Abstract
Background
We aimed to explore the potential association of body composition parameters measured by bioelectrical impedance analysis (BIA) with the incidence of sarcopenia in patients with acute myeloid leukemia (AML) (non-M3) after chemotherapy.
Patients and Methods
This was a single-center observational study. Sixty-nine patients with newly diagnosed AML underwent BIA at the time of initial diagnosis and after completion of three chemotherapy sessions. Pre- and post-chemotherapy BIA parameters were compared. Sarcopenia was defined as low skeletal muscle mass plus low muscle strength according to the Asian Working Group for Sarcopenia (AWGS). Association of sarcopenia with mid-arm muscle circumference (MAMC) and intracellular water (ICW) was assessed by multivariate logistic regression.
Results
There was a significant increase in the prevalence of sarcopenia after chemotherapy (39.1% vs 14.5%, P<0.001). Skeletal muscle mass (SMM), fat-free mass (FFM), and soft lean mass (SLM) showed a significant decrease after chemotherapy (P<0.05). MAMC, ICW, and total body water (TBW) significantly decreased after chemotherapy (P<0.05). BIA indices including appendicular skeletal muscle mass (ASM) (r=0.889, P<0.001), ICW (r=0.869, P<0.001), MAMC (r=0.849, P<0.001) showed a positive correlation with SMI. Moreover, ASM (r=−0.453 P=0.001), ICW (r=−0.322, P<0.05), and MAMC (r=−0.352, P<0.05) showed a negative correlation with sarcopenia. On multivariate logistic regression analysis, increased ICW was associated with decreased risk of sarcopenia [odds ratio (OR): 0.50; 95% confidence interval (CI) 0.30–0.82]. Each additional unit of MAMC after chemotherapy was associated with 71% lower risk of sarcopenia (OR: 0.29; 95% CI 0.13–0.66).
Conclusion
The incidence of sarcopenia was associated with chemotherapy of patients with AML (non-M3) as reflected by body composition changes.
Introduction
Acute myeloid leukemia (AML) is one of the most common forms of leukemia in adults.Citation1 In the absence of treatment, death usually occurs within months of diagnosis secondary to infection or bleeding.Citation2 Chemotherapy is the main treatment modality for AML. However, the side effects of chemotherapy, including body composition disorders, dramatically decrease the quality of life and lead to poor prognosis.Citation3 Therefore, prevention and early detection of post-chemotherapy changes in body composition is a key imperative for AML patients.
Muscle wasting is a critical component of the change in body composition. Sarcopenia is characterized by severe loss of muscle mass and decline in muscle function.Citation4 The incidence of sarcopenia in elderly patients with hematologic malignancies, especially in non-Hodgkin’s lymphoma (NHL), is higher than general elderly population.Citation5 Several studies have corroborated the poor prognostic impact of sarcopenia and body composition changes in patients with various solid cancers.Citation6–Citation8 Sun et al reported that progress of sarcopenia in patients with AML is associated with adverse outcomes, and the overall survival (OS) of patients with sarcopenia was shorter than non-sarcopenia patients.Citation9
Bioelectrical impedance analysis (BIA) has been widely used to evaluate body composition in various clinical fields.Citation10 Devices for BIA have a high accuracy and efficiency for evaluation of body composition at a relatively low cost.Citation11 In the contemporary literature, changes in body composition and occurrence of sarcopenia in AML (non-M3) patients after repeated sessions of chemotherapy are not well characterized.
The purpose of this study was to investigate the post-chemotherapy changes in body composition indicators of AML patients (non-M3), as measured by BIA, and to assess their potential association with the incidence of sarcopenia.
Patients and Methods
Study Design and Population
This was a single-center, observational study conducted from July 2020 to July 2021 in the Department of Hematology at the First Affiliated Hospital of Chongqing Medical University. During the study reference period () a total of 105 patients were hospitalized due to newly diagnosed AML.
Figure 1 Schematic illustration of the study design and patient-selection criteria.
