Abstract
Background
The coronavirus disease 2019 (COVID-19) has been shown to affect several systems, notably the respiratory system. However, there has been considerable evidence implicating the nervous system in COVID-19 infection. This study aims to investigate the clinical characteristics of patients whose cerebrospinal fluid (CSF) tested positive for SARS-CoV-2.
Methods
A comprehensive search of PubMed, EMBASE, Scopus, WHO Coronavirus database, bioRxiv, medRxiv, and Web of Science databases was carried out in August 2020. Original studies involving patients who tested positive for SARS-COV-2 in their CSF were included. Key search terms encompassed all variations of “COVID-19” AND “Cerebrospinal Fluid”.
Results
A total of 525 studies were identified. Fifty-six full-text articles were assessed, of which 14 were included. In total, 14 patients tested positive for SARS-CoV-2 in their CSF. 21.4% (3/14) of patients had negative nasopharyngeal (NP) swabs despite a positive CSF sample. About 14.2% (2/14) of patients who initially had positive NP swabs developed neurological deterioration after a supposed recovery as indicated by their negative NP swabs, but their CSF still tested positive for SARS-CoV-2. Common symptoms were headache (42.8%; 6/14), fever (35.6%; 5/14), vomiting (28.6%; 4/14), cough (28.6; 4/14), visual disturbances (28.6%; 4/14), diarrhea (21.4%; 3/14), and seizures (21.4%; 3/14). Four patients (28.6%) were admitted to ICU, one (7.14%) was admitted to a rehabilitation facility, and two (14.3%) died.
Conclusion
Physicians should be familiar with the presenting neurological features of COVID-19, and be aware that they can occur despite a negative NP swab. The results of this study are intended to aid in the development of informed guidelines to diagnose and treat COVID-19 patients with neurological manifestations.
Background
Coronavirus Disease 2019 (COVID-19) is a novel infectious disease capable of causing mild to severe illness, typically respiratory, in both humans and animals. The virus responsible for COVID-19, referred to as Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), resides primarily in the respiratory tract and causes symptoms ranging from mild cough, sore throat, and nasal congestion to more severe respiratory distress. Recently, it has been shown to have additional neuro-invasive potential.Citation1 Infected patients globally have been reported to have headaches, paraesthesia, anosmia, ageusia, neuralgia, and dizziness.Citation2
Additionally, several case reports and cohort studies have reported rare cases of meningoencephalitis, seizures, and immune-mediated neurological diseases.Citation3
SARS-CoV-2 can either infiltrate the peripheral nervous system (PNS) and migrate to the CNS or directly infect the CNS.Citation4 There are three postulated mechanisms of transmission of the SARS-CoV-2 virus via the PNS: the transcribial route, axonal transport, and trans-synaptic transfer, and hematogenous and/or lymphatic route.Citation5 The transcribial route involves a primary olfactory infection followed by infiltration into the subarachnoid space via the cribriform plates.Citation4,Citation6 The axonal transport and trans-synaptic transfer hypothesis suggests that an initial infection of peripheral nerve terminals results in a migration of the virus, up the nervous system, to the trigeminal, olfactory, and/or vagus nerve.Citation4,Citation6 It is important to note that both the gastrointestinal and the respiratory branches of the vagus nerve are susceptible to the infection.Citation7–Citation10 An infection of the CNS may occur via direct contact of the SARS-CoV-2 virus with the brain microvascular endothelial cells. This in turn leads to extracellular virus release into the CNS parenchyma. Lastly, compromised tight junctions at the blood brain barrier or virally infected leukocytes may provide viral access to the CNS via endocytosis.Citation9,Citation10 More research is needed to accurately map the neurologic pathogenesis of SARS-CoV-2, and how this may translate to clinical diagnosis, prognosis, and patient care.
