Abstract
Atrial fibrillation (AF) is the most common cardiac arrhythmia with a significant increase in morbidity and mortality worldwide. Rheumatoid arthritis (RA), as a systemic inflammatory disease, affecting 0.5–1.0% of the adult population, is associated with increased incidence of cardiac arrhythmias such as AF. Several epidemiologic studies find that the risk of AF is increased in RA when compared with the general population. Other studies are inconsistent. Considering that inflammation plays an important role in AF, RA may be involved in the occurrence and development of AF. This review summarizes the epidemiology, pathophysiology, and management of AF in patients with RA.
Introduction
Rheumatoid arthritis (RA) is a systemic inflammatory joint disease, affects 0.5–1.0% of the adult population, and is associated with an increased mortality rate.Citation1 RA is autoimmune and is generally characterized by autoantibodies to immunoglobulin G (IgG).Citation2 The utilization of biologic disease-modifying anti-rheumatic drugs (bDMARDs) improves long-term prognosis of RA patients.Citation3 Besides being a cause of joint structural damage,Citation4 many diseases are associated with RA, including osteoporosis,Citation5 depression,Citation6 pulmonary disease,Citation7 hypertension,Citation8 cardiovascular disease (CVD)Citation9 and cardiac arrhythmias.Citation10 The association between CVD and RA is well established and the risk of CVD in RA patients is 1.5 times higher compared to the general population.Citation11,Citation12 CVD is the most common cause of death in RA.Citation13 In addition to accelerating the development of atherosclerosis, RA is associated with increased incidence of cardiac arrhythmias, especially in women.Citation14 Inflammatory cytokines such as interleukin (IL)-1, IL-6 and tumor necrosis factor- alpha (TNF-α) may play an important role in the cardiac electrophysiology.Citation15,Citation16
Atrial fibrillation (AF), as the most common cardiac arrhythmia, affects more than 33 million people worldwide and is associated with a significant increase in morbidity and mortality.Citation17 The increased risk of stroke, dementia, heart failure, and death are associated with AF.Citation18 The management of AF mainly includes rate control, rhythm control, and stroke prophylaxis.Citation19 AF is promoted by advancing age, male sex, heart disease, hypertension, diabetes, obesity, stroke and alcohol overuse.Citation17,Citation20 However, some individuals develop AF with no apparent risk factors, which suggests some underlying risk factors to AF. In patients with AF, inflammatory infiltrates have been observed in atrial tissuesCitation21 and increased systemic inflammation appears to relate to the persistence of AF.Citation22 In the general population, inflammatory cytokines such as IL-1, IL-2, IL-6, IL-10 and TNF-α are associated with the presence, persistence and outcome of AF.Citation23,Citation24
Under the premise that a systemic inflammatory state may contribute to AF, patients with chronic inflammatory conditions such as RA might be at an increased risk of developing AF. Moreover, growing studies suggest that the incidence of AF may be significantly increased in RA than in the general population.Citation25 Nevertheless, other researches have yielded inconsistent results.Citation26,Citation27 Since systemic inflammation plays an important role in the pathogenesis of AF and RA is a disease characterized by chronic systemic inflammation, RA may play an important role in the occurrence and development of AF. In this article, we will review the epidemiology, pathophysiology, and management of AF in patients with RA.
Epidemiology
In a Danish nationwide cohort study, Lindhardsen et al report an overall 40% higher incidence of AF in patients with RA compared to the general population.Citation28 Besides, in patients with RA younger than 50, the relative risk of AF is increased threefold. It is worth noting that the study did not adjust for all cardiovascular risk factors due to the lack of relevant information. Using data from a large US commercial insurance plan, Kim et al show that the incidence of hospitalization for AF in patients with RA is 1.4 times higher than in non-RA patients.Citation26 After adjusting for various comorbidities, medications and healthcare utilization, the result shows no increased risk of AF associated with RA. These inconsistent findings could be due to a number of differences such as the age of patients, gender composition and duration of follow-up time between the two studies. Bacani et al also adjust the age, sex, calendar year, smoking, and hypertension.Citation29 Compared with non-RA subjects, the risk of AF during 9.6 years follow-up is higher among patients with RA. However, AF related mortality is equally both in patients with and without RA. In a senior RA cohort from the Korean National Health Insurance Service, a similar risk of AF is found by Jang et al when compared with the control.Citation27 Although patients in this study could represent the RA patients older than 65 years of age, the similar occurrence of new-onset AF in the senior patients with RA maybe due to the limited close surveillance of heart rhythm. Besides, in a recent study by Argnani et al, RA acts as an independent risk factor for the development of AF.Citation30 In this study, there are more women in the RA cohort. In conclusion, these studies suggest a link between AF and RA. A routine screening for AF should be part of assessment of patients with RA. The detailed characteristics of the epidemiologic studies are illustrated in .
Table 1 Epidemiologic Studies
Pathophysiological Pathways Between RA and AF
RA and Atrial Remodeling
Structural atrial remodeling is associated with the maintenance and recurrence of AF and electrical remodeling of atria may be the first stage in the onset of AF.Citation31,Citation32 RA is involved in both atrial electrical and structural remodeling.
