Abstract
Purpose
This study’s goal was to explore risk factors affecting short-term prognosis of cardiorenal syndrome type 1 (CRS1) in acute myocardial infarction (AMI) patients.
Methods
In this retrospective analysis of CRS1 in AMI patients hospitalized from January 2011 to December 2014, the patients were classified into deceased or survivor groups. Clinical data, including demographics, laboratory results, and 28-day outcomes, were collected.
Results
The incidence rate of CRS1 in AMI patients was 15.2% (274 in 1801). Ultimately, 88 patients were enrolled and 25 (28.4%) were classified into the deceased group, while 63 were classified into the survivor group. There were statistically significant differences between the groups for hypertension, mechanical ventilation, KIDGO stage, NT-proBNP, Hb, ALB, PCI, decreased LVEF, 7th-day SCr value, and the highest SCr value recorded within 7 days (all P < 0.05). Multivariate logistic regression showed that the following factors were significantly related to whether a patient died: requiring mechanical ventilation, increased NT-proBNP levels and 7th-day SCr values, and decreased LVEFs. The APACHE II, SOFA, and SASP II scores on the 7th day were significantly higher in the deceased group (all P < 0.05). The accuracy of APACHE II, SOFA, and SASP II scores on the 7th day for predicting death were 84.1%, 78.4% and 79.5%, respectively. The AUC of 7th-day APACHE II, SOFA, and SASP II scores was 0.844, 0.803, and 0.827, respectively, with no statistically significant differences between the three scores (P > 0.05).
Conclusion
The mortality rate of CRS1 in AMI patients was 28.4% (25 in 88) within 28 days. Mechanical ventilation, increased NT-proBNP levels, the 7th-day SCr value, and decreased LVEF were related to death in AMI patients with CRS1. APACHE II, SOFA, and SAPS II scores on the 7th day were satisfactorily accurate in predicting death within 28 days.
Introduction
The heart and the kidneys are two of the vital organs in the human body. Under physiological conditions, they are interdependent, and under pathological conditions, they can affect one another. Once one organ becomes diseased, the other organ often is involved. Cardiorenal syndrome (CRS) is a clinical syndrome in which these two organs are damaged in tandem to one another, and the prognosis is usually very poor. In 2008, the Acute Dialysis Quality Initiative (ADQI) defined the concept of CRS, pointing out that acute or chronic damage to heart or kidney function can lead to acute or chronic injury to the other organ’s function.Citation1 CRS often is related to prolonged hospitalization, a greater likelihood of rehospitalization, worse morbidity, and increased mortality.Citation2 CRS is divided into five subtypes of which CRS type I (CRS1) is the most common. CRS1, also known as acute CRS, refers to acute worsening of heart function leading to kidney injury and/or dysfunction. It is commonly seen after a patient experiences cardiac surgery, acute heart failure (AHF), cardiogenic shock, or an acute coronary syndrome (ACS) such as acute myocardial infarction (AMI).Citation1 In elderly Chinese patients, the incidence of CRS1 was 52.56% and the in-hospital mortality of CRS1 was 23.2%.Citation3 In Spain, the incidence of CRS1 was 9.2/1000 person-days of hospitalization, CRS1 increased the rate of death and readmission after discharge for ACS patients, and CRS1-related deaths accounted for 56.6% of all-cause mortality.Citation4
Until recently, there have been few studies on the risk factors of and prediction methods for the short-term prognosis of CRS1.Citation5 The goal of this study was to explore the risk factors that affect the short-term prognosis of CRS1 in AMI patients and to understand the predictive value of three scoring methods—Sequential Organ Failure Assessment (SOFA), Acute Physiology and Chronic Health Evaluation (APACHE) II, and Simplified Acute Physiology Scores (SAPS) II—on the mortality of AMI patients with CRS1 within 28 days after the onset of AMI, in order to provide clinical guidance.
