Abstract
Background
Challenges in selecting the right formulation of testosterone (TE) for young males with delayed puberty (DP) arise from the fact that there is limited evidence based guidelines in recommending the most efficient and safe formulation of TE.
Objective
To evaluate the existing evidence and systematically review the interventional effects of transdermal TE to other modes of TE administration for the treatment of DP among young and adolescent males.
Methods
All types of methodologies published in English were searched from the data sources including MEDLINE, Embase, Cochrane Reviews, Web of Science, AMED and Scopus from 2015 till 2022. Boolean operators with keywords “types of TE”, “modes of TE administration”, “DP”, “transdermal TE”, “constitutional delay of growth and puberty, (CDGP)” “adolescent boys” and “hypogonadism” to optimize the search results. The main outcomes of concern were optimal serum TE level, body mass index, height velocity, testicular volume, pubertal stage (Tanner), The secondary outcomes included in this study were adverse events and patient satisfaction.
Results
After screening 126 articles, 39 full texts were reviewed. Only five studies could be included after careful screening and rigid quality assessments. Most studies were at high or unclear risk of bias with short duration and follow up periods. Only one study was a clinical trial covering all the outcomes of interests.
Conclusion
This study points out the favorable effects of transdermal TE treatment for DP in boys, while the existence of the vast gap in research needs to be acknowledged. Despite the utmost demand in an appropriate TE treatment for young males with DP, scarce efforts and trials are being undertaken to provide clear clinical guidance of treatment. Quality of life, cardiac events, metabolic parameters, coagulation profiles are important aspects of the treatment are overlooked and under evaluated in most studies.
Systematic Review Registration
PROSPERO CRD 42022369699.
Introduction
Rationale
The clinical absence of first signs of pubertal developmental milestones is defined as delayed puberty (DP) in boys and girls.Citation1 Functional delay in the production of gonadotropin-releasing hormone (GnRH) from the hypothalamus considered responsible for DH.Citation2 CDGP due to individual genetic variations, malnutrition, chronic illness and other functional defects can be the underlying case for DP.Citation3 DP can present with socio physiological burden for patients and their families.
Diagnosis for DP among boys are based on the assessment of clinical signs and symptoms and a low testosterone (TE) concentrations in serum in the morning on at least two occasions (< 10.4 nmol/L).Citation4,Citation5 In conditions like hypogonadism, where enough hormones for masculine growth and development during puberty (TE) or enough sperm or bothCitation6 are not produced due to congenital or acquired conditions external hormonal treatments are required.Citation7 Based on causes of DP like CDGP or hypogonadism for young boys, chemically synthesized TE has been used since 1935 as a common clinical intervention, targeting genital maturation, adequate secondary sexual characteristics developments, attainting the optimal muscular and bone growth.Citation8,Citation9
The typical clinical approach for adolescent males with CDGP or hypogonadism is the prescription for Testosterone Replacement Therapy (TRT). Currently various choices of interventions for TE replacement and management for adults are available, including intramuscular (IM), subcutaneous, oral and transdermal preparations,Citation10–15 but the right tittered choice and mode of administration of TE for young adults are not available.Citation16–18 Pitfalls in the use of TE usages for adolescents have disclosed multitudes of risks, associated behavioral deviations in the pre pubertal group and below target height line achievement with non-advancement of one’s age which has generated a warning for its usage.Citation19–21 Earlier short acting esters of TE like Sustanon®, were used as IM preparations that have been used for many years for the treatment of pubertal TE deficiency.Citation22 Studies report the safety and efficacy of short-term use of TE Enanthateor oral TE undecanoate in inducing puberty and increasing growth in young males with CDGP.Citation23,Citation24
Though the long-term safety and efficacy of Transdermal testosterone therapy (TRT) for puberty completion and maintenance have not been established, reliable evidence on the use of Transdermal testosterone (TT) for adolescent boys to induce and maintain puberty are emerging.Citation25–27 It is optimal that all kinds of TE therapies for young males are administered and monitored to better mimic the physiologic pubertal development. A critical lack of scientific evidence based guidelines for prescribing transdermal TE for adolescent boys hinders achieving this and the current TRT regimens are based on consensus and expert opinion.