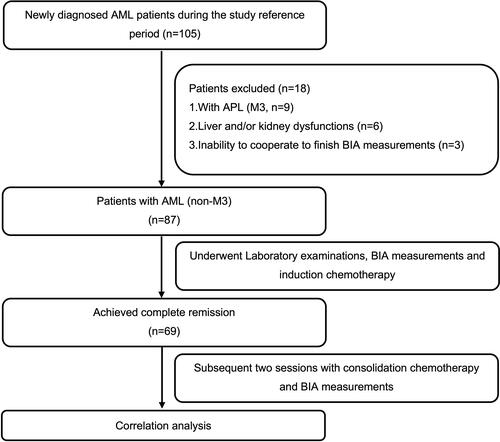
The inclusion criteria for this study were 1) adult patients (age ≥18 years) with diagnosis of AML confirmed by pathological examination or MICM classification and 2) indications for chemotherapy evaluated by the hematologist. The exclusion criteria were 1) patients with acute promyelocytic leukemia; 2) patients with liver and/or kidney dysfunction or other contraindications for chemotherapy, and those who still could not tolerate chemotherapy after active treatment for the above contraindications; 3) pregnant women; 4) any other reason that the investigator considered inappropriate to participate in this study. Finally, 69 initial diagnosis AML (non-M3) patients who reached complete remission (CR) were recruited in this study, followed by two sessions of consolidation chemotherapy. All AML (non-M3) patients were given the standard “3+7” induction chemotherapy with idarubicin (IDA, 10mg/m2·d) or daunorubicin (DNR, 60mg/m2·d) plus cytarabine (Ara-c, 100–200mg/m2·d). Consolidation chemotherapy consisted of high-dose cytarabine-based regimens (Ara-c, 1–3g/m2·q12).
The purpose and content of this study were explained to all participants and their written informed consents were obtained prior to their enrolment. The study was conducted in accordance with the principles of the Declaration of Helsinki. Ethical approval was obtained from the First Affiliated Hospital of Chongqing Medical University (approval number 2020-589).
Measurements and Definition of Body Composition Parameters
Anthropometric variables include height (cm), weight (kg) and hand grip strength (kg) were measured by doctors, following a standard protocol.Citation12 Body mass index (BMI) was calculated as body weight in kilograms divided by height in meters squared (kg/m2). Routine laboratory tests were performed in the Department of Laboratory at the First Affiliated Hospital of Chongqing Medical University. All measurements and tests were undertaken at the time of initial diagnosis of AML and after completion of three chemotherapy sessions. All participants were subjected to BIA measurements by Direct Segmental Multi-Frequency Bioelectrical Impedance Analyzer (DSM-BIA, Inbody S10, Korea). After admission to the hospital, BIA measurements were performed prior to initiation of any fluid therapy following the manufacturer’s instructions. All participants were instructed not to consume any food or drink and to avoid strenuous activity within 2 hours before the measurements.
All BIA parameters were obtained using a standard montage of outer and inner electrodes on the right hand and foot while patients lay down with legs apart. Body composition indicators including appendicular skeletal muscle mass (ASM), skeletal muscle mass (SMM), soft lean mass (SLM), intracellular water (ICW), total body water (TBW), arm-muscle circumference (MAMC), visceral fat area (VFA); fat mass (FM) and protein content were measured and recorded. Sarcopenia was defined as low muscle mass plus low muscle strength according to the criteria of Asia Working Group for Sarcopenia (AWGS).Citation13 Low muscle mass was defined as ASM corrected by height squared (appendicular skeletal muscle mass index, SMI) (<5.7 kg/m2 in women and <7.0 kg/m2 in men). Low muscle strength was determined by low hand grip strength (HGS) (<18 kg in women and <28 kg in men).
Statistical Analysis
Continuous variables were presented as median with upper and lower quartiles, the interquartile range, or mean ± stand deviation. Between-group differences with respect to normally distributed continuous variables were assessed using independent-samples t-test and those with respect to non-normally distributed variables were assessed using Mann–Whitney U-test. Categorical variables were presented as frequency (proportions) and compared using Chi-squared test. Multivariate logistic regression was performed to further test the strength of association of sarcopenia with ICW and MAMC, after controlling for potential confounding variables including age, sex, FM, VFA, WBC, HB, PLT, UA, LDH. All statistical analyses were performed using SPSS 25 (IBM, Armonk, New York, NY, USA). Two-sided P values < 0.05 were considered indicative of statistical significance for all comparisons.