Methods
Study Population
The study protocols were approved by the ethics committee (No. 202111216) of Xiangya hospital and complied with the tenets of the Declaration of Helsinki. The data collected were retrospective and anonymous, and the requirement for patients’ informed consent was, therefore, waived. The study retrospectively analyzed patients with AMI as the first diagnosis on admission and a secondary diagnosis of CRS1 during hospitalization in the Department of Cardiology, Xiangya Hospital, Central South University, from January 2011 to December 2014.
The inclusion criteria were 1) Diagnostic criteria for AMI were based on the AMI diagnostic criteria as described in the 2012 European Society of Cardiology (ESC) guidelines for the diagnosis and treatment of acute and chronic heart failure, and cardiac function classifications were based on the Killip classification method.Citation6 2) AKI diagnoses were made according to the 2012 Kidney Disease Improving Global Outcomes (KDIGO) AKI diagnostic criteriaCitation7 as follows: serum creatinine (SCr) levels increased ≥0.3 mg/dl (≥26.5 μmol/L) within 48 hours, or a SCr increase of ≥50% could be confirmed or inferred within 7 days, or urine production was less than 0.5 mL/kg/h for more than 6 hours’ duration; if any of those criteria were met, the diagnosis was confirmed and KDIGO stages were performed. 3) AMI patients who were admitted to the hospital’s emergency department within 12 hours of the onset of symptoms and were admitted to the cardiology intensive care unit within 24 hours.
The exclusion criteria were 1) Patients who were hospitalized for less than 7 days. 2) Patients whose SCr levels were not tested within 48 hours of admission. 3) Patients with at least two missing SCr results within the first 7 days of hospitalization. 4) Patients with malignant tumors. 5) Patients who had had any manifestations of acute or chronic kidney damage before admission. 6) Patients who used nephrotoxic drugs before admission. 7) Patients who had incomplete information in their charts.
Data Collection
According to their survival status within 28 days after the onset of AMI diagnosis, which was set as the observation end-point for a “short-term” prognosis, the patients were divided into either the deceased group or the survivor group.
The following general information was collected: sex, age, history of diabetes and hypertension, angiotensin-converting enzyme inhibitor (ACEI)/angiotensin II receptor blocker (ARB) use before admission, Killip Classification, and KDIGO stage.
The following hospitalization data were collected: hemoglobin (Hb); serum albumin (ALB); high-density lipoprotein (HDL); pro-brain natriuretic peptide (NT-proBNP); troponin I (cTnI); cystatin-C (Cys-C); triglycerides (TG); high-sensitivity C-reactive protein (hs-CRP) within 48 hours after admission; the highest value of SCr levels within 48 hours and 7 days after admission; the 7th-day SCr value; left ventricular ejection fraction value (LVEF) within 48 hours of admission; and whether the patient required blood purification, mechanical ventilation, and/or percutaneous coronary intervention (PCI) during their hospital stay. Scoring data for APACHE II, SOFA, and SAPS II were recorded within 48h and the 7th day after admission.
Statistical data were analyzed using SPSS 19.0 statistical software. Data with normal distributions were represented by the mean ± standard deviation, and the Student’s t-test was used for comparisons between groups. The median was used for data with non-normal distributions, and the Wilcoxon rank sum test was used for comparisons between those groups. Comparison of count data between the two groups was analyzed by the Pearson’s chi-square test, and the Fisher’s exact probability test was used when necessary. Logistic regression was used to analyze the independent risk factors of recent death in AMI patients with CRS1. The area under curve (AUC) of receiver operating characteristic (ROC) was used to compare and measure the ability of three scoring methods (ie, APACHE II, SOFA, and SAPS II) to predict the short-term prognosis of CRS1 in AMI patients. For all analyses, P < 0.05 was considered to be statistically significant.
Results
Data for a total of 1801 patients with AMI as their first diagnosis upon admission were collected in this study. Of those patients, 274 had CRS1 and the incidence rate of CRS1 in AMI patients was 15.2%. A total of 102 cases met the inclusion criteria but 14 cases were lost to follow-up. Ultimately, 88 cases were included in this study. Twenty-five patients (28.4%) died within 28 days after the onset of illness and were classified into the deceased group; 63 cases survived and were classified into the survivor group. The basic information of the two groups is shown in .