Dealing with a pandemic of this magnitude requires rapid and effective diagnostic tools to help combat the disease as early as possible. The diagnostic tool most widely accepted is the reverse transcriptase polymerase chain reaction (RT-PCR), used on a nasopharyngeal (NP) sample.Citation11 Although SARS-CoV-2 RT-PCR is typically conducted on an NP swab, it can also be conducted on a cerebrospinal fluid (CSF) sample obtained from a lumbar puncture (LP).Citation12–Citation14
In order to best understand the pathophysiology of SARS-CoV-2 as it relates to neuropsychiatric manifestations, it is important to explore viral presence in the nervous system, and how this may correlate—if at all—with clinical presentation and outcomes. Thus, this systematic review aims to compile and synthesize primary studies that report on patients who tested positive for SARS-CoV-2 via their CSF sample. Our study investigates the unique clinical manifestations and characteristics of this patient cohort, along with relevant outcomes, disease progression and management. Furthermore, we hope our findings will help identify when to consider PCR CSF tests despite a negative NP swab test.Citation15 By exploring the CNS involvement in SARS-CoV-2, this can aid in the development of new guidelines to diagnose and treat COVID-19 patients with neurological involvement.
Methods
Eligibility
We included primary research papers (case reports, case studies, cohort studies, cross-sectional studies, randomised control trials, letters to the editor reporting primary findings) that investigated the clinical course, outcomes, prognosis, management, and characteristics of patients who tested positive for SARS-COV-2 in their CSF using RT-PCR test. Exclusion criteria included non-English articles, animal studies, and non-original articles (eg, editorials that did not contain original data).
Search Strategy
We conducted our search in PubMed NCBI, Excerpta Medica dataBASE, Scopus, WHO COVID-19 Global literature on coronavirus disease database, Biorxiv and Medrxiv, and Web of Science on August 24th, 2020 using the following search terms: ((“Cerebrospinal fluid” [Mesh]) OR (“CSF” OR “Cerebrospinal fluid” OR “Cerebral spinal fluid” OR “Cerebro-spinal fluid” OR “Lumbar puncture” OR “Spinal tap”)) AND (“coronavirus” [MeSH] OR “coronavirus infections” [MeSH Terms] OR “coronavirus” [All Fields] OR “covid 2019” [All Fields] OR “SARS2” [All Fields] OR “SARS-CoV-2” [All Fields] OR “SARS-CoV-19” [All Fields] OR “severe acute respiratory syndrome coronavirus 2” [supplementary concept] OR “coronavirus infection” [All Fields] OR “severe acute respiratory pneumonia outbreak” [All Fields] OR “novel cov” [All Fields] OR “2019ncov” [All Fields] OR “sars cov2” [All Fields] OR “cov22” [All Fields] OR “ncov” [All Fields] OR “covid-19” [All Fields] OR “covid19” [All Fields] OR “coronaviridae” [All Fields] OR “corona virus” [All Fields]). The selection criteria were limited to papers published from December 2019 until August 2020 and papers written in English.
Study Selection
After deduplication of the titles, two reviewers independently screened all the titles and abstracts of the papers according to the predefined inclusion and exclusion criteria. Next, full texts of potentially eligible studies were retrieved and reviewed independently by two authors. A third author resolved any disagreement. Reviews that included patients who tested positive for SARS-CoV-2 in their CSF were cross checked to identify any studies that matched our eligibility criteria.
Data Extraction and Interpretation
Data was extracted via a dual approach by two independent reviewers and inserted into a standardized review sheet. Data collected includes study characteristics (study title, authors, date of publication, publication type, study site, number of subjects), population characteristics, clinical findings, radiological findings, management, and final outcome. A third author resolved any disagreement.
Risk of Bias in Individual Studies
Two authors assessed the quality of the selected articles utilizing the National Institutes of Health (NIH) quality assessment tool for observational cohort and cross-sectional studies. Quality assessment of case reports was carried out using Joanna Briggs Institute (JBI) critical appraisal checklist for case reports.
Meta-Analysis
A meta-analysis was not performed due to the preliminary nature of the study. Our aim was to review the literature in a scoping manner, and systemically gather and report the relevant data in the literature. In addition, due to the qualitative, heterogenic narrative nature of the outcomes, and the limited number of case reports (and absence of clinical studies), a meta-analysis would not be appropriate.
Results
Study Selection
An initial search of seven databases yielded a total of 525 publications. Fifty-six full-text articles were included and assessed for eligibility post abstract screening for relevance and deduplication, of which 14 were qualitatively analysed. After the application of the inclusion/exclusion criteria, they were narrowed down to 14. A Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram explaining the steps of identification, screening, inclusion, and exclusion is presented in .