The US Food and Drug Administration has currently approved only IM TE esters (TE enanthate) and subcutaneous TE for DP, while no preparation is approved for long-term use in the adolescent age.Citation17,Citation28–32 In adult males with hypogonadism several TE formulations like transdermal nasal, subcutaneous, and oral formulation have been are recently developed with improved pharmacokinetic profile and to ease the administration route increasing patient compliance.Citation33–36 These formulations are not approved for pediatric age, although some of them are used as off-label regimens.Citation37–40 Pediatric uses of exogenous TE has been reported for the treatment of microphallus in infants and the management of diminished or absent mini puberty.Citation41–43.
Critically limited evidences are available in support of the use of TT in the long term use boys with DP. Current practices lacks evidence based guidelines and the recommendations in selecting the route of administration and in the ideal preference of monitoring of the TE dose are based on expert opinions and consensus. It is of paramount importance clinically to identify and utilize an effective, safe, convenient to use and cost effective regimen of TE treatment which optimally mimics the physiological situation, as the majority of boys diagnosed with DP require longer periods of treatment.
TT for young males has not yet been a topic of any meta-analysis or a systematic review to this date and thus this review has a highlighted an importance in providing guidelines and evidence to the current TT treatment requirements to treat DP. Hence this study aims to compare the TT intervention to other types of TE interventions recommended to treat the symptoms of DP among young males.
In this study, a PRISMA guided systematic review is undertaken including randomized-controlled trials (RCTs) and non-randomized studies with an objective to evaluate the TT treatment options involving young male population diagnosed with DP. The review question formulated for this review is as follows. Participants (P): Boys younger than 19 years, Intervention (I): TT preparations for DP for young boys. Comparisons(C): Injectable TEs and oral TE. Outcomes (O): Serum TE levels, bone mineral density, height velocity and pubertal growth, adverse effect and quality of life.
Objectives
Using a broad set of eligibility criteria and an inclusive search strategy this systematic review sought to evaluate the existing evidence and systematically review the interventional effects of TT to other modes of TE administration for the treatment of DP among young and adolescent males.
Methods
Protocol and Registration
Detailed eligibility criteria and methods of analysis were specified in advance and documented in a published review protocol.Citation44 This review conforms to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelinesCitation45 and is registered with the International Prospective Register of Systematic Reviews (PROSPERO) (registration number CRD 42022369699). The institutional review board ruled out the need for an ethical approval for a systematic review.
Inclusion and Exclusion Criteria
The full text articles retrieved were assimilated that were relevant after review of the titles and abstracts. Final eligibility assessment was done independently for full text articles and all studies were individually appraised.
Inclusion criteria for the studies were (1) Methodologies of clinical trials without limitation on intervention design features, systematic reviews, meta-analysis, case reports and series, cross sectional studies on young and adolescent boys with DP (below 18 years) from 2015 until 2022, comparing TT to other modes of TE treatment. (2) Studies which included at least 3 of the following post-interventional outcomes helpful to assess the DP related clinical presentations: Serum TE levels, body mass index, bone mineral density, LSH, FSH and adverse effects. Studies on adults, studies on women, trans genders, animal studies, narrative reviews letters to editor, editorials, commentaries, and abstracts only available were excluded. summarizes the inclusion and exclusions criteria designed for this study.
Table 1 The Inclusion and Exclusion Criterias for the Study Selection
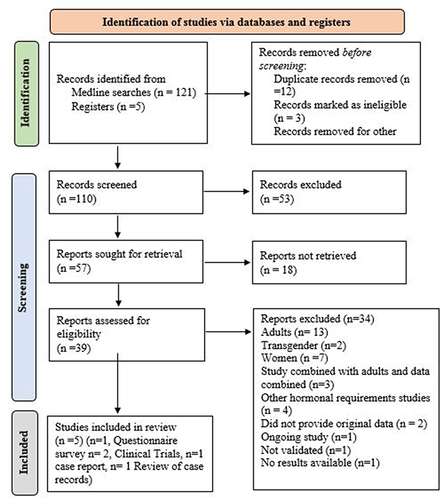
describes these criteria.The illustrates the PRISMA flow diagram for the studies selected in the search process and eligibility appraisal.