Table 1 Basic Information of the Survivor and Deceased Patients
Our results indicated that between the deceased group and the survivor group, there were statistically significant differences in the patients’ hypertension status; whether they received mechanical ventilation; their KIDGO stage; their levels of NT-proBNP, Hb, and ALB; whether they received PCI; their LVEFs within 48h; their 7th-day SCr values; and their highest SCr values within 7 days, as shown in (all P < 0.05). In contrast, there were no statistically significant differences between the deceased group and the survivor group in age; sex; diabetes status; receiving blood purification treatment during hospitalization; being on ACEI/ARB therapy; the length of their hospital stay; Killip classifications; levels of cTnI, Hs-CRP, Cys-C, TG, or HDL; basic SCr values; or the highest SCr level within 48 hours, as shown in (all P > 0.05).
To determine the independent factors related to death in AMI patients with CRS1, multivariate logistic regression analyses were performed. The threshold for entering variables into the multivariate models was P < 0.05. The results of the multivariate logistic regression are shown in . They showed that patients who needed mechanical ventilation during hospitalization (OR = 4.722, 95% CI 1.067–20.887), with significantly elevated NT-proBNP levels within 48 hours of admission (OR = 1. 025, 95% CI 1.002–1.048), with a 7th-day SCr value (OR = 4.841, 95% CI 1.107–21.161), and having a decreased LVEF (OR = 0.937, 95% CI 0.880–0.998), were significantly related to death in AMI patients with CRS1 (all P < 0.05, see for details). Through ROC curve analysis, the best cut-off values for NT-proBNP levels was 3345.2 pg/mL, 7th-day SCr levels were 176.8μmol/L, and LVEF was 41.5%, all indicating a higher risk of death in AMI patients with CRS1.
Table 2 Multivariate Logistic Regression Models for Independent Factors Related to Death
SOFA, APACHE II, and SAPS II scoring systems are commonly used to assess patients with acute and critical illnesses in the internal medicine ward and intensive care unit. The three scores’ differences were compared for their predictive value for risk of death in AMI patients with CRS1, as shown in . The APACHE II, SOFA, and SAPS II scores in AMI patients with CRS1 within 48 hours of admission were not statistically significant between the deceased group and the survivor group (P > 0.05). On the 7th day after admission, the APACHE II, SOFA, and SAPS II scores were statistically significantly higher in the deceased group compared to those in the survivor group (all P < 0.05).
Table 3 Three Scoring Methods Values for the Two Groups Patients
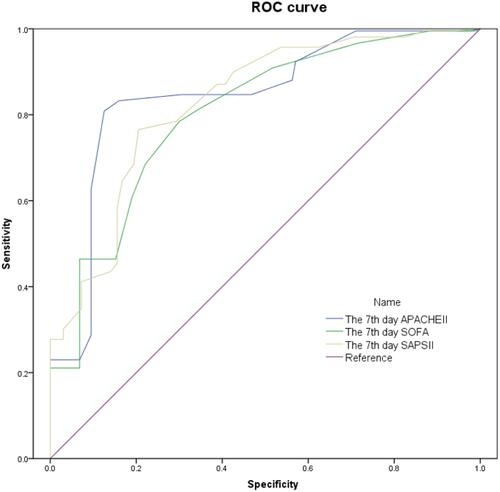
The 7th-day AUC of the APACHE II, SOFA, and SAPS II scores were 0.844 (P = 0.000, 95% CI: 0.807–0.881), 0.803 (P = 0.000, 95% CI: 0.763–0.842), and 0.827 (P = 0.000, 95% CI: 0.791–0.864), respectively. On the 7th day, the optimal thresholds for APACHE II, SOFA, and SAPS II scores to predict the death of CRS1 in AMI patients were 16 points, 6 points, and 34 points, respectively (). The accuracy of APACHE II, SOFA, and SAPS II scores on the 7th day in predicting death were 84.1%, 78.4% and 79.5%, respectively (). However, there was no statistically significant differences in the AUC of the three scoring methods (P > 0.05, ).