Study and Patients Characteristics
Of the 14 articles included in this study, nine were case reports,Citation10,Citation16–Citation23 three were retrospective studies,Citation24–Citation26 one was a letter to the editor reporting original data of a patient,Citation27 and one follow-up letter to the editor of the same latter patient.Citation28 All were published in 2020. The studies were conducted worldwide, including France, USA, Spain, Brazil, Japan, Turkey, Sweden, UAE, France, Germany, and Iran (). From the 14 eligible studies identified, the total sample size was 733. Out of these, only 14 patients tested positive for SARS-CoV-2 in their CSF samples. As the scope of this review is to investigate only patients who tested positive for SARS-CoV-2 in their CSF according to the eligibility criteria, we only described these 14 patients. The mean age of the patients was 40 (SD ±15.7) and the median was 47.5, with 50% of them being females. Comorbidities were present in 40% (4/10) of the patients, and were mainly hypertension (2/4),Citation16,Citation22 ischemic heart disease (1/4),Citation22 diabetes (1/4),Citation28 metastatic colorectal cancer (1/4),Citation22 migraines (1/4),Citation16 and one patient had prior pancreatic-kidney transplant surgery (1/4).Citation23
Figure 1 PRISMA flow diagram of literature search and selection.
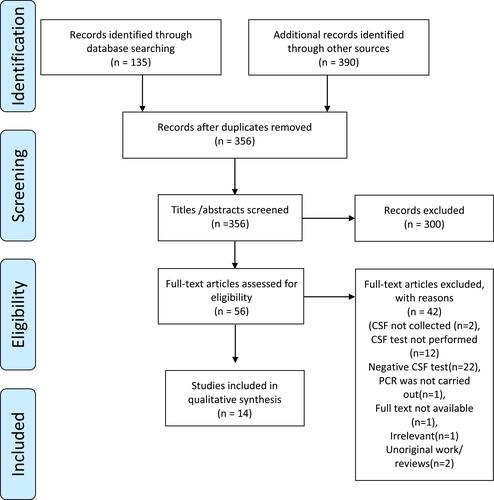
Table 1 Characteristics of Included Studies
Clinical Course and Diagnosis
In 21.4% (3/14) of cases, nasopharyngeal (NP) swabs initially tested negative despite a positive CSF sample.Citation10,Citation17,Citation18 14.2% (2/14) of positive cases as per NP swab tested negative after supposed recovery but progressed to neurological deterioration and positive CSF tests.Citation20,Citation22 10/14 patients had both positive nasopharyngeal sample and CSF sampleCitation16,Citation19–Citation25,Citation28 (in two of these cases CSF was not tested initially, but was found to be positive at post-mortem), however samples were not always positive on the first test; 3/14 cases demonstrated a positive nasopharyngeal test but an initially negative CSF test.Citation20,Citation22,Citation28 summarises the clinical and diagnostic findings.
Table 2 Summary of Presentation and Clinical Course of All Cases Testing Positive for SARS-CoV-2 in CSF Samples
Symptoms
Most commonly reported symptoms included: Headache (6/14),Citation10,Citation16,Citation17,Citation19,Citation21,Citation28 fever (5/14),Citation17,Citation20,Citation21,Citation23,Citation28 vomiting (4/14),Citation16,Citation21 cough (4/14),Citation10,Citation19,Citation21,Citation23 visual disturbances (4/14),Citation16,Citation19,Citation23,Citation28 diarrhoea (3/14),Citation18,Citation21,Citation23 and seizure (3/14)Citation17,Citation23,Citation28(). In two of the studies, the patients’ COVID status was identified as severeCitation25,Citation26 and in one of these cases the patient was noted to be suffering from acute respiratory distress syndrome.Citation25 Neurological symptoms were cited as the reason CSF test was carried out in 6/14 of the studies.Citation16–Citation18,Citation20,Citation22,Citation28
Lab Findings
Studies of the positive patients’ CSF samples () revealed leukocytosis in 2/14 patients,Citation17,Citation22 elevated CSF protein (hyperproteinorrachia) in 3/14,Citation22,Citation23,Citation28 hypoglycorrhachia in 1/14,Citation22 and an elevated red blood cells (RBCs) in 1/14 samples.Citation28 D-dimers were elevated in 3/14 blood samples.Citation16,Citation21,Citation22
Table 3 Blood and Cerebrospinal Fluid Lab Findings of Cases with SARS-CoV-2 Positive CSF Samples
Radiological Findings
Radiological findings (CXR, chest CT, systemic CT, Brain MRI, and head CT) were reported for 11/14 patients. However, we could not extract the data from one cohort study.Citation26 Radiological findings were normal in 2/14 patients.Citation18,Citation28 The most common findings on brain MRI FLAIR were hyperintense regions in different areas of the brain (6/14),Citation10,Citation17,Citation19–Citation21,Citation23 and the commonest finding on chest CT was ground glass opacities in the lungs (5/14)Citation10,Citation17,Citation20,Citation22,Citation23 ().
