Abstract
Emergomycosis is an emerging deadly infectious disease caused primarily by a little-known airborne pathogen Emergomyces africanus, which can cause clinical management challenge especially in patients with advanced HIV disease. This minireview describes Es. africanus as the main cause of emergomycosis in Africa as well as considers contributing factors to the difficulties encountered in managing this infection. Emergomycosis is common in HIV-positive persons with low CD4 lymphocyte count and has an estimated fatality of 50%. The infection exhibits airborne transmission with pulmonary and extrapulmonary manifestations leading to skin lesions. However, the pathogenesis of Es. africanus is still poorly understood. The management of the infection is complicated due to lack of defined diagnostic and therapeutic guidelines. Limited expertise, poor research funding, and lack of awareness and national surveillance are thought to impact the recognition and prioritisation of the infection. These factors may ultimately assign emergomycosis a ‘neglected infection status’ even as it is suspected to be prevalent in more African countries than previously recognised. Increased awareness and integrated and targeted strategies such as mobilising manpower in clinical mycology are of paramount importance in managing emergomycosis in Africa and beyond.
Introduction
Fungal pathogens are constantly evolving perhaps to develop new strategies to thrive in novel host niches and harsh environment leading to new species increasingly associated with human infections. Consequently, managing the resulting infections becomes a challenge. Emergomycosis is one of such observed infections caused by the recently described novel thermal dimorphic fungi genera, Emergomyces which is closely related to Histoplasma, Blastomyces, and Paracoccidioides.Citation1 The genus Emergomyces is made up of 5 unique species: Emergomyces pasteurianus, Emergomyces africanus, Emergomyces canadensis, Emergomyces orientalis, and Emergomyces europaeus.Citation1,Citation2 Emergomycosis is common in immunocompromised persons and characterised with widespread skin lesion and pulmonary disease.Citation3 Other clinical manifestations of the infection become apparent in HIV patients following the initiation of ART.Citation4 Cases of emergomycosis reported are mainly due to Es. africanusCitation5 which accounts for majority of the diagnosed cases in Africa.Citation6
Emergomycosis is an airborne infection which can be diagnosed using histological findings, serology, and/or molecular identification; however, no well-streamlined guideline is available for emergomycosis diagnosis. Consequently, clinicians and dermatologists usually misdiagnose the lesions for other conditions ranging from varicella to scrofluoderma,Citation3 which can significantly affect treatment outcome.
In Africa, Es. africanus causes systemic infection associated with skin lesionsCitation3,Citation4 with an estimated case fatality rate of 50% especially among patients with advanced HIV disease ().Citation3 In a recent study, 96% of patients with systemic emergomycosis infection had low CD4 lymphocyte counts (median CD4 lymphocyte count 16 cells/µL).Citation3 The infection is now recognised as the most prevalent endemic fungal infection in South Africa with cases reported in 6 of the 9 provinces. Emergomycosis has also been reported in a LesothoCitation3 and a Ugandan patient (caused by Es. pasteurianus).Citation7 Currently, South Africa has the highest burden of emergomycosis in the world. The reason for this concentration is unknown; however, it can be reasonably presumed to be due to the high prevalence of HIV with only less than half of those eligible for ART accessing it.Citation8 In addition, it could be because South Africa has the most advanced diagnostic capability in Africa besides the high clinical awareness and disease surveillance systems in the country.
Table 1 Description of Characteristics, Diagnosis, Treatment, and Outcome of Reported Cases of Emergomycosis Infection in Africa
Es. africanus can infect immunocompetent individuals ().Citation9 It is not clear whether the disease results from exposure to higher bioload of Es. africanus, infection with more virulent strains, or undiagnosed immune impairment. Limited clinical expertise on the management of this infection along with lack of specific diagnostic and therapeutic guidelines poses challenge to our current healthcare system’s responsiveness. Most importantly, there is generally a systemic lack of awareness of the infection and invasive fungal infections (IFIs) among clinicians, which further reduces proactive approaches to this emerging condition. This paper calls for an increase in awareness, investment, and targeted approach to improve the management of emergomycosis in order to give this deadly infection the deserved priority as it has almost fulfilled the criteria for a neglected disease status.
Infection and Manifestation
Emergomycosis is an airborne infection that is amongst the common complications in HIV-related immunocompromised patients which can worsen with antifungal and antiretroviral therapies (ART).Citation8
The environmental reservoir of Emergomyces is not known. However, the infection is thought to begin through the inhalation of Emergomyces species conidia dispersed by the saprophytic mycelia in the soilCitation14 which then undergoes temperature-dependent transition from vegetative structures that lodge in the lungs to budding yeasts that cause pulmonary infection and upon haematogenous dissemination, extrapulmonary infections ().