Table 4 The AUC of Three Scoring Methods on the 7th Day to Predict the Death in CRS1 Patients
Table 5 The AUC of Three Scoring Methods on the 7th Day When Compared Among Each Group
Discussion
CRS is used to identify a pathophysiologic disorder of the heart and kidneys whereby acute or chronic dysfunction in one organ may induce acute or chronic dysfunction in the other. CRS1 is the most common type of CRS.Citation8,Citation9 Previous studiesCitation8,Citation9 reported that the prevalence rate of CRS1 in acute coronary syndrome (ACS) patients was 9–19%. Importantly, the CRS1 in AMI patients led to an increased morbidity and longer hospital stay.Citation10 In ACS-hospitalized patients, the incidence of CRS1 was 9.2/1000 person-days of hospitalization, but these patients accounted for 56.6% of all-cause mortality. The risk of in-hospital mortality associated with CRS1 was greater than the sum of the risks associated with acute heart failure (AHF) and AKI independently.Citation4 In our study, the incidence rate of CRS1 in AMI patients was 15.2% in the 88 patients enrolled in the study. Of these patients, 25 patients died, resulting in a mortality rate of 28.4%. Our data are in accordance with previous studies. We used the KDIGO diagnostic criteria to diagnose AKI in our study. However, it is worth noting that there are differences in the current international diagnostic criteria for AKI and the determination of basic creatinine values. These discrepancies cause the incidence of AKI to vary greatly between different studies and do not accurately reflect the epidemiological characteristics of CRS1. At the same time, these discrepancies also highlight certain difficulties for clinicians in identifying AKI early. In a systematic literature review that included a total of 64 published studies, 10 different definitions for AKI were used.Citation11
CRS1 is defined as acute worsening of heart function leading to kidney injury and/or dysfunction.Citation1 It can be inferred that CRS1 is the manifestation of AKI that occurred in patients with AMI or other heart dysfunctions. Previous studies revealed that several factors were associated with the incidence of CRS1 in patients and their in-hospital mortality. Marenzi et al found that in patients with ST-segment elevation acute myocardial infarction (STEMI) complicated by cardiogenic shock, AKI occurred in 55% of the patients. Age >75 years, decreased LVEF, and the use of mechanical ventilation were independent predictors of AKI. STEMI patients with AKI had a longer hospital stay, a poor clinical outcome, and significantly higher mortality rate than patients without AKI.Citation12 Zhang et al found that the combination of NT-proBNP, estimated glomerular filtration rate (eGFR), and hs-CRP at presentation might help predict CRS1 in patients with AMI.Citation13 Fan et al found that age, diabetes, New York Heart Association (NYHA) class, eGFR, hs-CRP, and urinary angiotensinogen (uAGT) were independently associated with CRS1.Citation14 Hu found that the incidence of CRS1 in elderly Chinese patients was relatively high and related to a worse clinical outcome. In this study, decreased eGFR, lower ALB, and use of diuretics were risk factors for CRS1, while the use of diuretics, beta-blockers, and blood purification during hospitalization were predictors of in-hospital mortality in CRS1 patients.Citation3 Margolis et al reported that among STEMI patients, those with mid-range LVEF at presentation constituted a distinct group in terms of baseline characteristics, in-hospital outcomes, and long-term mortality.Citation15 Shacham et al also concluded that among STEMI patients, reduced LVEF, congestive heart failure, and a trend for increased time to coronary reperfusion emerged as independent predictors of AKI.Citation16