Table 4 Radiological Findings of Cases with SARS-CoV-2 Positive CSF Samples
EEG Findings
EEG findings were reported in two studies,Citation20,Citation28 two of which noted a similar generalised slowing of waves with no epileptic activity.Citation20,Citation28 One of these patients was noted to have a previous seizure.Citation28
Management and Treatment
The management of 4 patients was not discussed in their respective studies,Citation18,Citation24–Citation26 while the management for the remaining patients varied. Invasive intervention was required in two patients: Surgery was performed on 1/14 patients to remove the chronic subdural haematomaCitation21 and endotracheal intubation and mechanical ventilation was required on another patient with impaired consciousness.Citation17 The mainstay initial management in 4/14 patients was acyclovir.Citation10,Citation20,Citation22,Citation28 This was, however, discontinued in one patient following negative herpes simplex virus results.Citation28 Levetiracetam was given in 3/14 patientsCitation10,Citation23,Citation28 and hydroxychloroquine was administered to 5/14 patients.Citation10,Citation16,Citation22,Citation23,Citation28 shows the management and outcomes of the 14 SARS-CoV-2 CSF positive patients.
Table 5 The Management and Outcomes of SARS-CoV-2 CSF Positive Patients
Clinical Outcomes
The outcomes at the end of the study periods varied in these 14 SARS-CoV-2 CSF positive patients (). Overall, 2/14 deathsCitation24 and 4/14 ICU admissionsCitation17,Citation21,Citation25 were reported. Symptoms improved in 1/14 cases who remained admitted,Citation10 while 6/14 cases were discharged/recovered,Citation16,Citation18–Citation20,Citation23,Citation28 and 1/14 was transferred to a rehabilitation centre.Citation20 Outcomes were not stated for two of the 14 patients.Citation22,Citation26
Risk of Bias Across Studies
Bias assessment is documented in Appendix 1, Tables S1 and S2.
Discussion
In this systematic review, we identified 14 articles which described 14 patients with positive SARS-CoV-2 CSF out of 733 articles. We systematically reviewed all reports of RT-PCR positive SARS-CoV-2 CSF samples in the literature since the start of the outbreak in December. A mixed-methods exploratory approach was adopted for data analysis, making observations, and investigating any preliminary patterns and theories that can be extracted from the sporadic cases reported. Common symptoms were headache fever, vomiting, cough, visual disturbances, diarrhoea, and seizures. Four patients were admitted to ICU, one was admitted to a rehabilitation facility, and two died.
The paucity of case reports that reported CSF-positive SARS-CoV-2 patients may indicate that viral neuro-invasion by SARS-CoV-2 appears to be rare. This is in accordance with another systematic review in which 6%Citation17 tested positive out of 304 patients whose CSF was tested for SARS-CoV-2.Citation29
The low prevalence of CSF positive SARS-CoV-2 results can be attributed to several factors, the first is that CSF testing rate was initially low since it is done only in cases with serious CNS manifestations, if patients had no CNS manifestations they would therefore not be tested. Secondly, isolation of SARS-CoV-2 in CSF may be challenging because of rapid CSF clearance, low titters or delayed sampling.Citation30–Citation32 Further, CSF antibodies test was not always done for CSF negative patients which could have led to missing resolved infection. In a recent systematic review, it was reported that out of those who did not test positive for CSF but had CNS symptoms, 42/58 (72%) tested positive for SARS-CoV-2 antibodies in the CSF.Citation29
The discrepancy between CSF results and NP results could be attributed to the variability in the cycle threshold Ct (Ct; the number of amplification cycles required for the target gene to exceed the threshold) cut-off point (some used 40, some 37 and some 35).Citation17,Citation29 Preanalytical issues such as collection techniques, and inadequate sample storage/transportation, timing of sample throughout the course of the disease which could have led to serious diagnostic errors.Citation33 Further, serum antibodies were not always checked for NP PCR negative patients which could have verified the resolved infections. Additionally, CSF SARS-CoV-2 PCR testing is not 100% specific for intrathecal virus, in part because a sample can be contaminated from shed airborne virus or blood contamination.Citation34 Interestingly, PCR testing for the N2 gene target of SARS-CoV-2 was noted to have the highest sensitivity in CSF when compared with a nasopharyngeal swab, bronchoalveolar lavage, sputum, plasma, or stool.Citation37 Despite this, the clinical indications for performing LPs in patients with SARS-CoV-2 infection remain unclear. Additionally, how clinicians can use information gained from LPs, such as cell counts and infectious workup, in the management of COVID-19 and neurological symptoms has not been established.