Figure 1 PRISMA Flowchart elaborating on study retrieval and inclusion in this study.
Information Sources and Search Strategy
Based on the study eligibility criteria, a search strategy was designed consisting of a systematic, computer-assisted, literature search of existing evidence from several online databases like MEDLINE, Embase, Cochrane Reviews, Web of Science, the Allied and Contemporary Medicine (AMED) for articles published between 2015 to 2022. Boolean logics with keywords “types of TE”, “modes of TE administration”, “DP”, “TT”, “CDGP”, “adolescent boys” and “hypogonadism”, “androgens”, hypogonadal boys, IM TE injections, to which relevant subheadings like “administration & dosage”, modes of administration (buccal, cream, gel, implant, injections, oral, patch, transdermal) were applied in combination to optimize the search results.
Study Selection
The selected studies were independently identified, selected, and appraised. To avoid possible selection bias, grey literatures including published and unpublished thesis, was obtained by searching Web of Science, ProQuest Dissertations and clinicaltrials.gov. The reference sections of retrieved original articles and reviews were scanned for studies that might have been missed in the primary searches. Studies were filtered with regard to study design and methodological features, main and secondary reported outcomes evaluated under this study. Eligibility was tracked and arranged with EndNote (Version 5.0 Thompson Reuters, 2011).
Data Collection Process
Data extraction for all variables was independently completed and assessed using a customized data extraction spreadsheet. Search results were combined and duplicates were deleted. The selected titles and abstracts were reviewed, and full‐text reports were retrieved of those that were potentially relevant, and later classified as included or excluded. Data from the included studies were extracted into the predesigned spreadsheet.
Data Items
Full-data extraction was done for study design details including the publication details, methodological attributes, and post interventional outcomes featured in the study, TE duration and preparation used, remarks of the included study, participant demographics. and and are illustrative of this data extraction and synthesis.
Table 2 Main Outcome Comparison and Descriptions
Table 3 Adverse Events and Patient Satisfaction on Transdermal Testosterone Treatment for Young and Adolescent Boys with Delayed Puberty
A meta-analysis was ruled out due to the heterogeneous nature of the limited amount of studies which are available. This there was no further statistical analysis involved and the review was summarized narratively after the explicit quality assessment process. This directs to a key research gap of the lack of evidence in this topic and contributes to the medical decisions based on weaker scientific support.
The review was themed into main outcomes and secondary outcomes. Main outcomes of concern were the achievement of optimal serum TE levels, height velocity, bone mineral density and pubertal growth. The secondary outcomes evaluated were adverse events and quality of life.
Risk of Bias in Individual Studies
Due to the heterogeneous nature of the included studies, Mixed Methods Appraisal Tool (MMAT)
2018Citation46 was used to appraise the quality of the different study designs included in this study. The
MMAT, consists of a checklist which appraises the methodological quality of included studies in systematic reviews, qualitative, quantitative, and mixed methods studies.Citation47 Checklists for nonrandomized and mixed method designs were used. Each study was screened with 2 questions and further a 5 assessments criteria check list to answered with Yes, No, cannot tell was used. A total score of 7 constitutes a Yes response to the screening and assessment criteria.Citation48 Subsequently the spreadsheet was updated for the final review and summarization of the included studies.
Summary Measures and Additional Analyses
A tabular as well as narrative summary of all study design features, participant details, outcome variables of interests are presented in this review. The outcomes of interests were compared between the IM TE dose and TT dose.
Results
Study Selection
This systematic review search ended only with 5 studies included from an initial hit of 126 studies Following the removal of duplicates, 110 studies were screened (titles and abstracts) to identify and 39 articles were assessed for full eligibility and only 5 studies were included in this systematic review. Full-text review led to the removal of 34 articles. The PRISMA flow diagram of study selection procedure is shown in . A total of 842 participants were included from these included studies and provides the summary of the attributes included studies. Out of the included studies one was a questionnaire based survey,Citation49 two were clinical trials,Citation50,Citation51 and one a retrospective case reports reviewCitation52 one a case report.Citation53 This is an explicit manifestation of the dire insufficiency of clinical trials and on young males with TE requirements.
Table 4 Summary of the Attributes Included Studies
Study Characteristics
A summary of the attributes included studies included studies are comprehensively presented in .