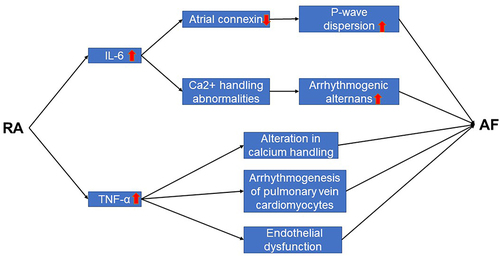
P-wave indices (P-wave duration, P-wave dispersion, and P-wave standard deviation) are associated with atrial remodeling33 and involved in the increased risk of AF.Citation34 In patients with inflammatory condition such as RA, indices of P-wave dispersion are increased.Citation24 IL-6-induced downregulation of atrial connexin is responsible for this change. As a systemic inflammatory disease, patients with RA have ten times higher levels of IL-6 in serum than healthy human.Citation35 Furthermore, IL-6-mediated-Ca2+ handling abnormalities contributes to the development of AF by increasing propensity for arrhythmogenic alternans.Citation36 Guler et al find that P-wave duration and P-wave dispersion, signs for the prediction of AF, are higher in RA patients than healthy control subjects.Citation37 These rapidly induce atrial electrical remodeling. Prolonged atrial electromechanical conduction is associated with the decreased plasma prolidase activity in patients with AF.Citation38 Meanwhile, in patient with RA, collagen turnover and fibrosis are decreased with lower prolidase activity.Citation39 Atrial conduction time is one of the factors that represent left atrial remodeling and is also a predictive factor of AF recurrence.Citation40 The prolonged atrial conduction time is observed in collagen-induced arthritis (CIA) rat, a widely used animal model to study the pathophysiology of human RA.Citation41 TNF-α, as a major proinflammatory factor in RA,Citation42 mediates inflammation-related AF by altering calcium handling and increasing arrhythmogenesis of pulmonary vein cardiomyocytes.Citation43
As early as in the 1976, study has observed a link between left atrial enlargement and AF.Citation44 The late stage of atrial remodeling is mainly characterized by structural remodeling.Citation45 In CIA rat after primary immunization, atrial structural remodeling is observed and AF inducibility and duration are substantially increased.Citation41 Through the same animal model, Zhang et al find that the inducibility and duration of AF are significantly reduced by resveratrol, a type of polyphenol antioxidant.Citation46 Meanwhile, there is a trend for left atrial (LA) enlargement in cardiac asymptomatic RA patients.Citation47 Atrial fibrosis is a hallmark of atrial structural remodeling.Citation48 In patients with RA, extensive atrial fibrosis is observed.Citation49 The degree of LA fibrosis is not only associated with the occurrence of AF,Citation50 but also a good predictor of AF recurrence post-AF ablation.Citation51 It is worth noting that when compared with the control group, the degree of fibrosis by cardiac magnetic resonance imaging is similar or lower in RA patients with low to moderate disease activity.Citation52 Furthermore, insulin resistance (IR) contributes to increased AF susceptibility by engendering both atrial structural remodeling and abnormal intracellular calcium homeostasis.Citation53 Elevated IR has been observed in patients with RA and is consistent with high disease activity.Citation54
RA and Autonomic Nerve System
After activation of the autonomic nervous system (ANS), significant changes in atrial electrophysiology lead to the occurrence of AF.Citation55 Methods that reduce autonomic innervation or outflow may improve the incidence of AF. At the same time, studies have suggested an imbalance of the ANS in patients with RA.Citation56 Sympathetic nerve activity (SNA) is thought to play an important role in provoking AF.Citation57 It makes the atria more vulnerable to arrhythmogenic factors from the pulmonary veins. There is evidence of heightened sympathetic outflow in patients with RA and it’s associated with increases in both pain and inflammation in RA.Citation58 ANS dysfunction in RA is not only present in the resting state, study has shown an enhanced sympathetic response to exercise in post-menopausal women with RA.Citation59 In addition, the use of tocilizumab, an IL-6 blockade, does have the potential to improve autonomic dysfunction in RA.Citation60
RA and Renin Angiotensin System
Renin angiotensin system (RAS) is suggested to play a key role in the occurrence and development of AF through structural and electrical remodeling.Citation61,Citation62 In addition, RAS blockade therapy has been shown to reduce the relative risk of recurrent AF by 39%.Citation63 Angiotensin-converting enzyme (ACE) and angiotensin II (Ang II) are the major components of RAS and there is intracellular RAS in cardiac myocytes.Citation64 The stimulation of angiotensin II may induce AF through inflammation, epicardial fat accumulation, and electrical cardiac remodeling.Citation65 Previous study has found that the expression of ACE and Ang II are up-regulated in the synovium of the joints from patients with RA.Citation64 Research shows that in women with RA, serum levels of RAS components such as ACE and Ang II are higher than healthy females and this difference is not altered by the use of ACE inhibitors.Citation66 Furthermore, the levels of Ang II are higher in active RA when compared with the remission group.Citation67
RA and Endothelial Dysfunction
Through years of follow-up, studies have found that endothelial dysfunction is associated with increased risk of AF.Citation68,Citation69 Besides, the incidence of AF is significantly higher in patients with coronary endothelial dysfunction when compared with those with normal coronary endothelial function and similar AF risk factors.Citation70 Possible mechanisms include inflammation and oxidative stress.Citation71,Citation72 From microvascular to macrovasculature, several studies have shown that endothelial dysfunction is observed in patients with RA.Citation73–75 Increased TNF-α levels induce the endothelial dysfunction in RA.Citation76 This is associated with reduced nitric oxide (NO) bioavailability and decreased cyclic guanosine monophosphate (cGMP) levels by increasing vascular oxidized low density lipoprotein (ox-LDL) content and activation of the lectin-like oxidized low-density lipoprotein receptor-1/ nuclear factor-κB/arginase 2 (LOX-1/NFκB/Arg2) pathway. Meanwhile, the addition of TNF-α inhibitors improves endothelial dysfunction in RA.Citation77,Citation78 Apart from peripheral arteries, Ciftci et al find that coronary endothelial dysfunction is also present in patients with RA.Citation79 The role of inflammatory factors between RA and AF are shown in .