Figure 1 A model for Emergomyces infection and clinical manifestation. Emergomyces species (Es. africanus, Es. pasteurianus, Es. canadensis, Es. europaeus and Es. orientalis) have been reported - using Es. africanusCitation5 - to exist in the soil as saprophytic moulds that produce spores (conidia). The conidia adapt for wind dispersal and can remain airborne for lengthy periods. Humans inhale the conidia (1) which then lodged in the terminal regions of the lungs where they undergo morphological transition to the infective phase - budding yeast (2). The yeast phase causes pulmonary infection in susceptible individuals especially in patients with advanced HIV infection which can lead to disseminated infections. From the lungs, the yeast cells can spread (3) throughout the body through haematogenous dissemination in neutrophils and infect virtually any organ including the skin, heart, bone marrow, spleen, and lymph node. Figure created with BioRender.com.
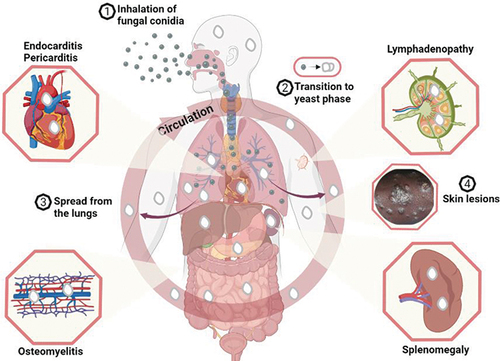
Emergomycosis also affects patients with other immune impairments including those with haematological malignancies, solid organ transplant, and those using immunosuppressants;Citation2,Citation9 however, little is known about the infection pathogenesis, but the infection manifestation is fairly well understood. Patients with Es. africanus infection often present with skin lesions manifesting as verrucous lesions, papules, plaques, nodules, or ulcers that are widespread.Citation11 Emergomycosis lesions are seen to become more numerous or erupt following ART initiation, suggesting that unmasking of immune reconstitution inflammatory syndrome (IRIS) is often involved.Citation4 Pulmonary infection is common, and chest radiograph shows manifestations that include effusions, consolidation, reticulonodular disease, or lymphadenopathy.Citation3 Emergomycosis has also been reported to affect other organs, such as bone marrow, gastrointestinal tract, and the liver.
Pathogenesis
Once the pathogen’s conidia are inhaled, they are lodged in the terminal regions of the lungs where they undergo morphological transition to budding yeasts which are the infective phase. The phenotypic switch is similar to the mechanisms regulating dimorphism in Blastomyces dermatitidis and Histoplasma capsulatum, though genes regulating the process in Emergomyces species have yet to be identified.
Genomic analysis revealed that virulence factors are conserved in Es. africanus and Es. pasteurianusCitation15 (causes emergomycosis in Europe). The roles of the virulence genes in pathogenesis are still unknown. However, Es. africanus expresses urease,Citation16 a known virulence factor in Cryptococcus neoformans. Experiments in mice demonstrated dose-dependent susceptibility to emergomycosis infection.Citation14 An infectious dose of 102 conidia was unable to cause infection in a mice model of systemic emergomycosis; however, Es. africanus was recovered from their livers and spleens. Es. africanus was fatal at an infectious dose of 106 conidia.Citation14 Currently, research in emergomycosis and Es. africanus is limited and understanding the pathogen’s pathogenicity traits holds potential in understanding the infection, its diagnosis and treatment.
Diagnosis and Setbacks
In spite of the ubiquity and fatal nature of many fungal infections, the field of medical mycology has been heavily underfunded compared to research in bacteriology, parasitology, or virology.Citation17,Citation18 This deficiency exists despite the more than 1.2 billion cases of fungal infection reported worldwide with about 2 million fatalities per annum. As a result of this chronic underfunding, medical mycologists have been left with limited tools to diagnose and treat emergomycosis. Additionally, the global response to the SARS-CoV-2 pandemic has left the existing healthcare structure for fungal infections in a rather bad state in terms of diagnostics, therapeutics, and funding.Citation19 The unavailability of appropriate diagnostics may explain the under-reporting of emergomycosis cases in other parts of Africa. Current diagnostics present a lot of setbacks ( and ):Citation20 Firstly, histology or tissue culture can be invasive, slow, and insensitive with Es. africanusthat is indistinguishable from Histoplasma species when diagnosing these infections in patients with advanced HIV conditions () and even in an otherwise health individual.Citation21 Microscopically, Emergomyces mycelia morphology is also indistinguishable from Sporothrix schecnkii, though this does not form a basis for diagnosis. Serological tests or serum biomarkers for early diagnosis are not yet available for emergomycosis. Cross-reactivity has been observed with urine Histoplasma galactomannan antigen test, and PCR-based assays have not been validated for emergomycosis. Emergomyces species have been reported to cross-react with the available DNA probe for B. dermatitidis (). Sequencing of rDNA internal transcribed spacer region is the most reliable diagnostic method, but the technology remains sophisticated and expensive for routine diagnosis in Africa. However, the current effort to expand genomics tools and research in Africa in response to the Covid-19 pandemic could be useful in addressing some of the challenges to emergomycosis diagnosis and treatment.