Risk of Bias Within Studies
The methodological quality of the included articles are shown in the . Of the included reports, only 2 studies were rated as moderately strong, and the other 3 studies were moderately weak, respectively.
Table 5 Quality Assessment of the Included Studies Using Mixed Methods Appraisal Tool (MMAT)
Synthesis of Results
Due to the high heterogeneous nature of the included studies a meta-analysis was excluded and therefore progressed with a narrative synthesis.
Participant Details
In total, this review represents 842 participants, 40.02% (n = 337) were identified as having CDGP, where 20.19% (n=170) had hypogonadism and 0.83% (n=7) had hypogonadotropic hypogonadism. The mean age of the participants were 14.84 and the duration of the TE treatment were all short term with a mean of 6 months.
Main Outcomes
The main outcomes of concern were optimal serum TE level, body mass index, height velocity, testicular volume, pubertal stage (Tanner).
Serum Testosterone Level
Raising Serum TE to an optimal level is the utmost goal of TE treatment, which alleviates the symptoms of DP. The transdermal TE gel treatment is shown to be promising than IM TE for young boys with DPCitation50,Citation51 and suggests it to be an alternative to other modes of TE treatments. It can be seen that both gel and IM TE preparations are able to achieve desirable levels of TE within a short duration with minimal fluctuations.Citation50–53 Studies on adult menCitation54–57 have reported an average level of TE levels are reported by the weekly usage of short acting IM TE. Women and girls studiesCitation32,Citation58–61 reports the favorable pharmacokinetic profile of transdermal gel compared to achieve an optimal peak of TE level.
The limited amount of evidences available in selecting TT against other modes of TE is evident in the sparse amount of studies included in this study. It is evident that there exists, a critical need for explicit clinical guidance that can be applied to monitor the safety as well as efficacy of transdermal therapy in this age group. which is descriptive of all the main outcomes covered in this study.
Pubertal Growth
Evaluation of BMI (kg/m2) testicular volume, testicular volume, bone mineral density, pubertal stages as per tanner stages, were included to identify the growth. is inclusive of these observed outcomes from the included study.
It can be seen that all the included studiesCitation49–53 reported the comparable efficacy of TT treatment in all the included main outcome observations.The heterogeneous nature of outcome reporting and the extremely limited availably of clinical trials makes it hard to make a definite statement.
TE changes the BMI and the included studiesCitation51–53 reports TT to be at par with IM TE preparations. Though from a limited amount to studies, BMI increase was apparent in all typesCitation49–53 of TE treatment. Clinical trials should be initiated to cover the anthropometric parameters, metabolic changes, changes in fat mass, lean body mass, and muscle strength comparing transdermal approach to other modes of TE treatment. It is noteworthy that in all studies with the transdermal gel approachCitation49–53 bone age is reported to be a gradual and progressive increase during treatment more apparently.
Secondary Outcomes
Secondary outcomes included in this review were the adverse events of the interventions and the reported patient satisfaction.
Adverse Events and Patient Satisfaction
Only one studyCitation53 assessed the effect of TE therapy on patient satisfaction. Increase aggression and mood swings was reported by a study,Citation52 whereas the other studies does not significantly draws notice to any adverse effects. The impact of TE therapy on quality of life among boys with DP is difficult to quantify due to the significant heterogeneity in study population, study duration, and the measures to identify it. One studyCitation49 reports acne or oily skin, aggressive behavior with IM TE and erythema was reportedCitation53 for a patient under IM TE treatment, while there no significant adverse events reported on TT by the other included studies. It is evident that clear conclusion cannot be derived from such limited sources on the different formulations for young boys with DP. briefs all these observations.
Strengths and Limitations
This review has explicitly followed the PRISMA protocols and presented a transparent review, drawing on recommendations and highlighting the research gaps found. In addition, the breadth of the study inclusion criteria has ensured a comprehensive coverage of the TE intervention under review. A rigorous use of screening process, data abstraction, and quality appraisal increases the strength of conclusions has been ensured in this study. Based on these this review support the evidence of the use of TT for the clinical management of DP in boys.