RA and Other Potential Cardiovascular Risk Factors
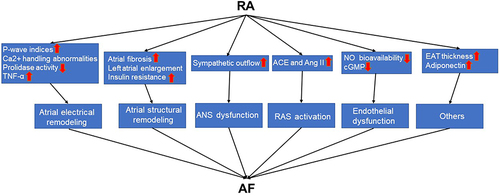
Epicardial adipose tissue (EAT) is located between visceral pericardium and epicardium surface.Citation80 Increased EAT thickness is an independent predictor of AF and associated with the severity and recurrence of AF.Citation81 In patients with RA, EAT thickness is higher than in healthy controls.Citation82 Furthermore, EAT thickness is positively correlated with the severity of RA.Citation83 Higher adiponectin concentrations are independently associated with increased risk of AF, specifically among older individuals.Citation84 Circulating adiponectin levels are significantly higher in patients with RA and they play an important pro-inflammatory role in the pathogenesis of RA.Citation85,Citation86 The pathophysiological pathways between RA and AF are shown in .
Figure 2 The pathophysiological pathways between RA and AF.
Effects of Drugs Used to Treat RA on AF
Biologicals on AF
TNF-α plays an important role in atrial structural, electrical, contractile, and autonomic remodeling.Citation87 Anti-TNF-α therapy, a cornerstone of RA treatment, has been demonstrated to improve cardiovascular outcomes.Citation88–90 Therefore, TNF-α antagonists may have a positive effect on the treatment of AF. IL-6, another inflammatory factor in RA, is elevated in serum and synovial fluid of affected joints.Citation91 On the other hand, IL-6 is a risk factor for AF and high levels of IL-6 predict poor prognosis in patients with AF.Citation23,Citation92 The underlying mechanisms include atrial fibrosis and atrial electrical remodeling.Citation93 Considering that the inhibition of IL-6 significantly reduces cardiovascular event rates,Citation94 patients with AF may benefit from anti-IL-6 therapeutics. In addition, endothelial function is improved by tocilizumab, an IL-6–blocking agent.Citation95 This is perhaps a mechanism by which anti-IL-6 therapeutics may improve AF. IL-1β is an independent risk factor of sustained AF.Citation96,Citation97 Besides, it has been found to mediate chronic inflammatory responses in RA.Citation98–100 In the treatment of RA, IL-1 blockers are safe and effective.Citation101 In consideration of the pathogenic role of IL-1β in AF, further studies are needed to determine the effect of IL-1 blockers on AF. Janus kinase (JAK) inhibitor has enabled the treatment of RA to enter a new stage by suppressing the action of JAK, an intracellular tyrosine kinase.Citation102 On the other hand, JAK signaling pathway plays an important role in cardiac pathophysiology and has been implicated in atrial myocytes hypertrophy, one of the structural remodeling features in AF.Citation103 The occurrence and development of AF in RA may be affected by JAK inhibitor.
Glucocorticoids and Hydroxychloroquine on AF
Glucocorticoids, as a short-term bridge, have been used for decades to manage symptoms of RA.Citation104 However, 30 to 50% of patients with RA still use glucocorticoids for long-term treatment.Citation105 In a large case-control study, current glucocorticoid use is associated with an almost 2-fold increased risk of AF.Citation106 Subsequent study has shown that glucocorticoid-induced biochemical modification of cardiac ion channels and effective refractory period shortening may be the underlying mechanisms of AF.Citation107 Hydroxychloroquine is especially used in RA due to its cost-effectiveness, safety and efficacy.Citation108 In patients with systemic lupus erythematosus, the use of hydroxychloroquine is associated with an 88% decrease in the risk of incident AF.Citation109 Further studies would be needed to confirm the anti-fibrillatory benefit of this medication in patients with RA.
Managements of AF in RA
Patients with AF have a four-fold to five-fold increase in the risk of stroke.Citation110 Meanwhile, the risk of stroke is increased in RA, especially in younger patients.Citation111 However, in an international clinical audit, a large proportion of RA patients with AF who have high risk of stroke do not receive anticoagulant therapy.Citation112 Anticoagulant treatment among RA patients with AF deserves more attention. Catheter ablation with pulmonary vein isolation (PVI) is an effective treatment for AF.Citation113 In patients with RA, catheter ablation is safe and effective when compared with non-RA patients.Citation114,Citation115 However, AF recurrence is more common in RA and is associated with preablation C-reactive protein (CRP) levels and erythrocyte sedimentation rates (ESRs).Citation115 Inflammation plays a negative role in AF recurrence and maintenance after ablation.Citation116 Study has shown that glucocorticoids reduce the rate of immediate AF recurrence after catheter ablation by suppressing inflammation.Citation117 Control of RA disease activity before ablation and use of antiarrhythmic drugs after ablation may help to reduce AF recurrence. Most worthy of mention is that catheter ablation might be considered as the first-line therapy in patients with RA, because some antiarrhythmic drugs are difficult to apply in RA. For instance, both amiodarone and RA cause the development of pulmonary fibrosis.Citation118,Citation119 In the prevention of AF, patients with hypertension and heart failure may benefit from angiotensin-converting enzyme inhibitor (ACEI) and angiotensin II receptor blocker (ARB).Citation120 Further research is needed to determine whether patients with RA benefit from ACEI and ARB.
Conclusion and Perspective
In conclusion, patients with RA may have higher incidence of AF than patients without RA. Inflammation is a common driver of both disease processes.Citation121,Citation122 Compared with the general population, the mortality in patients with RA is increased.Citation123 Elevated incidence of AF due to RA may be one of the reasons. However, two retrospective cohort studies have found no link between AF and RA.Citation26,Citation27 This may be due to the differences in patient characteristics, follow-up time and treatment.
In addition to synovitis, patients with RA are also at high risk of CVD, including AF.Citation28–30,Citation124 The major pathophysiological mechanisms of AF include atrial remodeling, autonomic nervous system dysfunction, activation of RAS, endothelial dysfunction, increased EAT thickness, higher adiponectin concentrations and IR.Citation31,Citation32,Citation53,Citation55,Citation62,Citation68,Citation69,Citation81,Citation84 RA is widely involved in these pathophysiological processes. Controversially, some studies have shown an absence of association between RA and the occurrence of new-onset AF.Citation26,Citation27 This may be due to the lack of close surveillance of heart rhythm.