In our study, we did not analyze the predictors of CRS1 in AMI patients, but we determined the risk factors associated with mortality within 28 days in AMI patients with CRS1. Our data showed that mechanical ventilation during hospitalization, increased NT-proBNP levels, the 7th-day SCr value, and decreased LVEF levels were significantly related to death in AMI patients with CRS1. In fact, mechanical ventilation indicated that the patients were in respiratory failure or heart failure, increased NT-proBNP levels and 7th-day SCr values indicated that the patients had kidney dysfunction or heart failure, and decreased LVEF levels indicated heart failure or unstable hemodynamics. All of these changes reflected that the patients had multiple-organ dysfunction, which were the likely causes of death. Few studies have reported the risk factors leading to death in CRS1 patients. In a cohort of 147 patients with CRS1 who received peritoneal dialysis, all patients experienced respiratory failure, while the 30-day mortality rate was 73.4%. Increased age, unstable hemodynamics, and positive total fluid balance over the first 5 days of peritoneal dialysis were factors for an increased risk of death.Citation17
APACHE II,Citation18 SOFA,Citation19 and SAPS IICitation20 scores are the most widely used scores in the clinic to assess a patient’s prognosis. In our study, the three scores at 48 hours had no value in predicting death. Possible reasons for this outcome might be associated with the diagnostic time-window of AKI. The KDIGO diagnostic criteria defined that the time-window for AKI was within 7 days. AKI occurring within 48 hours is early-stage AKI, while AKI occurring after 48 hours is advanced AKI. In our study, some patients who died did not develop AKI within 48 hours of admission. Therefore, the three sets of scores for this group of patients were lower than those of advanced AKI patients. In contrast, on the 7th day, APACHE II scores had better accuracy in predicting death than SOFA and SAPS II scores, although there were no statistically significant differences between them. The SOFA score had the lowest accuracy. A reason for this result might be because SOFA scores are mainly used for sepsis patients,Citation21 while in our study, the underlying disease was AMI, not infection or sepsis. In a study to assess the three scoring criteria for discharge from an intensive care unit (ICU) in patients who required continuous renal replacement therapy (CRRT) during extracorporeal membrane oxygenation (ECMO), it was found that APACHE II and SAPS II scores had better potential utility than the SOFA score in predicting mortality in patients treated with both ECMO and CRRT.Citation22 In predicting mortality in ICU patients after cardiac surgery, APACHE II and SAPS II had better discriminatory power compared to SOFA.Citation23 In a study to predict in-hospital mortality of critically ill patients with AKI, APACHE II scored showed favorable AUC, sensitivity, and specificity values.Citation24
Limitations
Firstly, one limitation was that we only conducted a single-center, retrospective study. Secondly, we only analyzed the CRS1 of patients with AMI; we did not also analyze STEMI and NSTEMI patients. Lastly, there are several classification methods for diagnosing AKI; we only used the KDIGO classification of renal impairment.
Conclusions
The incidence rate of CRS1 in AMI patients was 15.2% and the mortality rate was 28.4% (25 in 88) within 28 days. Mechanical ventilation, increased NT-proBNP levels, 7th-day SCr values, and decreased LVEFs were all related to mortality in AMI patients with CRS1. The APACHE II, SOFA, and SAPS II scores on the 7th day had satisfactory accuracy in predicting death within 28 days.
Data Sharing Statement
All data generated or analyzed during this study are included in the manuscript.
Consent for Publication
All authors have approved the manuscript for submission.
Disclosure
The authors declare that there are no conflicts of interest.