Table 2 Some Diagnostic Characteristics of Emergomycosis Pathogen
Treatment and Antifungal Availability
Even after a successful diagnosis, management of this fungal infection remains difficult (). Surgical debridement may be necessary to treat severely infected and necrotic skin, but this can lead to disfigurement.Citation3,Citation4,Citation8 However, systemic antifungal therapy is the mainstay of treatment to restore severely infected skin to normal. Amphotericin B, a nephrotoxic antifungal, is the first-line therapy for emergomycosis, usually for 1–2 weeks followed by itraconazole or similar azole for maintenance ().Citation6 Currently, according to the WHO guideline for cryptococcosis, liposomal amphotericin B is preferred because of its low toxicity and substituted for deoxycholate amphotericin B for treating emergomycosisCitation27 but it is prohibitively costly or unavailable in most resource poor settings. Only 7 countries (Benin, South Africa, Egypt, Tanzania, Mauritania, Eswatini and Ethiopia) have access to liposomal amphotericin B in Africa to dateCitation28 (www.gaffi.org). The few alternativesCitation14 are either unavailable or expensive (voriconazole, itraconazole) or completely out of reach (posaconazole) in Africa. Currently, treatment recommendations, in addition to antifungal therapy, rely on expert opinion and observational studies because of the complex clinical manifestations of emergomycosis.
Epidemiology and Clinical Surveillance
The seriousness of the already diagnosed cases of emergomycosis in South Africa and the possibility that the infection might be more prevalent in more African countries than it is known should bring attention to the pathogen. The true burden of emergomycosis in South Africa and its socioeconomic impacts are unknown. National surveillance data in South Africa and other African countries remain unavailable; however, the pathogen is airborne which brings a certain level of predictability and cause for concern. The inherent ability of airborne Es. africanus vegetative structures to survive in the air for long periods of timeCitation5 and the potential for long-distance dispersal indicate that the pathogen may be common across African countries. These potentials suggest that the infection might be part of the healthcare problems most sub-Saharan African countries currently face but fail to recognize. The last published report on emergomycosis in Africa was in 2019 even though there is visible manifestation of skin lesions in HIV-positive persons in HIV care centres in most sub-Saharan African countries which may be due to Emergomyces species.
Research Efforts and Gaps
A significant amount of work has been done to inform our current understanding of emergomycosis. The molecular and cellular mechanisms that govern the infection pathogenesis and host immune response or susceptibility to Emergomyces species remain a mystery and an interesting research focus. Our current understanding of the manifestation, management of the disease, and risk factors associated with the infection and its epidemiology remain elusive. Concomitantly, there is a need for the development, testing, and uptake of accurate diagnostic tools, development of treatment guidelines, and increased access to effective antifungals. Appropriate timing of ART commencement after a positive emergomycosis diagnosis in ART naïve patients’ needs to be defined, as a measure to mitigate the development of IRIS. Several possible interactions occur between ART and antifungals; thus, optimal dose adjustment must be established. Finally, epidemiological surveillance of emergomycosis is important to understand the distribution and then mount proper responses especially in the African context before it becomes a major healthcare crisis.
Perspective
Emergomycosis is an emerging infection, that is rapidly achieving a “neglected infection status” though not there yet. The infection is becoming prevalent in Africa. In our opinion, the time for action on emergomycosis is now. The possibility that the infection is common in most African countries is high due to the high burden of HIV infection, misdiagnosis, and missed diagnosis due to both limited expertise and laboratory infrastructure in sub-Saharan Africa. An online survey conducted in Africa involving 165 researchers in 40 institutions from 21 countries showed that only 5 institutions’ laboratories met the minimum laboratory standards.Citation29 The paper also reported suboptimal diagnostic and therapeutic capacities to manage fungal infections in general. Furthermore, the problem of awareness is a major challenge especially amongst clinicians.Citation30 Public awareness of fungal infections in the African populace, especially in those living in rural communities, is generally low. These deficits may partly explain the poor response to IFIs and why most of the infections that are endemic in Africa are fast achieving a “neglected infection status”.