Both RA and AF promote stroke,Citation110,Citation111 but in RA patients with AF, a small proportion of them receive anticoagulant therapy.Citation112 Given the high risk of stroke, anticoagulation or left atrial appendage closure procedure is important in the management of RA patients with AF when required. Although catheter ablation is safe in patients with RA, a higher rate of AF recurrence is a problem.Citation115 Systemic inflammation may be responsible, this would need further study in the future.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Houge IS, Hoff M, Halsan O, Videm VJCR. Exercise Self-Efficacy and patient global assessment were associated with 6-min walk test distance in persons with rheumatoid arthritis. Clin Rheumatol. 2022;41(12):3687–3696. doi:10.1007/s10067-022-06309-6
- Sharma A, Goel A. Pathogenesis of rheumatoid arthritis and its treatment with anti-inflammatory natural products. Mol Biol Rep. 2023;6:1–20.
- Neycheva S, Naseva E, Batalov Z, Karalilova R, Batalov A. Adherence to biological therapies in patients with rheumatoid arthritis: a retrospective cohort study. Rheumatology. 2023;19:1–10.
- Patanè M, Carmisciano L, Hysa E, et al. Engineered glove to evaluate hand disability in rheumatoid arthritis: a pilot-study. Joint Bone Spine. 2022;89(1):105272. doi:10.1016/j.jbspin.2021.105272
- Paolino S, Hysa E, Stoian SA, et al. Metabolic profile and bone status in post-menopausal women with rheumatoid arthritis: a monocentric retrospective survey. Nutrients. 2021;13(9):3168.
- Ln A, Ss A, Mi A, Pjc B. Rheumatoid arthritis and depression: an inflammatory perspective - ScienceDirect. Lancet Psychiatry. 2019;6:164–173.
- Jung J, Lim J, Bang C, Seok H, Song G. Prevalence of chronic obstructive pulmonary disease in patients with rheumatoid arthritis: a cross-sectional study. Int J Rheum Dis. 2021;24(6):774–780.
- Qiu S, Li M, Jin S, Lu H. Rheumatoid arthritis and cardio-cerebrovascular disease: a Mendelian randomization study. Front Genet. 2021;12:745224.
- Kerola AM, Kazemi A, Rollefstad S, et al. All-cause and cause-specific mortality in rheumatoid arthritis, psoriatic arthritis and axial spondyloarthritis: a nationwide registry study. Rheumatology. 2022;61(12):4656–4666. doi:10.1093/rheumatology/keac210
- Gawako M, Balsam P, Lodziński P, Grabowski M, Kosiuk J. Cardiac arrhythmias in autoimmune diseases. Circ J. 2020;84:5.
- Dijkshoorn B, Raadsen R. Nurmohamed, cardiovascular disease risk in rheumatoid arthritis anno 2022. J Clin Med. 2022;11(10):2704.
- Johnson TM, Sayles HR, Baker JF, George MD. England, Investigating changes in disease activity as a mediator of cardiovascular risk reduction with methotrexate use in rheumatoid arthritis. Ann Rheum Dis. 2021;80(11):1385–1392.
- Federico LE, Johnson TM, England BR, et al. Circulating adipokines and associations with incident cardiovascular disease in rheumatoid arthritis. Arthritis Care Res. 2022;75:768–777.
- Hegazy H, Folke F, Coronel R, Torp-Pedersen C, Gislason GH, Eroglu T. Risk of out-of-hospital cardiac arrest in patients with rheumatoid arthritis: a nationwide study. Open Heart. 2022;9(1):e001987. doi:10.1136/openhrt-2022-001987
- Lazzerini PE, Capecchi PL. Systemic inflammation and arrhythmic risk: lessons from rheumatoid arthritis. Eur Heart J. 2017;38(22):1717–1727.
- Patel KHK, Jones TN, Sattler S, Mason JC, Ng FS. Proarrhythmic electrophysiological and structural remodeling in rheumatoid arthritis. Am J Physiol Heart Circ Physiol. 2020;319(5):H1008–H1020. doi:10.1152/ajpheart.00401.2020
- Andersen JH, Andreasen L. Atrial fibrillation—a complex polygenetic disease. Eur J Hum Genet. 2021;29(7):1051–1060.
- Weng L-C, Choi SH, Klarin D, et al. Heritability of atrial fibrillation. Circ Cardiovasc Genet. 2017;10(6):e001838. doi:10.1161/CIRCGENETICS.117.001838
- Chung MK, Refaat M, Shen W-K, et al. Atrial fibrillation: JACC council perspectives. J Am Coll Cardiol. 2020;75(14):1689–1713.
- Kalstø SM, Siland JE, Rienstra M. Atrial fibrillation genetics update: toward clinical implementation. Front Cardiovasc Med. 2019;6:127.
- Harada M, Van Wagoner DR. Role of inflammation in atrial fibrillation pathophysiology and management. Circ J. 2015;79(3):495–502.
- Wu L, Emmens R, van Wezenbeek J, et al. Atrial inflammation in different atrial fibrillation subtypes and its relation with clinical risk factors. Clin Res Cardiol. 2020;109(10):1271–1281.
- Amdur RL, Mukherjee M, Go A, et al. Interleukin-6 is a risk factor for atrial fibrillation in chronic kidney disease: findings from the CRIC study. PLoS One. 2016;11(2):e0148189. doi:10.1371/journal.pone.0148189
- Lazzerini PE, Laghi‐Pasini F, Acampa M, et al. Natale, systemic inflammation rapidly induces reversible atrial electrical remodeling: the role of interleukin‐6–mediated changes in connexin expression. J Am Heart Assoc. 2019;8(16):e011006. doi:10.1161/JAHA.118.011006
- Ungprasert P, Srivali N. Risk of incident atrial fibrillation in patients with rheumatoid arthritis: a systematic review and meta‐analysis. Int J Rheum Dis. 2017;20(4):434–441.