A potential limitation of this review is the volume of included literature included in this study. Although short term TE preparations are being marketed on a high scale, there is a clear lack of scientific evidence and support for the consumption and prescription of these products. Due to the high volume of research con ducted with mixed samples of adults and adolescents an inability to obtain segregated data from the articles is highlighted here.
Considering the various TE preparations and modes of administration used in the literature as well as the different participant samples, this review has a great deal of clinical heterogeneity. Variation in response to the intervention was infrequently reported. Consequently, complete statistical analysis and quantitative integration of the data cannot be assimilated.
Recommendations for Future Research
Future studies recruiting sufficient sample population with longer follow up periods should be considered. These studies should report unified measurements of outcome measurements. Finally, further research exploring the potential mechanisms mediating the transdermal preparations pharmacokinetics is warranted to better understand the interventional outcomes. Such mechanisms include, but are not limited to, vascular indices of health and function as well as patient satisfaction.
Conclusion
Positive treatment effects of TE for adult men, women and transgender are well-researched and has been explored. This study exposes the critical lack of experimental researches, meta-analysis for comparing TT delivery with other TE administration routes for young males with DP. It is very imminent that there should be clinical trials undertaken in this field as there are many new combination of TE dosages and administration routes emerging in the market. These new preparations of TE should be according to the patient’s preference, cost, availability, and formulation-specific properties for the young boys with clinically evident DP.
In conclusion, though both transdermal gel andIM TRT appear to be effective preparations for the treatment of DP in young males, TT shows more promising in the mode of administration and in better outcomes of serum TE elevation and hence recommended for treating DP among young boys. However, any clinical recommendations on TT treatment needs more clinical trial and meta analyses and thus this study only provides a clinical support and not a guideline. The research gap identified needs to be filled by future clinical trials comparing TT with other modes of TE administration for young males with DP.
Patient and Public Involvement
This study being a systematic literature review, the patients selected were recruited by the researchers of the included studies. All the patient and families related aspects involved in design and implementation of the interventions were priori addressed by the authors of the selected studies.
Data Sharing Statement
This review has utilized the published data from the included studies and thus a full dataset is not openly available.
Informed Consent
For this type of study, formal consent is not required.
Disclosure
The author has no conflict of interest to disclose.
Additional information
Funding
References
- Jin H-Y, Lim J-S, Lee Y, et al. Growth, puberty, and bone health in children and adolescents with inflammatory bowel disease. BMC Pediatr. 2021;21(1). doi:10.1186/s12887-021-02496-4
- Wu H-M, Chang H-M, Leung PCK. Gonadotropin-releasing hormone analogs: mechanisms of action and clinical applications in female reproduction. Front Neuroendocrinol. 2021;60:100876. doi:10.1016/j.yfrne.2020.100876
- Witchel SF, Plant TM. Neurobiology of puberty and its disorders. Human Hypothal Neuroendocr Disord. 2021;463–496. doi:10.1016/b978-0-12-820683-6.00033-6
- Matsumoto AM. Diagnosis and Evaluation of Hypogonadism. Endocrinol Metab Clin North Am. 2022;51(1):47–62. doi:10.1016/j.ecl.2021.11.001
- Persani L, Bonomi M, Cools M, et al. Endo-ERN Expert opinion on the differential diagnosis of pubertal delay. Endocrine. 2021;71(3):681–688. doi:10.1007/s12020-021-02626-z
- Jayasena CN, Anderson RA, Llahana S, et al. Society for endocrinology guidelines for testosterone replacement therapy in male hypogonadism. Clin Endocrinol. 2021;96(2):200–219. doi:10.1111/cen.14633
- Federici S, Goggi G, Quinton R, et al. New and consolidated therapeutic options for pubertal induction in hypogonadism: in-depth review of the literature. Endocr Rev. 2021;43(5):824–851. doi:10.1210/endrev/bnab043