Results
Changes in Clinical Indicators After Chemotherapy
Eighty-seven patients with AML (non-M3) at initial diagnosis were eligible for inclusion. Of these, 69 (79.3%) participants achieved CR and subsequently accepted both BIA and three consolidation chemotherapy sessions. shows the comparison of pre- and post-chemotherapy clinical and laboratory data. Post-chemotherapy BMI, white blood cell count (WBC), uric acid (UA), serum albumin (ALB), and lactate dehydrogenase (LDH) levels were significantly lower than the corresponding baseline levels (P<0.05 for all), while post-chemotherapy platelet count (PLT) was significantly higher than the baseline PLT (median 117 vs 51, P<0.001). There was no significant difference between the two groups with respect to other indices, including systolic blood pressure, diastolic blood pressure, or hemoglobin (HB).
Table 1 Changes in Clinical Indicators After Chemotherapy
Changes in Body Composition Indices After Chemotherapy
The BIA parameters are shown in . Post-chemotherapy ASM and HGS levels were significantly lower than those before chemotherapy. The prevalence of sarcopenia in our cohort after chemotherapy was significantly higher than that before chemotherapy (39.1% vs 14.5%, P<0.001). After chemotherapy, there was a significant decrease in the three muscle indices (ie, SMM, FFM, and SLM). In addition, the value of MAMC, ICW, and TBW significantly decreased after chemotherapy (P<0.05). There was no significant effect of chemotherapy on FM or VFA (P>0.05).
Table 2 Pre- and Post-Chemotherapy Bioelectric Impedance Analysis Parameters
Correlation Between Sarcopenia and BIA Indices
Correlation between sarcopenia and BIA indices was assessed by Spearman correlation analysis (). BIA indices including ASM (r=0.889, P<0.001), ICW (r=0.869, P<0.001), and MAMC (r=0.849, P<0.001) showed a positive correlation with SMI. ASM (r=−0.453 P=0.001), ICW (r=−0.322, P<0.05), MAMC (r=−0.352, P<0.05) showed a negative correlation with sarcopenia. VFA and FM showed no correlation with SMI or sarcopenia (P>0.05).
Table 3 Correlation Coefficients (Spearman’s Rho) for Sarcopenia and BIA Indicators
Logistic Regression Analysis of the Association of Sacropenia with MAMC and ICW
Logistic regression analysis was performed to evaluate the strength of the association of sarcopenia with ICW and MAMC (). In model 2, after adjusting for age, sex, VFA, FM, WBC, HB, PLT, UA, and LDH, increased ICW showed a significant association with decreased sarcopenia [odds ratio (OR): 0.50; 95% confidence interval (CI) 0.30–0.82]. Each additional unit of MAMC was associated with 71% lower risk of sarcopenia (OR: 0.29; 95% CI 0.13–0.66) in AML (non-M3) patients after chemotherapy.
Table 4 Results of Logistic Regression Analysis Showing the Association of BMI, AMC, and ICW with the Incidence of Sarcopenia
Discussion
The key findings of our study are as follows: a) In AML patients, the increased incidence of sarcopenia after chemotherapy depends on weight reduction, which is mainly due to the loss of muscle mass and water content rather than fat mass. b) MAMC and ICW showed a significant association with the incidence of sarcopenia after adjusting for age, sex, and clinical indicators.