In this review, comorbidities were not commonly present among CSF positive patients (60%) which re-emphasizes that otherwise healthy individuals may present with CSF viral neuro-infiltration in the absence of co-morbidities in the setting of COVID-19.Citation35
Patients whose CSF samples tested positive for SARS-CoV-2 reported a range of symptoms, with respiratory distress not always being reported. Headache, fever, vomiting, coughing, and visual disturbances were commonly reported, before progressing to more severe/intense neurological symptoms.Citation36 This might have an implication on CSF testing for diagnostic purposes. Further studies are required to define whether CSF SARS-CoV-2 testing is warranted in certain clinical contexts.Citation16,Citation20,Citation28
High levels of lymphocytes and protein were reported in 2/14 and 3/14 CSF samples of COVID-19 positive patients, respectively. CSF Pleocytosis is expected and occurs secondary to an inflammatory and/or infectious process.Citation29 Also, the observation of hyperproteinorrachia may indicate axonal injury and the existence of intrathecal antibodies.Citation29 There were no striking blood findings except for leucocytosis and hyperglycaemia. Both are expected and reflects the inflammatory process due to the disease. However, due to the lack of data, it is not known whether patients with hyperglycaemia in these case reports had pre-existing diabetes or not.
The EEG findings showed two patients with generalised slowing of waves with no epileptic activity. One of them had a history of pre-existing seizures. It is well documented in the literature that COVID-19 can result in EEG changes, and it is correlated with disease severity.Citation37,Citation38 EEG findings in COVID-19 may indicate localized dysfunction, non-specific encephalopathy, and cortical irritability.Citation38 In fact, frontal findings are common and have been proposed as a biomarker for COVID-19 encephalopathy.Citation38 Diffuse EEG changes in the context of COVID-19 have been speculated to result from systemic involvement or diffuse viral involvement of the brain while frontal EEG findings suggest direct brain involvement.Citation38
There are several limitations to this review mainly attributed to lack of data from original case series such as description of test technique, time at which sample is collected, CSF analysis and SARS CoV antibodies. Lastly, this review is limited by publication bias and the paucity of published case reports.
More studies are needed to describe how results of LP influence clinical decision-making in a case series of patients with COVID-19 even if SARS-CoV-2 is not detected in the CSF.
Conclusions
This review describes the unique characteristics of patients who tested positive for SARS-CoV-2 in their CSF sample, regardless of the test outcome of the NP sample. Nevertheless, there are not enough data in the literature for guideline formation, especially given the fact that COVID-19 is a novel virus and an emergent crisis. Hence, more evidence is needed to improve our understanding regarding how results of LP influence clinical decision-making in a case series of patients with COVID-19 even if SARS-CoV-2 is not detected in the CSF. Additionally, how clinicians can use information gained from LPs, such as cell counts and infectious workup, in the management of COVID-19.
Abbreviations
COVID-19, coronavirus disease 2019; CSF, cerebrospinal fluid; NP, nasopharyngeal; RT-PCR, reverse transcriptase polymerase chain reaction; LP, Lumbar Puncture; PNS, peripheral nervous system; NIH, National Institutes of Health; JBI, Joanna Briggs Institute; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Data Sharing Statement
All data generated or analysed during this study are included in this published article and its supplementary information files.