- Grinspon RP, Castro S, Brunello FG, et al. Diagnosis of Male central hypogonadism during childhood. J Endocr Soc. 2021;5(11). doi:10.1210/jendso/bvab145
- Harrington J, Palmert MR. An approach to the patient with delayed puberty. J Clin Endocrinol Metab. 2022;107(6):1739–1750. doi:10.1210/clinem/dgac054
- Ehrensaft D, Tishelman AC. Take the T out, Put the T in: gender‐affirming hormones in youth. Andrology. 2021;9(6):1698–1706. doi:10.1111/andr.13055
- Grannis C, Leibowitz SF, Gahn S, et al. Testosterone treatment, internalizing symptoms, and body image dissatisfaction in transgender boys. Psychoneuroendocrinology. 2021;132:105358. doi:10.1016/j.psyneuen.2021.105358
- Desai A, Yassin M, Cayetano A, et al. Understanding and managing the suppression of spermatogenesis caused by Testosterone Replacement Therapy (TRT) and Anabolic–Androgenic Steroids (AAS). Ther Adv Urol. 2022;14:175628722211050. doi:10.1177/17562872221105017
- Corona G, Vena W, Pizzocaro A, et al. Testosterone supplementation and bone parameters: a systematic review and meta-analysis study. J Endocrinol Invest. 2022;45(5):911–926. doi:10.1007/s40618-021-01702-5
- Lanier NE, Nguyen CM, Mihalopoulos NL, et al. 54. Adolescent transgender boys: effects of 2 years of testosterone therapy. J Adolesc Health. 2022;70(4):S29. doi:10.1016/j.jadohealth.2022.01.167
- Al Samahy O, Othman D, Gad D, et al. Efficacy of topical testosterone in management of scrotal hypoplasia and agenesis. J Pediatr Urol. 2021;17(4):515.e1–515.e8. doi:10.1016/j.jpurol.2021.02.014
- Cohen J, Nassau DE, Patel P, et al. Low testosterone in adolescents & young adults. Front Endocrinol. 2020;10. doi:10.3389/fendo.2019.00916
- Kresch E, Patel M, Lima TF, Ramasamy R. An update on the available and emerging pharmacotherapy for adults with testosterone deficiency available in the USA. Expert Opin Pharmacother. 2021;22(13):1761–1771. doi:10.1080/14656566.2021.1918101
- Patel M, Muthigi A, Ramasamy R. JATENZO®: challenges in the development of oral testosterone. Int J Impot Res. 2021;34(7):721–724. doi:10.1038/s41443-021-00461-4
- Figueiredo MG, Gagliano-Jucá T, Basaria S. Testosterone therapy with subcutaneous injections: a safe, practical, and reasonable option. J Clin Endocrinol Metab. 2021;107(3):614–626. doi:10.1210/clinem/dgab772
- Suarez AMC, Israeli JM, Kresch E, et al. Testosterone therapy in children and adolescents: to whom, how, when? Int J Impot Res. 2022;34(7):652–662. doi:10.1038/s41443-021-00525-5
- Zacharin MR, Warne GL. Treatment of hypogonadal adolescent boys with long acting subcutaneous testosterone pellets. Arch Dis Child. 1997;76(6):495–499. doi:10.1136/adc.76.6.495
- Vogiatzi M, Tursi JP, Jaffe JS, et al. Testosterone use in adolescent males: current practice and unmet needs. J Endocr Soc. 2020;5(1). doi:10.1210/jendso/bvaa161
- Pastuszak AW, Gittelman M, Tursi JP, et al. Pharmacokinetics of testosterone therapies in relation to diurnal variation of serum testosterone levels as men age. Andrology. 2021;10(2):209–222. doi:10.1111/andr.13108
- Abildgaard J, Petersen JH, Bang AK, et al. Long‐term testosterone undecanoate treatment in the elderly testosterone deficient male: an observational cohort study. Andrology. 2021;10(2):322–332. doi:10.1111/andr.13124
- Shankara Narayana N, Ly LP, Jayadev V, et al. Optimal injection interval for testosterone undecanoate treatment of hypogonadal and transgender men. Endocr Connect. 2021;10(7):758–766. doi:10.1530/EC-21-0109
- White WB, Dobs A, Carson C, et al. Effects of a novel oral testosterone undecanoate on ambulatory blood pressure in Hypogonadal Men. J Cardiovasc Pharmacol Ther. 2021;26(6):630–637. doi:10.1177/10742484211027394
- Ahmad SW, Molfetto G, Montoya D, et al. Is oral testosterone the new frontier of testosterone replacement therapy? Cureus. 2022;14(8). doi:10.7759/cureus.27796
- Khodamoradi K, Khosravizadeh Z, Parmar M, et al. Exogenous testosterone replacement therapy versus raising endogenous testosterone levels: current and future prospects. F&S Rev. 2021;2(1):32–42. doi:10.1016/j.xfnr.2020.11.001
- Hashim PH, Kinnear HM, Cruz CD, et al. Pharmacokinetic comparison of three delivery systems for subcutaneous testosterone administration in female mice. Gen Comp Endocrinol. 2022;327:114090. doi:10.1016/j.ygcen.2022.114090
- Barbonetti A, D’Andrea S, Francavilla S. Testosterone replacement therapy. Andrology. 2020;8(6):1551–1566. doi:10.1111/andr.12774
- Ghosh T, Abraham W, Jasti B. Transdermal and topical drug delivery systems. In: Theory and Practice of Contemporary Pharmaceutics. CRC Press; 2004:423–455.