Sarcopenia caused by changes in body composition is often associated with poor prognosis in cancer patients. Sarcopenia is characterized by loss of skeletal muscle mass and performance and is a risk factor for frailty, morbidity, and mortality in older people.Citation14,Citation15 Moreover, low skeletal muscle has been shown to be a prognostic marker in the context of many diseases such as malignant cancer, heart failure, and chronic obstructive pulmonary disease.Citation16–Citation18 Several studies have shown that body composition, as assessed by CT, is useful in predicting the prognosis; in addition, CT-detected sarcopenia was associated with lower overall survival in patients with AML and acute lymphoblastic leukemia (ALL).Citation19,Citation20
Our study revealed changes in body composition and increased prevalence of sarcopenia after chemotherapy. There was a simultaneous decline in BMI, muscle and water content without changes in fat content, although the biochemical parameters (WBC, PLT, ALB and LDH) improved after three chemotherapy sessions. Myelosuppression is a common side effect of anthracyclines. For prevention of complications such as bleeding and infection, patients are often advised bed rest.Citation21 Previous studies have shown that bed rest substantially reduces skeletal muscle mass without affecting lipid content and is associated with changes in metabolic phenotype including decline in resting fat oxidation, basal metabolic rate,Citation22 glucose tolerance and insulin sensitivity,Citation23 and increase in mitochondrial production of reactive oxygen species (ROS).Citation24 Therefore, treatment regimens of sarcopenia may be for individual and selected cases, including physical training, modifications of nutritional intake, and pharmacological treatment.Citation25
There is a distinct difference between body composition disorder induced by chemotherapy and cachexia. Cancer cachexia is related to poor prognosis and is defined as atrophy of skeletal muscle and adipose tissue resulting in progressive weight loss.Citation26 It has been frequently related to involuntary weight loss, decreased muscle mass as well as biochemical changes, such as decreased ALB.Citation27 Of note, the post-chemotherapy weight loss in our cohort was mainly due to loss of muscle mass and water instead of fat. However, the underlying mechanism of this phenomenon is not clear. Several reasons may explain this phenomenon. Firstly, both adipocytes and fat tissue affect metabolism and inactivation of anthracyclines. Secondly, this phenomenon may be related to the inflammatory response of AML with sarcopenia. A substantial body of literature suggests that inflammatory cytokines activate many molecular pathways in skeletal muscle rather than adipose tissue,Citation28 and high level of inflammatory cytokines was shown to negatively correlate with skeletal muscle mass.Citation29
BIA is a simple, relatively cheap, and effective approach to evaluate body composition. High BMI is associated with increased risk of most hematological malignancies.Citation30 Although BMI is most frequently used to assess nutritional status, it does not precisely reflect body composition, nor does it differentiate skeletal muscle from body fat.Citation31 Owing to its advantages, BIA has been widely used for body composition analysis in research of various diseases over the past few decades.Citation11,Citation32 Many BIA indicators including segmental skeletal muscle, body fat and water content provide useful supportive information to facilitate clinical diagnosis.Citation33,Citation34 Here, we measured body composition indicators including skeletal muscle mass using BIA with the aim of more accurate characterization of the link between sarcopenia and AML.
MAMC measured by BIA can reflect muscle mass and performance and has been used as an indicator of nutritional status.Citation35 Many previous studies have demonstrated a correlation between MAMC and skeletal muscle index, which has been proven valuable to assess the outcomes in patients with hematological malignancies.Citation36,Citation37 Due to better predictable effects of reflecting muscle performance, intracellular water (ICW) may be used as a supplementary indicator together with MAMC for the diagnosis of sarcopenia.Citation38,Citation39 In the present study, both MAMC and ICW measured by BIA showed a strong correlation with post-chemotherapy sarcopenia in AML patients, which is consistent with previous studies. This association persisted after adjustment for a variety of potential confounding factors.
Some limitations of our study should be acknowledged. Firstly, this was a single center cross-sectional study with a small sample size. Secondly, we used BIA to measure body composition instead of other modalities such as dual-energy X-ray absorptiometry (DEXA), computed tomography (CT), and magnetic resonance imaging (MRI); the accuracy of BIA is still controversial. Thirdly, we only observed the incidence of sarcopenia based on changes in BIA indicators. We were unable to call the patients back for a comprehensive assessment of sarcopenia.
Conclusion
We found increased incidence of sarcopenia in AML (non-M3) patients after chemotherapy. Both MAMC and ICW measured by BIA showed a strong association with sarcopenia. Our findings further our understanding of sarcopenia in AML patients.
Acknowledgment
This study was funded by grants from Chongqing Medical Research Project: Study on early cardiotoxicity of antitumor drugs in lymphoma patients (Grant No. 2021MSXM276); and Chongqing Natural Science Foundation general project: the mechanism of doxorubicin promoting atherosclerosis in lymphoma patients through NF-κB/miR-33 signaling pathway (Grant No. cstc2019jcyj-msxmX0043).
Disclosure
The authors report no conflicts of interest in this work.