Author Contributions
MS conceived of the idea. MS, SIM, WK, LRM, and YA drafted one or more sections of the manuscript. MS, SIM, and GAJ reviewed and edited the manuscript. GAJ supervised the work. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Acknowledgments
We would like to thank Mr. Niall O’Brien for assisting us with our search strategy.
Disclosure
The authors declare no conflicts of interest in this work.
Additional information
Funding
References
- World Health Organization Organisation TWH. Q&A on coronaviruses (COVID-19); April 17, 2020. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/q-a-coronaviruses. Accessed December 7, 2021.
- Ellul MA, Benjamin L, Singh B, et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19(9):767–83{Ellul, 2020 #17}. doi:10.1016/S1474-4422(20)30221-0
- Chen X, Laurent S, Onur OA, et al. A systematic review of neurological symptoms and complications of COVID-19. J Neurol. 2021;268(2):392–402.
- Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995–998. doi:10.1021/acschemneuro.0c00122
- Cain MD, Salimi H, Diamond MS, Klein RS. Mechanisms of pathogen invasion into the central nervous system. Neuron. 2019;103(5):771–783. doi:10.1016/j.neuron.2019.07.015
- Baig AM. Emerging insights for better delivery of chemicals and stem cells to the brain. ACS Chem Neurosci. 2017;8(6):1119–1121. doi:10.1021/acschemneuro.7b00106
- Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008;82(15):7264–7275. doi:10.1128/JVI.00737-08
- Desforges M, Le Coupanec A, Dubeau P, et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12(1):14. doi:10.3390/v12010014
- Bohmwald K, Gálvez NMS, Ríos M, Kalergis AM. Neurologic alterations due to respiratory virus infections. Front Cell Neurosci. 2018;12:386. doi:10.3389/fncel.2018.00386
- Demirci Otluoglu G, Yener U, Demir MK, Yilmaz B. Encephalomyelitis associated with covid-19 infection: case report. Br J Neurosurg. 2020;1–3. doi:10.1080/02688697.2020.1787342
- Burki TK. Testing for COVID-19. Lancet Respir Med. 2020;8(7):e63–e64. doi:10.1016/S2213-2600(20)30247-2
- Prevention CfDCa. CDC 2019-Novel Coronavirus (2019-nCoV) real-time RT-PCR diagnostic panel; July 13, 2020 [updated July 13, 2020]. Available from: https://www.fda.gov/media/134922/download. Accessed December 7, 2021.
- World Health Organization (WHO) TWHO. Protocol: real-time RT-PCR assays for the detection of SARS-CoV-2; 2020. Available from: https://www.who.int/docs/default-source/coronaviruse/real-time-rt-pcr-assays-for-the-detection-of-sars-cov-2-institut-pasteur-paris.pdf?sfvrsn=3662fcb6_2. Accessed December 7, 2021.
- Doherty CM, Forbes RB. Diagnostic lumbar puncture. Ulster Med J. 2014;83(2):93–102.
- Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18–22. doi:10.1016/j.bbi.2020.03.031
- Cebrián J, Gonzalez-Martinez A, García-Blanco MJ, et al. Headache and impaired consciousness level associated with SARS-CoV-2 in CSF: a case report. Neurology. 2020;95(6):266–268. doi:10.1212/WNL.0000000000010213
- Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi:10.1016/j.ijid.2020.03.062
- Domingues RB, Mendes-Correa MC, de Moura Leite FBV, et al. First case of SARS-COV-2 sequencing in cerebrospinal fluid of a patient with suspected demyelinating disease. J Neurol. 2020;267(11):3154–3156. doi:10.1007/s00415-020-09996-w
- Fadakar N, Ghaemmaghami S, Masoompour SM, et al. A first case of acute cerebellitis associated with Coronavirus Disease (COVID-19): a case report and literature review. Cerebellum. 2020:1–4. doi:10.1007/s12311-019-01083-9
- Rostami E, Virhammar J, Kumlien E, et al. Data from: acute necrotizing encephalopathy with SARS-CoV-2 RNA confirmed in cerebrospinal fluid. Dryad. 2020;95(10):445–449.