Conclusion
In conclusion, development and uptake of accurate diagnostic tools, increasing funding for research, raising awareness, plus integrated and targeted efforts are needed to tackle the suspected multifaceted healthcare challenges of emergomycosis in Africa. The pathogenesis of the infection is poorly understood in Africa. Data reflecting the true epidemiology of the infection are also lacking, and there are no surveillance systems established for the infection.
Increasing awareness is important to mobilise critical mass to boost mycology research in Africa. Studies to understand the epidemiology of the infection are required as well as clinical research to understand the disease manifestation and associated complications. The outcome of research will help to mount effective prevention and control measures against the infection. It will also help to enhance our understanding of the infection manifestation and guide the establishment of diagnostic and therapeutic guidelines. Additionally, research outcome will help us understand the environmental reservoir of emergomycosis. Government and non-governmental agencies in Africa need to prioritise the infection, which can be done through public-private sector partnership to mount and improve surveillance through data and resource sharing. These actions are important especially in ensuring that the infection does not take a “neglected infection status”.
Future research should aim to identify the pathogens’ virulence attribute to understand the disease pathogenesis and host–pathogen interaction especially person with advanced HIV infection.
Disclosure
The authors declare no competing interests.
Acknowledgment
IC thanks Abia State University for support.
Additional information
Funding
References
- Dukik K, Muñoz JF, Jiang Y, et al. Novel taxa of thermally dimorphic systemic pathogens in the Ajellomycetaceae (Onygenales). Mycoses. 2017;60:296–309. doi:10.1111/myc.12601
- Schwartz IS, Maphanga TG, Govender NP. Emergomyces: a new genus of dimorphic fungal pathogens causing disseminated disease among immunocompromised persons globally. Curr Fungal Infect Rep. 2018;12:44–50. doi:10.1007/s12281-018-0308-y
- Schwartz IS, Govender NP, Corcoran C, et al. Clinical characteristics, diagnosis, management, and outcomes of disseminated emmonsiosis: a retrospective case series. Clin Infect Dis. 2015;61(6):1004–1012. doi:10.1093/cid/civ439
- Schwartz IS, Kenyon C, Lehloenya R, et al. AIDS-related endemic mycoses in Western Cape, South Africa, and clinical mimics: a cross-sectional study of adults with advanced HIV and recent-onset, widespread skin lesions. Open Forum Infect Dis. 2017;4(4):1–7. doi:10.1093/ofid/ofx186
- Schwartz IS, McLoud JD, Berman D, et al. Molecular detection of airborne Emergomyces africanus, a thermally dimorphic fungal pathogen, in Cape Town, South Africa. PLoS Negl Trop Dis. 2018;12(1):1–12. doi:10.1371/journal.pntd.0006174
- Maphanga T, Britz E, Zulu TG, et al. In vitro antifungal susceptibility of yeast and mold phases of isolates of dimorphic fungal pathogen Emergomycesafricanus (Formerly Emmonsia sp.) from HIV-Infected South African Patients. J Clin Microbiol. 2017;56:1812–1820. doi:10.1128/JCM.02524-16
- Schwartz IS, Govender NP, Sigler L, et al. Emergomyces: the global rise of new dimorphic fungal pathogens. PLoS Pathog. 2019;15(9):e1007977. doi:10.1371/journal.ppat.1007977
- Crombie K, Spengane Z, Locketz M, et al. Paradoxical worsening of Emergomyces africanus infection in an HIV-infected male on itraconazole and antiretroviral therapy. PLoS Negl Trop Dis. 2018;12(3):1–7. doi:10.1371/journal.pntd.0006173
- Heys I, Taljaard J, Orth H. An Emmonsia species causing disseminated infection in South Africa. N Eng J Med. 2014;370:283–284.