References
- Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136–1152. doi:10.1056/NEJMra1406184
- De kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016;6(7):e441–e441. doi:10.1038/bcj.2016.50
- Kadia TM, Ravandi F, Cortes J, Kantarjian H. New drugs in acute myeloid leukemia. Ann Oncol. 2016;27(5):770–778. doi:10.1093/annonc/mdw015
- Bauer J, Morley JE, Schols AMWJ, et al. Sarcopenia: a time for action. An SCWD position paper. J Cachexia Sarcopenia Muscle. 2019;10(5):956–961. doi:10.1002/jcsm.12483
- Kamiya T, Mizuno K, Ogura S, et al. A prospective observational study evaluating sarcopenia by using the bioelectrical impedance analysis in elderly patients with hematologic malignancies. Blood. 2018;132(Supplement 1):4851. doi:10.1182/blood-2018-99-114545
- Martin L, Senesse P, Gioulbasanis I, et al. Diagnostic criteria for the classification of cancer-associated weight loss. J Clin Oncol. 2015;33(1):90–99. doi:10.1200/JCO.2014.56.1894
- Dewys WD, Begg C, Lavin PT, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Am J Med. 1980;69(4):491–497. doi:10.1016/S0149-2918(05)80001-3
- van Lieshout R, Tick LW, Dieleman JP, et al. Changes in body weight and serum liver tests associated with parenteral nutrition compared with no parenteral nutrition in patients with acute myeloid leukemia during remission induction treatment. Support Care Cancer. 2020;28(9):4381–4393. doi:10.1007/s00520-019-05251-9
- Sun Q, Ming H, Qian S, Li JY. Sarcopenia as a significant prognostic factor in acute myeloid leukemia: validation of a novel scoring system. Blood. 2019;134(Supplement_1):5896. doi:10.1182/blood-2019-130517
- Kyle UG, Bosaeus I, De Lorenzo AD, et al. Bioelectrical impedance analysis—part II: utilization in clinical practice. Clin Nutr. 2004;23(6):1430–1453. doi:10.1016/j.clnu.2004.09.012
- Ward LC. Bioelectrical impedance analysis for body composition assessment: reflections on accuracy, clinical utility, and standardisation. Eur J Clin Nutr. 2019;73(2):194–199. doi:10.1038/s41430-018-0335-3
- Mathiowetz V, Kashman N, Volland G, Weber K, Dowe M, Rogers S. Grip and pinch strength: normative data for adults. Arch Phys Med Rehabil. 1985;66(2):69–74.
- Chen LK, Woo J, Assantachai P, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21(3):300–307.e2. doi:10.1016/j.jamda.2019.12.012
- Pin F, Barreto R, Couch ME, Bonetto A, O’Connell TM. Cachexia induced by cancer and chemotherapy yield distinct perturbations to energy metabolism. J Cachexia Sarcopenia Muscle. 2019;10(1):140–154. doi:10.1002/jcsm.12360
- Argilés JM, Busquets S, Stemmler B, López-Soriano FJ. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer. 2014;14(11):754–762. doi:10.1038/nrc3829
- Hopkins JJ, Reif RL, Bigam DL, Baracos VE, Eurich DT, Sawyer MB. The impact of muscle and adipose tissue on long-term survival in patients with stage I to III colorectal cancer. Dis Colon Rectum. 2019;62(5):549–560. doi:10.1097/DCR.0000000000001352
- Huang BT, Peng Y, Liu W, et al. Lean mass index, body fat and survival in Chinese patients with coronary artery disease. QJM. 2015;108(8):641–647. doi:10.1093/qjmed/hcv013
- Kou HW, Yeh CH, Tsai HI, et al. Sarcopenia is an effective predictor of difficult-to-wean and mortality among critically ill surgical patients. PLoS One. 2019;14(8):e0220699. doi:10.1371/journal.pone.0220699
- Surov A, Wienke A. Sarcopenia predicts overall survival in patients with malignant hematological diseases: a meta-analysis. Clin Nutr. 2021;40(3):1155–1160. doi:10.1016/j.clnu.2020.07.023
- Jung J, Lee E, Shim H, Park JH, Eom HS, Lee H. Prediction of clinical outcomes through assessment of sarcopenia and adipopenia using computed tomography in adult patients with acute myeloid leukemia. Int J Hematol. 2021;114(1):44–52. doi:10.1007/s12185-021-03122-w
- Alibhai SMH, O’Neill S, Fisher-Schlombs K, et al. A clinical trial of supervised exercise for adult inpatients with acute myeloid leukemia (AML) undergoing induction chemotherapy. Leuk Res. 2012;36(10):1255–1261. doi:10.1016/j.leukres.2012.05.016