- Al-olama M, Rashid A, Garozzo D. COVID-19-associated meningoencephalitis complicated with intracranial hemorrhage: a case report. Acta Neurochir. 2020;162(7):1495–1499. doi:10.1007/s00701-020-04402-w
- Mardani M, Nadji SA, Sarhangipor KA, Sharifi-Razavi A, Baziboroun M. COVID-19 infection recurrence presenting with meningoencephalitis. New Microbes New Infect. 2020;37:100732. doi:10.1016/j.nmni.2020.100732
- Westhoff TH, Seibert FS, Bauer F, et al. Allograft infiltration and meningoencephalitis by SARS-CoV-2 in a pancreas-kidney transplant recipient. Am J Transplant. 2020;20(11):3216–3220. doi:10.1111/ajt.16223
- Destras G, Bal A, Escuret V, Morfin F, Lina B, Josset L. Systematic SARS-CoV-2 screening in cerebrospinal fluid during the COVID-19 pandemic. Lancet Microbe. 2020;1(4):e149. doi:10.1016/S2666-5247(20)30066-5
- Helms J, Kremer S, Merdji H, et al. Delirium and encephalopathy in severe COVID-19: a cohort analysis of ICU patients. Crit Care. 2020;24(1):491. doi:10.1186/s13054-020-03200-1
- Kremer S, Lersy F, de Sèze J, et al. Brain MRI findings in severe COVID-19: a retrospective observational study. Radiology. 2020;297(2):E242–E251.
- Duong L, Xu P, Liu A. Meningoencephalitis without respiratory failure in a young female patient with COVID-19 infection in downtown los angeles, early April 2020. Brain, Behavior, and Immunity. 2020;87:33. doi:10.1016/j.bbi.2020.04.024
- Huang YH, Jiang D, Huang JT. SARS-CoV-2 detected in cerebrospinal fluid by PCR in a case of COVID-19 encephalitis. Brain Behav Immun. 2020;87:149. doi:10.1016/j.bbi.2020.05.012
- Lewis A, Frontera J, Placantonakis DG, et al. Cerebrospinal fluid in COVID-19: a systematic review of the literature. J Neurol Sci. 2021;421:117316. doi:10.1016/j.jns.2021.117316
- Zanin L, Saraceno G, Panciani PP, et al. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir. 2020;162(7):1491–1494. doi:10.1007/s00701-020-04374-x
- Ye M, Ren Y, Lv T. Encephalitis as a clinical manifestation of COVID-19. Brain Behav Immun. 2020;88:945–946. doi:10.1016/j.bbi.2020.04.017
- Panciani PP, Saraceno G, Zanin L, et al. SARS-CoV-2: “three-steps” infection model and CSF diagnostic implication. Brain Behav Immun. 2020;87:128–129. doi:10.1016/j.bbi.2020.05.002
- Peñarrubia L, Ruiz M, Porco R, et al. Multiple assays in a real-time RT-PCR SARS-CoV-2 panel can mitigate the risk of loss of sensitivity by new genomic variants during the COVID-19 outbreak. Int J Infect Dis. 2020;97:225–229. doi:10.1016/j.ijid.2020.06.027
- Needham EJ, Chou SH, Coles AJ, Menon DK. Neurological implications of COVID-19 infections. Neurocrit Care. 2020;32(3):667–671. doi:10.1007/s12028-020-00978-4
- Lindan CE, Mankad K, Ram D, et al. Neuroimaging manifestations in children with SARS-CoV-2 infection: a multinational, multicentre collaborative study. Lancet Child Adolesc Health. 2021;5(3):167–177. doi:10.1016/S2352-4642(20)30362-X
- Alimohamadi Y, Sepandi M, Taghdir M, Hosamirudsari H. Determine the most common clinical symptoms in COVID-19 patients: a systematic review and meta-analysis. J Prev Med Hyg. 2020;61(3):E304–E312. doi:10.15167/2421-4248/jpmh2020.61.3.1530
- Menon U, Fine L, Chimakurthy A, Khan F, Ramsay E. EEG findings in COVID-19 positive patients: a case series (4816). Neurology. 2021;96(15 Supplement):4816.
- Antony AR, Haneef Z. Systematic review of EEG findings in 617 patients diagnosed with COVID-19. Seizure. 2020;83:234–241. doi:10.1016/j.seizure.2020.10.014
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71