Additional information
Funding
References
- Ronco C, McCullough P, Anker SD, et al. Cardio-renal syndromes: report from the consensus conference of the acute dialysis quality initiative. Eur Heart J. 2010;31(6):703–711. doi:10.1093/eurheartj/ehp507
- Ong LT. Evidence based review of management of cardiorenal syndrome type 1. World J Methodol. 2021;11(4):187–198. doi:10.5662/wjm.v11.i4.187
- Hu W, He W, Liu W, et al. Risk factors and prognosis of cardiorenal syndrome type 1 in elderly Chinese patients: a retrospective observational cohort study. Kidney Blood Press Res. 2016;41(5):672–679. doi:10.1159/000447936
- Pimienta GR, Couto CP, Rodriguez EM, et al. Incidence, mortality and positive predictive value of type 1 cardiorenal syndrome in acute coronary syndrome. PLoS One. 2016;11(12):e0167166. doi:10.1371/journal.pone.0167166
- Gembillo G, Visconti L, Giusti MA, et al. Cardiorenal syndrome: new pathways and novel biomarkers. Biomolecules. 2021;11(11):1581. doi:10.3390/biom11111581
- McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33(14):1787–1847. doi:10.1093/eurheartj/ehs104
- Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–830. doi:10.7326/0003-4819-158-11-201306040-00007
- Uduman J. Epidemiology of cardiorenal syndrome. Adv Chronic Kidney Dis. 2018;25(5):391–399. doi:10.1053/j.ackd.2018.08.009
- Mavrakanas TA, Khattak A, Singh K, Charytan DM. Epidemiology and natural history of the cardiorenal syndromes in a cohort with echocardiography. Clin J Am Soc Nephrol. 2017;12(10):1624–1633. doi:10.2215/CJN.04020417
- Bagshaw SM, Cruz DN, Aspromonte N, et al. Epidemiology of cardio-renal syndromes: workgroup statements from the 7th ADQI Consensus Conference. Nephrol Dial Transplant. 2010;25(5):1406–1416. doi:10.1093/ndt/gfq066
- Vandenberghe W, Gevaert S, Kellum JA, et al. Acute kidney injury in cardiorenal syndrome type 1 patients: a systematic review and meta-analysis. Cardiorenal Med. 2016;6(2):116–128. doi:10.1159/000442300
- Marenzi G, Assanelli E, Campodonico J, et al. Acute kidney injury in ST-segment elevation acute myocardial infarction complicated by cardiogenic shock at admission. Crit Care Med. 2010;38(2):438–444. doi:10.1097/CCM.0b013e3181b9eb3b
- Zhang DQ, Li HW, Chen HP, et al. Combination of amino-terminal pro- BNP, estimated GFR, and high-sensitivity CRP for predicting cardiorenal syndrome type 1 in acute myocardial infarction patients. J Am Heart Assoc. 2018;7(19):e009162. doi:10.1161/JAHA.118.009162
- Fan Z, Li Y, Ji H, Jian X. Nomogram model to predict cardiorenal syndrome type 1 in patients with acute heart failure. Kidney Blood Press Res. 2018;43(6):1832–1841. doi:10.1159/000495815
- Margolis G, Khoury S, Ben-Shoshan J, et al. Prognostic implications of mid-range left ventricular ejection fraction on patients presenting with ST-segment elevation myocardial infarction. Am J Cardiol. 2017;120(2):186–190. doi:10.1016/j.amjcard.2017.04.005
- Shacham Y, Leshem-Rubinow E, Gal-Oz A, et al. Acute cardio-renal syndrome as a cause for renal deterioration among myocardial infarction patients treated with primary percutaneous intervention. Can J Cardiol. 2015;31(10):1240–1244. doi:10.1016/j.cjca.2015.03.031
- Parapiboon W, Kingjun T, Wongluechai L, Leawnoraset W. Outcomes after acute peritoneal dialysis for critical cardiorenal syndrome type 1. Cardiorenal Med. 2021;11(4):184–192. doi:10.1159/000517362
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. doi:10.1097/00003246-198510000-00009
- Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–710. doi:10.1007/BF01709751
- Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–2963. doi:10.1001/jama.1993.03510240069035
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
- Hiramatsu T, Shimizu S, Koga H. Prognostic factors in patients treated with extracorporeal membrane oxygenation and continuous renal replacement therapy. Perfusion. 2021;2676591211011039. doi:10.1177/02676591211011039
- Schoe A, Bakhshi-Raiez F, de Keizer N, van Dissel JT, de Jonge E. Mortality prediction by SOFA score in ICU-patients after cardiac surgery; comparison with traditional prognostic-models. BMC Anesthesiol. 2020;20(1):65. doi:10.1186/s12871-020-00975-2
- Gong Y, Ding F, Zhang F, Gu Y. Investigate predictive capacity of in-hospital mortality of four severity score systems on critically ill patients with acute kidney injury. J Investig Med. 2019;67(8):1103–1109. doi:10.1136/jim-2019-001003