- Kim SC, Liu J, Solomon DH. The risk of atrial fibrillation in patients with rheumatoid arthritis. Ann Rheum Dis. 2014;73(6):1091–1095. doi:10.1136/annrheumdis-2013-203343
- Jang S-Y, Kang K-W, Jo M, Park M. Risk of New-onset acute coronary syndrome and atrial fibrillation in patients with rheumatoid arthritis compared with a risk-set and propensity score-matched cohort―a Nationwide cohort study. Circ J. 2021;85(2):194–200. doi:10.1253/circj.CJ-20-0825
- Lindhardsen J, Ahlehoff O, Gislason GH, et al. Risk of atrial fibrillation and stroke in rheumatoid arthritis: Danish nationwide cohort study. BMJ. 2012;8:344.
- Bacani AK, Crowson CS, Roger VL, Gabriel SE, Matteson EL. Increased incidence of atrial fibrillation in patients with rheumatoid arthritis. Biomed Res Int. 2015;2015. doi:10.1155/2015/809514
- Argnani L, Zanetti A, Carrara G, Silvagni E, Guerrini G, Zambon A. Scirè, Rheumatoid arthritis and cardiovascular risk: retrospective matched-cohort analysis based on the RECORD study of the Italian society for rheumatology. Front Med. 2021;8:745601.
- Beyer C, Tokarska L, Stühlinger M, et al. Structural cardiac remodeling in atrial fibrillation. Cardiovasc Imaging. 2021;14(11):2199–2208.
- Grosu AI, Radulescu D, Grosu LC. Remodelling in atrial fibrillation: the impact of amiodarone. Cardiovasc J Afr. 2019;30(3):174–180.
- Li TY, Yeo LLL, Ho JSY, et al. Association of electrocardiographic P-wave markers and atrial fibrillation in embolic stroke of undetermined source. Cerebrovasc Dis. 2021;50(1):46–53.
- Maheshwari A, Norby FL, Soliman EZ, et al. Refining prediction of atrial fibrillation risk in the general population with analysis of P-wave axis (from the Atherosclerosis Risk in Communities Study). Am J Card. 2017;120(11):1980–1984. doi:10.1016/j.amjcard.2017.08.015
- Narazaki M, Tanaka T. The role and therapeutic targeting of IL-6 in rheumatoid arthritis. Expert Rev Clin Immunol. 2017;13(6):535–551.
- Liao J, Zhang S, Yang S, et al. Interleukin-6-mediated-Ca2+ handling abnormalities contributes to atrial fibrillation in sterile pericarditis rats. Front Immunol. 2021;12:5446.
- Guler H, Seyfeli E, Sahin G, Duru M, Akgul F, Saglam H. P wave dispersion in patients with rheumatoid arthritis: its relation with clinical and echocardiographic parameters. Rheumatol Int. 2007;27(9):813–818.
- Tascanov M, Screening HT. The relationship between prolidase activity and atrial electromechanical changes in patients with paroxysmal atrial fibrillation. Comb Chem High Throughput Screen. 2019;22(1):69–75.
- Uçar D, Em S, Bozkurt M, et al. M. Disorders, Serum prolidase activity in ankylosing spondylitis and rheumatoid arthritis. Clin Med Insights. 2013;6:S12602.
- Hori Y, Nakahara S, Fukuda R, et al. Atrial reverse remodeling represented by the atrial conduction time in persistent atrial fibrillation patients after catheter ablation: its impact on predicting late atrial fibrillation recurrence. J Cardiol. 2020;75(5):521–528.
- Dai H, Wang X, Yin S, et al. Atrial fibrillation promotion in a rat model of rheumatoid arthritis. J Am Heart Assoc. 2017;6(12):e007320. doi:10.1161/JAHA.117.007320
- Berthold E, Månsson B, Gullstrand B, Geborek P, Saxne T, Bengtsson A. Tumour necrosis factor-α/etanercept complexes in serum predict long-term efficacy of etanercept treatment in seronegative rheumatoid arthritis. J Rheumatol. 2018;47(1):22–26.
- Lee S-H, Chen Y-C, Chen Y-J, et al. Tumor necrosis factor-α alters calcium handling and increases arrhythmogenesis of pulmonary vein cardiomyocytes. Life Sci. 2007;80(19):1806–1815. doi:10.1016/j.lfs.2007.02.029
- Henry WL, Morganroth J, Pearlman AS, et al. Relation between echocardiographically determined left atrial size and atrial fibrillation. Circulation. 1976;53(2):273–279.
- Qiu D, Peng L, Ghista DN, Wong K. Technology, Left atrial remodeling mechanisms associated with atrial fibrillation. Cardiovasc Eng Technol. 2021;12(3):361–372.
- Zhang Y, Zhang S, Liu Z, Zhao X, Yuan Y, Sheng L. Resveratrol prevents atrial fibrillation by inhibiting atrial structural and metabolic remodeling in collagen-induced arthritis rats. Naunyn-Schmiedeb Arch Pharmacol. 2018;391(11):1179–1190. doi:10.1007/s00210-018-1554-9
- Meune C, Wahbi K, Assous N, Weber S, Kahan A. Myocardial dysfunction in rheumatoid arthritis: a controlled tissue-Doppler echocardiography study. J Rheumatol. 2007;34(10):2005–2009.
- Li CY, Zhang JR, Hu WN. Atrial fibrosis underlying atrial fibrillation. Int J Mol Med. 2021;47(3):1.