- Zeng J, Xie T-F, Huang T, et al. Preparation and in vitro and in vivo evaluation of a testosterone film forming gel for the treatment of hypoactive sexual desire disorder in women. AAPS Pharm Sci Tech. 2022;23(3). doi:10.1208/s12249-021-02201-9
- Dubey R, Pothuvan U. Transdermal patches: an emerging mode of drug delivery system in Pulmonary arterial hypertension. J Drug Deliv Ther. 2021;11(4–S):176–186. doi:10.22270/jddt.v11i4-S.4925
- Farooqui H, Upadhyay P, Upadhyay S. Transdermal patches approach towards self-nano-emulsifying drug delivery system (SNEDDS) using essential oil as penetration enhancer. Micro Nanosyst. 2022;14(4):314–340. doi:10.2174/1876402914666220221105304
- Al Hanbali OA, Khan HM, Sarfraz M, Arafat M, Ijaz S, Hameed A. Transdermal patches: design and current approaches to Painless Drug Delivery. Acta Pharmaceutica. 2019;69(2):197–215. doi:10.2478/acph-2019-0016
- Mani SB, Clavijo RI. Medical treatment of hypogonadism in men. Urol Clin North Am. 2022;49(2):197–207. doi:10.1016/j.ucl.2021.12.008
- Smurawa TM, Congeni JA. Testosterone precursors: use and abuse in pediatric athletes. Pediatr Clin North Am. 2007;54(4):787–796. doi:10.1016/j.pcl.2007.05.002
- Malik RD, Liu DB. Survey of pediatric urologists on the preoperative use of testosterone in the surgical correction of hypospadias. J Pediatr Urol. 2014;10(5):840–843. doi:10.1016/j.jpurol.2014.02.008
- Funderburgh LJ, Zipf W, Sotos JF. [PDF] direct measurement of testosterone in a pediatric center, with use of a radioimmunoassay kit and unextracted serum.: semantic scholar. Clin Chem. 1983;29(10):1796–1798. doi:10.1093/clinchem/29.10.1796
- Arisaka O, Hoshi M, Kanazawa S, et al. Systemic effects of transdermal testosterone for the treatment of microphallus in children. Pediatr Int. 2001;43(2):134–136. doi:10.1046/j.1442-200x.2001.01353.x
- Rey RA, Grinspon RP. Androgen treatment in adolescent males with hypogonadism. Am Jo Men’s Health. 2020;14(3):155798832092244. doi:10.1177/1557988320922443
- Takeuchi H, Okubo H. Clinical efficiency of combination therapy using testosterone replacement therapy, phosphodiesterase 5 inhibitors and Kampo herbal medicine for EUGONADAL patients with late-onset hypogonadism syndrome. Exp Ther Med. 2021;22(4). doi:10.3892/etm.2021.10608
- Lee J, Brock G, Barkin J, et al. The my-T study: patient satisfaction and preference comparing topical and nasal testosterone therapies. Can Urol Assoc J. 2019;13(11). doi:10.5489/cuaj.5680
- Schiavo JH. Prospero: an international register of systematic review protocols. Med Ref Serv Q. 2019;38(2):171–180. doi:10.1080/02763869.2019.1588072
- Liberati A, Altman D, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of clinical epidemiology. 2009;62(10). doi:10.1136/bmj.b2700
- Hong QN, Pluye P, Fàbregues S, et al. Improving the content validity of the mixed methods appraisal tool: a modified e-delphi study. J Clin Epidemiol. 2019;109:111. doi:10.1016/j.jclinepi.2019.01.012
- Harrison R, Jones B, Gardner P, Lawton R. Quality Assessment with diverse studies (Quads): an appraisal tool for methodological and reporting quality in systematic reviews of mixed- or multi-method studies. BMC Health Serv Res. 2021;21(1):1–20.
