Abstract
Background
The Great Chinese Famine, as the famine of 1959–1961 was often known. Famine exposure during early life was proven to be associated with some kidney diseases but has not been studied with kidney stone. We aimed to investigate the relationship between exposure to the Great Chinese Famine in early life and the incidence of kidney stone in adulthood.
Methods
From 1 January 2017 to 31 December 2018, a total of 19,658 eligible adults were recruited in a cross-sectional survey who were born between 1 October 1952 and 30 September 1964 in Guangdong, China. Participants were separated into kidney stone and none-kidney stone groups based on kidney stone status. According to birth data, participants were divided into non-exposed, fetal-exposed, early-, mid-, and late-childhood-exposed groups. Multivariate logistic regression, subgroup analysis and interaction test were used to estimate the odds ratios (ORs) and confidence intervals (CIs) between famine exposure and kidney stone.
Results
In total, 19,658 (12,246 female, mean age 59.31 ± 3.68 years) subjects were enrolled, and 3219 (16.38%) participants with kidney stone. The prevalence of kidney in none-, fetal-, early-, mid-, and late-childhood-exposed groups were 645 (14.9%), 437 (15.9%), 676 (16.3%), 743 (17.0%), and 718 (17.6%), respectively (P<0.001). When compared with the unexposed group, the fully adjusted ORs for kidney stone from fetal-exposed, early-, mid- to late-childhood-exposed groups were 1.37 (95% CI: 1.13, 1.68, P=0.002), 1.98 (95% CI: 1.45, 2.72, P<0.001), 2.94 (95% CI: 1.96, 4.42, P<0.001), and 3.48 (95% CI: 2.11, 5.72, P<0.001), respectively (P for trend<0.001). Subgroup analyses revealed no interactions between the famine effect on kidney stones and body mass index, gender, smoking status, history of diabetes or hypertension (all P for interaction >0.05).
Conclusion
This study found that exposure to the Great Chinese Famine during early life was independently associated with the increased incidence of kidney stone in adulthood.
Introduction
Kidney stone disease is one of the oldest diseases known to medicine and a global health care problem, with a high recurrence rate after stone removal.Citation1 Despite numerous preventive measures were taken, the prevalence of kidney stone disease was increasing worldwide.Citation2 Importantly, severe kidney stone could lead to serious complications such as hydronephrosis and kidney failure,Citation3 and it was also one of the important risk factors for chronic kidney disease.Citation3 Therefore, it was thus crucial to develop effective strategies to prevent the formation of new or recurrent stone. It was now generally accepted that urinary supersaturation and crystallization, Randall’s plaques formation, sex hormones dysfunction, the microbiome and the immune response were five entirely different main mechanisms for kidney stone formation.Citation4 However, the mechanisms of stone formation and development remain largely unclear.
As a common chronic kidney disease, kidney stone is also a disease closely related to the environment and nutrition.Citation5,Citation6 In recent years, exposure famine in early life was significantly related to the increased risk for chronic kidney diseases that have been confirmed in the Great Chinese Famine,Citation7,Citation8 the Dutch famineCitation9 and the Great Ethiopian Famine.Citation10 The Great Ethiopian Famine demonstrated that exposure to famine during prenatal stage was significantly related to decreased estimated glomerular filtration rate and higher risk of developing chronic kidney disease in adulthood after adjusting for systolic blood pressure, fasting blood glucose and body mass index (BMI).Citation10 The Dutch famine found that people exposed to famine in mid gestation had more microalbuminuria in later life.Citation9 Studies from Chinese population also suggested that prenatal and fetus-stage exposure to famine may have long-term effects on estimated glomerular filtration rate decline after adjusting for numerous potential confounding factors in humans during in later life.Citation7,Citation8,Citation11 In addition, low birth weight due to poor maternal nutrition was related to low podocyte endowment and glomerulosclerosis, and also was an important risk factor for chronic kidney disease.Citation12 Taken together, although malnutrition or famine exposure was closely linked with some chronic kidney diseases, the relationship between famine exposure during early life and kidney stone in adults remains unclear until now. Therefore, in the present study, the aim was to explore the relationship between famine exposure to the Great Chinese Famine during early life and kidney stone in adulthood, and to further explore whether this relationship was modified by gender, smoking, hypertension, diabetes and overweight or obesity status.