- Alcalde Ó, Cabrera GS, Vallès GE, Benito VB, Zuccarino F. Martí-almor, rheumatoid arthritis with severe atrial fibrosis and multiple atrial arrhythmias: chronic atrial myocarditis? Rev Esp Cardiol. 2017. doi:10.1016/j.rec.2017.04.003
- Bertelsen L, Diederichsen SZ, Haugan KJ, et al. Left atrial late gadolinium enhancement is associated with incident atrial fibrillation as detected by continuous monitoring with implantable loop recorders. Cardiovasc Imaging. 2020;13(8):1690–1700.
- Ma J, Chen Q, Ma S. Left atrial fibrosis in atrial fibrillation: mechanisms, clinical evaluation and management. J Cell Mol Med. 2021;25(6):2764–2775. doi:10.1111/jcmm.16350
- Bradham W, Ormseth MJ, Elumogo C, et al. Absence of fibrosis and inflammation by cardiac magnetic resonance imaging in rheumatoid arthritis patients with low to moderate disease activity. J Rheumatol. 2018;45(8):1078–1084.
- Chan Y-H, Chang G-J, Lai Y-J, et al. Atrial fibrillation and its arrhythmogenesis associated with insulin resistance. Cardiovasc Diabetol. 2019;18(1):1–14.
- Verma AK, Bhatt D, Goyal Y, Dev K, Beg MMA, Alsahli MA. Association of rheumatoid arthritis with diabetic comorbidity: correlating accelerated insulin resistance to inflammatory responses in patients. J Multidiscip Healthc. 2021;12:809–820.
- Chen P-S, Chen LS, Fishbein MC, Lin S-F, Nattel S. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res. 2014;114(9):1500–1515. doi:10.1161/CIRCRESAHA.114.303772
- Koopman F, Van Maanen M, Vervoordeldonk MJ. Balancing the autonomic nervous system to reduce inflammation in rheumatoid arthritis. J Intern Med. 2017;282(1):64–75.
- Kawasaki M, Yamada T, Furukawa Y, et al. Are cardiac sympathetic nerve activity and epicardial adipose tissue associated with atrial fibrillation recurrence after catheter ablation in patients without heart failure? Int J Cardiol. 2020;303:41–48. doi:10.1016/j.ijcard.2019.11.092
- Adlan AM, Paton JF, Lip GY, Kitas GD, Fisher JP. Increased sympathetic nerve activity and reduced cardiac baroreflex sensitivity in rheumatoid arthritis. J Physiol. 2017;595(3):967–981. doi:10.1113/JP272944
- Peçanha T, Meireles K, Pinto AJ, et al. Increased sympathetic and haemodynamic responses to exercise and muscle metaboreflex activation in post‐menopausal women with rheumatoid arthritis. J Physiol. 2021;599(3):927–941. doi:10.1113/JP280892
- Syngle A, Verma I, Krishan P. Interleukin-6 blockade improves autonomic dysfunction in rheumatoid arthritis. Acta Reumatol. 2015;40(1):85–88.
- Zhao J, Chen M, Zhuo C, Huang Y, Zheng L, Wang Q. The effect of renin-angiotensin system inhibitors on the recurrence of atrial fibrillation after catheter ablation a systematic review and meta-analysis. Int Heart J. 2020;61(6):1174–1182.
- Yu Z, Zhang D, Ji Q, Yi F. Inhibition of the renin-angiotensin-aldosterone system prevents and cures atrial fibrillation: an overview of systematic reviews. Medicine. 2021;100:18.
- Han M, Zhang Y, Sun S, et al. Renin–angiotensin system inhibitors prevent the recurrence of atrial fibrillation: a meta-analysis of randomized controlled trials. J Cardiovasc Pharmacol. 2013;62(4):405–415.
- Miller AJ. The renin–angiotensin system in cardiovascular autonomic control: recent developments and clinical implications. Clin Auton Res. 2019;29(2):231–243.
- Mascolo A, Urbanek K, De Angelis A, et al. Angiotensin II and angiotensin 1–7: which is their role in atrial fibrillation? Heart Fail Rev. 2020;25:367–380.
- Braz NFT, Pinto MRC, Vieira ÉLM, et al. Renin–angiotensin system molecules are associated with subclinical atherosclerosis and disease activity in rheumatoid arthritis. Mod Rheumatol. 2021;31(1):119–126.
- Pour SK, Scoville C, Tavernier SS, Aghazadeh-Habashi A. Plasma angiotensin peptides as biomarkers of rheumatoid arthritis are correlated with anti-ACE2 auto-antibodies level and disease intensity. Inflammopharmacology. 2022;30(4):1295.
- Shaikh AY, Wang N, Yin X, et al. Relations of arterial stiffness and brachial flow–mediated dilation with new-onset atrial fibrillation: the Framingham heart study. Hypertension. 2016;68(3):590–596.
- O’Neal WT, Efird JT, Yeboah J, Nazarian S, Alonso A, Heckbert SR. Brachial flow-mediated dilation and incident atrial fibrillation: the multi-ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34(12):2717–2720. doi:10.1161/ATVBAHA.114.304560
- Corban MT, Godo S, Burczak DR, et al. Coronary endothelial dysfunction is associated with increased risk of incident atrial fibrillation. J Am Heart Assoc. 2020;9(8):e014850. doi:10.1161/JAHA.119.014850
- Guazzi M, Arena R. Endothelial dysfunction and pathophysiological correlates in atrial fibrillation. Heart. 2009;95(2):102–106.
- Münzel T, Sinning C, Post F, Warnholtz A. Pathophysiology, diagnosis and prognostic implications of endothelial dysfunction. Ann Med. 2008;40(3):180–196.
- Bordy R, Totoson P, Prati C, Marie C, Wendling D, Demougeot C. Microvascular endothelial dysfunction in rheumatoid arthritis. Nat Rev Rheumatol. 2018;14(7):404–420.