- Lizarondo L, Stern C, Apostolo J, et al. Five common pitfalls in mixed methods systematic reviews: lessons learned. J Clin Epidemiol. 2022;148:178–183. doi:10.1016/j.jclinepi.2022.03.014
- Stancampiano MR, Lucas-Herald AK, Russo G, et al. Testosterone therapy in adolescent boys: the need for a structured approach. Horm Res Paediatr. 2019;92(4):215–228. doi:10.1159/000504670
- Mastromattei S, Todisco T, Chioma L, et al. Efficacy of short-term induction therapy with low-dose testosterone as a diagnostic tool in the workup of delayed growth and puberty in boys. J Endocrinol Invest. 2022;45(12):2377–2384. doi:10.1007/s40618-022-01879-3
- Chioma L, Papucci G, Fintini D, et al. Use of testosterone gel compared to intramuscular formulation for puberty induction in males with constitutional delay of growth and puberty: a preliminary study. J Endocrinol Invest. 2017;41(2):259–263. doi:10.1007/s40618-017-0726-7
- Lucas-Herald AK, Mason E, Beaumont P, et al. Single-centre experience of testosterone therapy for boys with hypogonadism. Horm Res Paediatr. 2018;90(2):123–127. doi:10.1159/000490738
- Contreras MF, Raisingani M, Prasad K, Franklin B, Shah B. Transdermal testosterone gel for induction and continuation of puberty in adolescent boys with hepatic dysfunction. J Pediatr Endocrinol Metabol. 2017;30(1). doi:10.1515/jpem-2016-0201
- Johansen N, Lindén Hirschberg A, Moen MH. The role of testosterone in menopausal hormone treatment. What is the evidence? Acta Obstet Gynecol Scand. 2020;99(8):966–969. doi:10.1111/aogs.13819
- Lin L-T, Li C-J, Tsui K-H. Serum testosterone levels are positively associated with serum anti-mullerian hormone levels in infertile women. Sci Rep. 2021;11(1):6336.
- Diem SJ, Greer NL, MacDonald R, et al. Efficacy and safety of testosterone treatment in men: an evidence report for a clinical practice guideline by the American College of Physicians. Ann Intern Med. 2020;172(2):105. doi:10.7326/M19-0830
- Nolan BJ, Proietto J, Sumithran P. Single‐Center Real‐life experience with testosterone treatment in adult men with Prader–Willi syndrome. Am J Med Genet A. 2022;188(9):2637–2641. doi:10.1002/ajmg.a.62770
- Madsen MC, van Dijk D, Wiepjes C, et al. M. Erythrocytosis in a large cohort of trans men using testosterone: a long term follow up study on prevalence, determinants, and the effect of years of exposure. J Endocr Soc. 2021;5(Supplement_1):A726–A726. doi:10.1210/jendso/bvab048.1477
- Bhasin S, Lincoff AM, Basaria S, et al. Effects of long-term testosterone treatment on cardiovascular outcomes in men with hypogonadism: rationale and design of the Traverse Study. Am Heart J. 2022;245:41–50. doi:10.1016/j.ahj.2021.11.016
- Salamin O, Nicoli R, Langer T, et al. Longitudinal evaluation of multiple biomarkers for the detection of testosterone gel administration in women with normal menstrual cycle. Drug Test Anal. 2021;14(5):833–850. doi:10.1002/dta.3040
- Donaldson M, Kriström B, Ankarberg-Lindgren C, et al. Optimal pubertal induction in girls with Turner syndrome using either oral or transdermal estradiol: a proposed modern strategy. Horm Res Paediatr. 2019;91(3):153–163. doi:10.1159/000500050