- Bergouignan A, Schoeller DA, Normand S, et al. Effect of physical inactivity on the oxidation of saturated and monounsaturated dietary fatty acids: results of a randomized trial. PLOS Clin Trial. 2006;1(5):e27. doi:10.1371/journal.pctr.0010027
- Dirks ML, Wall BT, van de Valk B, et al. One week of bed rest leads to substantial muscle atrophy and induces whole-body insulin resistance in the absence of skeletal muscle lipid accumulation. Diabetes. 2016;65(10):2862–2875. doi:10.2337/db15-1661
- Gram M, Vigelsø A, Yokota T, Helge JW, Dela F, Hey-Mogensen M. Skeletal muscle mitochondrial H 2 O 2 emission increases with immobilization and decreases after aerobic training in young and older men: impaired human mitochondrial function after immobilization. J Physiol. 2015;593(17):4011–4027. doi:10.1113/JP270211
- Denison HJ, Cooper C, Sayer AA, Robinson SM. Prevention and optimal management of sarcopenia: a review of combined exercise and nutrition interventions to improve muscle outcomes in older people. Clin Interv Aging. 2015;10:859–869. doi:10.2147/CIA.S55842
- Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489–495. doi:10.1016/S1470-2045(10)70218-7
- Fearon KC, Voss AC, Hustead DS. Definition of cancer cachexia: effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr. 2006;83(6):1345–1350. doi:10.1093/ajcn/83.6.1345
- Edward J, Sang-Rok L, Bong-Sup P, Jeong-Su K. Potential mechanisms underlying the role of chronic inflammation in age-related muscle wasting. Aging Clin Exp Res. 2012;24:5. doi:10.3275/8464
- Ito S, Nakashima H, Ando K, et al. Association between low muscle mass and inflammatory cytokines. Biomed Res Int. 2021;2021:1–7. doi:10.1155/2021/5572742
- De Ridder J, Julián-Almárcegui C, Mullee A, et al. Comparison of anthropometric measurements of adiposity in relation to cancer risk: a systematic review of prospective studies. Cancer Causes Control. 2016;27(3):291–300. doi:10.1007/s10552-015-0709-y
- Sarkar SR, Kuhlmann MK, Kotanko P, et al. Metabolic consequences of body size and body composition in hemodialysis patients. Kidney Int. 2006;70(10):1832–1839. doi:10.1038/sj.ki.5001895
- Kilic MK, Kizilarslanoglu MC, Arik G, et al. Association of bioelectrical impedance analysis–derived phase angle and sarcopenia in older adults. Nutr Clin Pract. 2017;32(1):103–109. doi:10.1177/0884533616664503
- Irisawa H, Mizushima T. Correlation of body composition and nutritional status with functional recovery in stroke rehabilitation patients. Nutrients. 2020;12(7):1923. doi:10.3390/nu12071923
- Chen J, Lu K, Chen H, et al. Trunk skeletal muscle mass and phase angle measured by bioelectrical impedance analysis are associated with the chance of femoral neck fracture in very elderly people. CIA. 2020;15:889–895. doi:10.2147/CIA.S250629
- Landi F, Russo A, Liperoti R, et al. Midarm muscle circumference, physical performance and mortality: results from the aging and longevity study in the Sirente geographic area (ilSIRENTE study). Clin Nutr. 2010;29(4):441–447. doi:10.1016/j.clnu.2009.12.006
- Chang PK, Chen WL, Wu LW. Mid-arm muscle circumference: a significant factor of all-cause and cancer mortalities in individuals with elevated platelet-to-lymphocyte ratio. PLoS One. 2018;13(12):e0208750. doi:10.1371/journal.pone.0208750
- Noori N, Kopple JD, Kovesdy CP, et al. Mid-arm muscle circumference and quality of life and survival in maintenance hemodialysis patients. CJASN. 2010;5(12):2258–2268. doi:10.2215/CJN.02080310
- Serra-Prat M, Lorenzo I, Palomera E, Yébenes J, Campins L, Cabré M. Intracellular water content in lean mass is associated with muscle strength, functional capacity, and frailty in community-dwelling elderly individuals. A cross-sectional study. Nutrients. 2019;11(3):661. doi:10.3390/nu11030661
- Hetherington-Rauth M, Baptista F, Sardinha LB. BIA-assessed cellular hydration and muscle performance in youth, adults, and older adults. Clin Nutr. 2020;39(8):2624–2630. doi:10.1016/j.clnu.2019.11.040