- Bassu S, Zinellu A, Sotgia S, et al. Oxidative stress biomarkers and peripheral endothelial dysfunction in rheumatoid arthritis: a monocentric cross-sectional case-control study. Molecules. 2020;25(17):3855. doi:10.3390/molecules25173855
- Sarli B, Baktir AO, Cebicci M, et al. Predictors of endothelial dysfunction in patients with rheumatoid arthritis. Angiology. 2014;65(9):778–782.
- Akhmedov A, Crucet M, Simic B, et al. TNFα induces endothelial dysfunction in rheumatoid arthritis via LOX-1 and arginase 2: reversal by monoclonal TNFα antibodies. Cardiovasc Res. 2022;118(1):254–266.
- Bosello S, Santoliquido A, Zoli A, Di Campli C, Flore R, Tondi P. TNF-alpha blockade induces a reversible but transient effect on endothelial dysfunction in patients with long-standing severe rheumatoid arthritis. Clin Rheumatol. 2008;27(7):833–839.
- Capria A, De Nardo D, Baffetti F, Barbini U, Violo A, Tondo T. pharmacology, Long-term anti-TNF-α treatments reverse the endothelial dysfunction in rheumatoid arthritis: the biological coherence between synovial and endothelial inflammation. Int J Immunopathol Pharmacol. 2010;23(1):255–262.
- Ciftci O, Yilmaz S, Topcu S, et al. Impaired coronary microvascular function and increased intima-media thickness in rheumatoid arthritis. Atherosclerosis. 2008;198(2):332–337.
- Konwerski M, Gąsecka A, Opolski G, Grabowski M, Mazurek T. Role of epicardial adipose tissue in cardiovascular diseases: a review. Biology. 2022;11(3):355.
- Iten L, Carroz P, Domenichini G, et al. Epicardial adipose tissue and atrial fibrillation. Rev Med Suisse. 2022;18(783):1048–1051. doi:10.53738/REVMED.2022.18.783.1048
- Fatma E, Bunyamin K, Savas S, et al. Epicardial fat thickness in patients with rheumatoid arthritis. Afr Health Sci. 2015;15(2):489–495.
- Alpaydın S, Buyukterzi Z, Akkurt HE, Yılmaz H. Impaired left ventricular diastolic functions and thickened epicardial adipose tissue in rheumatoid arthritis patients is correlated with DAS-28 score. Acta Cardiol Sin. 2017;33(2):182.
- Macheret F, Bartz TM, Djousse L, et al. Higher circulating adiponectin levels are associated with increased risk of atrial fibrillation in older adults. Heart. 2015;101(17):1368–1374. doi:10.1136/heartjnl-2014-307015
- Lee YH, Bae S-C. Circulating adiponectin and visfatin levels in rheumatoid arthritis and their correlation with disease activity: a meta‐analysis. Int J Rheum Dis. 2018;21(3):664–672. doi:10.1111/1756-185X.13038
- Szumilas K, Szumilas P, Słuczanowska-Głąbowska S, Zgutka K, Pawlik A. Role of adiponectin in the pathogenesis of rheumatoid arthritis. Int J Mol Sci. 2020;21(21):8265. doi:10.3390/ijms21218265
- Ren M, Li X, Hao L. Role of tumor necrosis factor alpha in the pathogenesis of atrial fibrillation: a novel potential therapeutic target? Ann Med. 2015;47(4):316–324.
- Bili A, Tang X, Pranesh S, et al. Research, Tumor necrosis factor α inhibitor use and decreased risk for incident coronary events in rheumatoid arthritis. Arthritis Care Res. 2014;66(3):355–363.
- Ljung L, Rantapää-Dahlqvist S, Jacobsson LT, Askling J. Response to biological treatment and subsequent risk of coronary events in rheumatoid arthritis. Ann Rheum Dis. 2016;75(12):2087–2094. doi:10.1136/annrheumdis-2015-208995
- Ozen G, Pedro S. The risk of cardiovascular events associated with disease-modifying antirheumatic drugs in rheumatoid arthritis. J Rheumatol. 2021;48(5):648–655.
- Pandolfi F, Franza L, Carusi V, Altamura S, Andriollo G, Nucera E. Nucera, Interleukin-6 in rheumatoid arthritis. Int J Mol Sci. 2020;21(15):5238. doi:10.3390/ijms21155238
- Jia X, Cheng X, Wu N, et al. Prognostic value of interleukin-6 in atrial fibrillation: a cohort study and meta-analysis. Anatol J Cardiol. 2021;25(12):872.
- Jalloul Y, Refaat MM. Refaat, Il‐6 rapidly induces reversible atrial electrical remodeling by downregulation of cardiac connexins. Am Heart Assoc. 2019;8(16):e013638. doi:10.1161/JAHA.119.013638
- Ridker PM, Rane M. Interleukin-6 signaling and anti-interleukin-6 therapeutics in cardiovascular disease. Circ Res. 2021;128(11):1728–1746. doi:10.1161/CIRCRESAHA.121.319077
- Bacchiega BC, Bacchiega AB, Usnayo MJG, Bedirian R, Singh G, Pinheiro GDRC. Interleukin 6 inhibition and coronary artery disease in a High‐Risk population: a prospective Community‐Based clinical study. J Am Heart Assoc. 2017;6(3):e005038. doi:10.1161/JAHA.116.005038
- Liu Q, Zhang F, Yang M. Increasing level of interleukin-1β in epicardial adipose tissue is associated with persistent atrial fibrillation. J Interferon Cytokine Res. 2020;40(1):64–69.
- Matsushita N, Ishida N, Ibi M, et al. IL-1β plays an important role in pressure overload-induced atrial fibrillation in mice. Biol Pharm Bull. 2019;42(4):543–546.
