi:10.1194/jlr.P067686
- Genkel V, Dolgushin I, Baturina I, et al. Associations between hypertriglyceridemia and circulating neutrophil subpopulation in patients with dyslipidemia. Int J Inflam. 2021;2021:6695468. doi:10.1155/2021/6695468
- Andersen CJ, Murphy KE, Fernandez ML. Impact of obesity and metabolic syndrome on immunity. Adv Nutr. 2016;7(1):66–75. doi:10.3945/an.115.010207
- Pirillo A, Catapano AL, Norata GD. HDL in infectious diseases and sepsis. Handb Exp Pharmacol. 2015;224:483–508. doi:10.1007/978-3-319-09665-0_15
- Andersen CJ, Lee JY, Blesso CN, Carr TP, Fernandez ML. Egg intake during carbohydrate restriction alters peripheral blood mononuclear cell inflammation and cholesterol homeostasis in metabolic syndrome. Nutrients. 2014;6(7):2650–2667. doi:10.3390/nu6072650
- Thompson PA, Kitchens RL. Native high-density lipoprotein augments monocyte responses to lipopolysaccharide (LPS) by suppressing the inhibitory activity of LPS-binding protein. J Immunol. 2006;177(7):4880–4887. doi:10.4049/jimmunol.177.7.4880
- Ma SD, Mussbacher M, Galkina EV. Functional role of B cells in atherosclerosis. Cells. 2021;10(2):270. doi:10.3390/cells10020270
- Wang F, Beck-García K, Zorzin C, Schamel WW, Davis MM. Inhibition of T cell receptor signaling by cholesterol sulfate, a naturally occurring derivative of membrane cholesterol. Nat Immunol. 2016;17(7):844–850. doi:10.1038/ni.3462
- Bagley J, Yuan J, Chandrakar A, Iacomini J. Hyperlipidemia alters regulatory T cell function and promotes resistance to tolerance induction through costimulatory molecule blockade. Am J Transplant. 2015;15(9):2324–2335. doi:10.1111/ajt.13351
- Mailer RKW, Gisterå A, Polyzos KA, Ketelhuth DFJ, Hansson GK. Hypercholesterolemia enhances T cell receptor signaling and increases the regulatory T cell population. Sci Rep. 2017;7(1):15655. doi:10.1038/s41598-017-15546-8
- Maganto-García E, Tarrio ML, Grabie N, Bu DX, Lichtman AH. Dynamic changes in regulatory T cells are linked to levels of diet-induced hypercholesterolemia. Circulation. 2011;124(2):185–195. doi:10.1161/CIRCULATIONAHA.110.006411
- Baardman J, Lutgens E. Regulatory T cell metabolism in atherosclerosis. Metabolites. 2020;10(7):279. doi:10.3390/metabo10070279