- van Hougenhouck-Tulleken WG, Papavarnavas NS, Nel JS, et al. HIV-associated disseminated emmonsiosis, Johannesburg, South Africa. Emerg Infect Dis. 2014;20(12):2164–2166. doi:10.3201/eid2012.140902
- Kenyon C, Bonorchis K, Corcoran C, et al. A dimorphic fungus causing disseminated infection in South Africa. N Eng J Med. 2013;369(15):1416–1424. doi:10.1056/NEJMoa1215460
- Lochan H, Naicker P, Maphanga T, et al. A case of emmonsiosis in an HIV-infected child. South Afr J HIV Med. 2015;16(1). doi:10.4102/sajhivmed.v16i1.352
- Rooms I, Mugisha P, Gambichler T, et al. Disseminated emergomycosis in a person with HIV infection, Uganda. Emerg Infect Dis. 2019;25(9):1750–1751. doi:10.3201/eid2509.181234
- Schwartz IS, Lerm B, Hoving JC, et al. Emergomyces africanus in Soil, South Africa. Emerg Infect Dis. 2018;24(2):377–380. doi:10.3201/eid2402.171351
- Muñoz JF, McEwen JG, Clay OK, Cuomo CA. Genome analysis reveals evolutionary mechanisms of adaptation in systemic dimorphic fungi. Sci Rep. 2018;8:1–13. doi:10.1038/s41598-018-22816-6
- Lerm B, Kenyon C, Schwartz IS, et al. First report of urease activity in the novel systemic fungal pathogen Emergomyces africanus: a comparison with the neurotrope Cryptococcus neoformans. FEMS Yeast Res. 2017;17. doi:10.1093/femsyr/fox069
- Rodrigues ML, Albuquerque PC. Searching for a change: the need for increased support for public health and research on fungal diseases. PLoS Negl Trop Dis. 2018;12:1–5. doi:10.1371/journal.pntd.0006479
- Ibe C. The fight against mycoses in Africa, are we making progress? Clin Microbiol Infect. 2022;28:9–12. doi:10.1016/j.cmi.2021.09.004
- Nargesi S, Bongomin F, Hedayati MT. The impact of covid-19 pandemic on aids-related mycoses and fungal neglected tropical diseases: why should we worry? PLoS Negl Trop Dis. 2021;15:e0009092. doi:10.1371/journal.pntd.0009092
- Govender NP, Grayson W. Emergomycosis (Emergomyces africanus) in advanced HIV disease. Dermatopathology. 2019;6:63–69. doi:10.1159/000495405
- Mah J, Bakker A, Tseng C, et al. Isolated Pulmonary Emergomycosis in an Immunocompetent Patient in Alberta, Canada. Open Forum Infect Dis. 2022;9(3). doi:10.1093/ofid/ofac021
- Moodley A, Mosam A, Govender NP, Mahabeer Y, Chateau AV. Emergomyces africanus: the Mimicking Fungus. Dermatopathology. 2019;6:157–162. doi:10.1159/000497608
- Linder KA, Kauffman CA. Histoplasmosis: epidemiology, Diagnosis, and Clinical Manifestations. Curr Fungal Infect Rep. 2019;13(3):120–128. doi:10.1007/s12281-019-00341-x
- Maphanga TG, Naicker SD, Gómez BL, et al. Cross-reactivity of a Histoplasma capsulatum antigen enzyme immunoassay in urine specimens from persons with emergomycosis in South Africa. Med Mycol. 2021;59(7):672–682. doi:10.1093/mmy/myaa100
- Azar MM, Hage CA. Laboratory diagnostics for histoplasmosis. J Clin Microbiol. 2017;55:1612–1620. doi:10.1128/JCM.02430-16
- Schwartz IS, Sanche S, Wiederhold NP, Patterson TF, Sigler L. Emergomyces canadensis, a Dimorphic Fungus Causing Fatal Systemic Human Disease in North America. Emerg Infect Dis. 2018;24:758–761. doi:10.3201/eid2404.171765
- World Health Organisation (WHO). Guidelines for diagnosing, preventing and managing cryptococcal disease among adults, adolescents and children living with HIV. World Health Organisation; 2022.
- Ibe C, Okoye CA. Integrated healthcare approach can curb the increasing cases of cryptococcosis in Africa. PLoS Negl Trop Dis. 2022;16:e0010625. doi:10.1371/journal.pntd.0010625
- Driemeyer C, Falci DR, Oladele RO, et al. The current state of clinical mycology in Africa: a European Confederation of Medical Mycology and International Society for Human and Animal Mycology survey. Lancet Microbe Preprint. 2022;3(6):e464–e470. doi:10.1016/S2666-5247(21)00190-7
- Oladele R, Otu AA, Olubamwo O, et al. Evaluation of knowledge and awareness of invasive fungal infections amongst resident doctors in Nigeria. Pan Af Med J. 2020;36:1–11. doi:10.11604/pamj.2020.36.297.23279