- Levescot A, Chang MH, Schnell J, et al. Blaustein, IL-1β–driven osteoclastogenic Tregs accelerate bone erosion in arthritis. J Clin Invest. 2021;131:18.
- Chen J, Wu W, Zhang M, Chen C. Taraxasterol suppresses inflammation in IL-1β-induced rheumatoid arthritis fibroblast-like synoviocytes and rheumatoid arthritis progression in mice. Int Immunopharmacol. 2019;70:274–283.
- Rong H, He X, Wang L, et al. Association between IL1B polymorphisms and the risk of rheumatoid arthritis. Int Immunopharmacol. 2020;83:106401. doi:10.1016/j.intimp.2020.106401
- Arnold DD, Yalamanoglu A, Boyman O. Systematic review of safety and efficacy of IL-1-targeted biologics in treating immune-mediated disorders. Front Immunol. 2022;13:888392. doi:10.3389/fimmu.2022.888392
- Morinobu A. JAK inhibitors for the treatment of rheumatoid arthritis. Immunol Med. 2020;43(4):148–155. doi:10.1080/25785826.2020.1770948
- Hao L, Ren M, Rong B, Xie F. m. medicine, TWEAK/Fn14 mediates atrial‐derived HL‐1 myocytes hypertrophy via JAK 2/STAT 3 signalling pathway. J Cell Mol Med. 2018;22(9):4344–4353. doi:10.1111/jcmm.13724
- Moore MN. Glucocorticoid and opioid use in rheumatoid arthritis management. Curr Opin Rheumatol. 2021;33(3):277–283.
- Ruyssen-Witrand A, Constantin A. Controversies in rheumatoid arthritis glucocorticoid therapy. Jt Bone Spine. 2018;85(4):417–422.
- Christiansen CF, Christensen S, Mehnert F, Cummings SR, Chapurlat RD, Sørensen HT. Glucocorticoid use and risk of atrial fibrillation or flutter: a population-based, case-control study. Arch Intern Med. 2009;169(18):1677–1683. doi:10.1001/archinternmed.2009.297
- Iwasaki YK, Sekiguchi A, Kato T, Yamashita T. Glucocorticoid induces atrial arrhythmogenesis via modification of ion channel gene expression in rats molecular evidence for stress-induced atrial fibrillation. Int Heart J. 2022;63(2):375–383. doi:10.1536/ihj.21-677
- Nirk EL, Reggiori F. Hydroxychloroquine in rheumatic autoimmune disorders and beyond. EMBO Mol Med. 2020;12(8):e12476.
- Gupta A, Shields KJ, Manzi S, Wasko MC, Sharma T. Association of Hydroxychloroquine use with decreased incident atrial fibrillation in systemic lupus erythematosus. Arthritis Care Res. 2021;73(6):828–832.
- Healey JS, Amit G. Atrial fibrillation and stroke: how much atrial fibrillation is enough to cause a stroke? Curr Opin Neurol. 2020;33(1):17–23.
- Liu W, Ma W, Liu H, et al. Stroke risk in arthritis: a systematic review and meta-analysis of cohort studies. PLoS One. 2021;16(3):e0248564. doi:10.1371/journal.pone.0248564
- Semb AG, Rollefstad S, Sexton J, et al. Oral anticoagulant treatment in rheumatoid arthritis patients with atrial fibrillation results of an international audit. Int J Cardiol. 2022;42:101117. doi:10.1016/j.ijcha.2022.101117
- Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. EP Eur. 2018;20(1):e1–e160.
- Wen S-N, Liu N, Li S-N, et al. Catheter ablation of atrial fibrillation in patients with rheumatoid arthritis. J Cardiol. 2015;66(4):320–325.
- Haq IU, Lodhi FK, Anan AR, et al. Safety and efficacy outcomes of atrial fibrillation ablation in patients with rheumatoid arthritis. Heart Rhythm O2. 2022;3(3):261–268. doi:10.1016/j.hroo.2022.03.001
- Korodi S, Toganel R, Benedek T, et al. Impact of inflammation-mediated myocardial fibrosis on the risk of recurrence after successful ablation of atrial fibrillation–the FIBRO-RISK study: protocol for a non-randomized clinical trial. Medicine. 2019;98:9.
- Koyama T, Sekiguchi Y, Tada H, et al. Comparison of characteristics and significance of immediate versus early versus no recurrence of atrial fibrillation after catheter ablation. J Am Coll Cardiol. 2009;103(9):1249–1254.
- Budin CE, Cocuz IG, Sabău AH, et al. Pulmonary fibrosis related to amiodarone—is it a standard pathophysiological pattern? A case-based literature review. Diagnostics. 2022;12(12):3217.
- Diesler R, Cottin V. Pulmonary fibrosis associated with rheumatoid arthritis: from pathophysiology to treatment strategies. Expert Rev Respir Med. 2022;16(5):541–553.
- Si-Qi L, Yan-Min Y, Jun Z, et al. Effects of angiotensin-converting enzyme inhibitor and angiotensin II receptor blocker on one-year outcomes of patients with atrial fibrillation: insights from a multicenter registry study in China. JGC. 2020;17(12):750.
- Testa D, Calvacchi S, Petrelli F, et al. One year in review 2021: pathogenesis of rheumatoid arthritis. Int J Mol Sci. 2021;39(3):445–452.
- Harada M. Implications of inflammation and fibrosis in atrial fibrillation pathophysiology. Risk Factor Atrial Fibrillation. 2021;13(1):25–35.
- Meyer PW, Anderson R, Ker JA. Rheumatoid arthritis and risk of cardiovascular disease. Cardiovasc J Afr. 2018;29(5):317–321.
- Semb AG, Ikdahl E, Wibetoe G, Crowson C, Rollefstad S. Atherosclerotic cardiovascular disease prevention in rheumatoid arthritis. Nat Rev Rheumatol. 2020;16(7):361–379.