Methods
Study Population
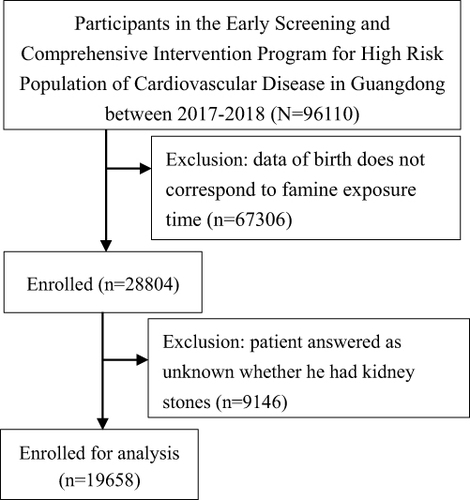
Data were from the Early Screening and Comprehensive Intervention Program for High-Risk Population of Cardiovascular Disease (CVD) between 1 January 2017 and 31 December 2018 in Guangdong province, China. The Early Screening and Comprehensive Intervention Program for High-Risk Population of CVD was an essential part of China-PEACE (Patient-Centered Evaluative Assessment of Cardiac Events). Million Persons Project was a government-funded public health program and an ongoing prospective population-centered national screening initiative to detect population at high CVD risk in China.Citation13 Detailed descriptions of the study design and methods were previously reported.Citation13,Citation14 At baseline, there were total of 96,110 individuals were recruited from the Early Screening and Comprehensive Intervention Program for High-Risk Population of CVD in Guangdong province between 1 January 2017 and 31 December 2018 in Guangdong province. Individuals who wereborn between 1 October 1952 and 30 September 1964 and have received a kidney stone response questionnaire were enrolled. But the start and end of the Great Chinese Famine was not exact, participants who were born from 1 October 1958 to 30 September 1959 and from 1 October 1961 to 30 September 1962 were excluded to minimize misclassification. In addition, when participants answered that it was unknown whether they had kidney stone were also excluded. Finally, a total of 19,658 subjects were enrolled for analysis ().
Definition of Famine Exposure and Grouping
It was now generally believed that the great famine in China occurred from 1959 to 1961, namely, 3 years of severe food shortages occurred in China between the spring of 1959 and the fall of 1961, and the “Three-year Natural Disaster” or “Great Chinese Famine” affected 600 million Chinese people, caused 30 million premature deaths, as well as the loss or delay of the birth of 30 million children, the majority of whom died from hunger-related causes.Citation15 Participants were divided into five groups based on date of birth according to previous studies.Citation14,Citation16 (1) no exposure group: born from 1 October 1962 to 30 September 1964, n=4319; (2) fetal exposure group: born from 1 October 1959 to 1961/9/30, n=2743; (3) early-childhood exposure group: born from 1 October 1956 to 30 September 1958, n=4149; (4) mid-childhood exposure group: born from 1 October 1954 to 30 September 1956, n=4368; (5) late-childhood exposure group: born from 1 October 1952 to 30 September 1954, n=4079.
Assessment of Kidney Stone
A highly skilled and qualified doctor and nurse used a questionnaire to gather basic data from the participants. The survey’s major components were general information, sociodemographic data, a combination of chronic conditions, dietary patterns, and medication use. Information on kidney stone was collected from face-to-face interviews by asking whether you have been diagnosed with kidney stone by a professional physician. The answer results were mainly as follows: “1. Yes; 2. No; 3. Unknown”. Furthermore, kidney stone acquisition could be detected from previously self-reported data by imaging examinations such as urine ultrasonography, abdominal X-ray, computed tomography, or magnetic resonance imaging.
Assessment of Covariates
The socio-demographic information, personal diseases history, lifestyle behaviors were collected during face-to-face interviews by trained interviewers according to a standardized structured questionnaire. The socio-demographic information mainly including birth data, gender, education level, income level, marriage status, and residential area. Lifestyle behaviors mainly including smoking and drinking. Personal chronic disease history, primarily hypertension, stroke, coronary heart disease, and diabetes. Current medications include hypoglycemic, antihypertensive, and lipid-lowering agents. The weight and height of participants without shoes and wearing light clothing were then measured by trained technicians. Blood pressure was measured by using an electronic blood pressure monitor (Omron HEM-7430; Omron Corp) on the right upper arm. Fasting blood glucose, total cholesterol, triglyceride, low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) were measured by fasting blood. BMI was calculated based on weight (kilograms) divided by height (meters squared). BMI≥25kg/m2 was defined as overweight.Citation17 Diabetes was defined as fasting blood glucose ≥126 mg/dl, self-reported status or taking glucose-lowering drugs.Citation18 Hypertension was defined as systolic/diastolic blood pressure ≥140/90 mmHg, self-reported status or taking antihypertensive drugs.Citation19
Statistical Analysis
The continuous and categorial variables were reported as mean ± standard deviation, and a frequency or percentage, respectively. A normality test was first performed on all continuous variable data; comparisons that passed the normality test were analyzed by a Students t-test, and those that did not were analyzed with Mann–Whitney U-test. For comparing the baseline categorical characteristics, the Chi-square test was used for categorical variables. Multiple group comparisons passing normality test were analyzed using analysis of variance (ANOVA) with post hoc tests, whereas nonparametric multiple group comparisons were analyzed using the Kruskal–Wallis test with Dunnett’s post hoc testing, when ANOVA assumptions were not met. Crude and adjusted odds ratio (OR) and 95% confidence interval (CI) values were estimated to assess the relationships between famine exposure and kidney stone. Multivariate logistic regression and interaction test was used to estimate the ORs and 95% CI between famine exposure and kidney stone. In model I, none of the covariates were adjusted. In model II, age and gender were adjusted. In model III, BMI, region, education, income, smoking, drinking, BMI, LDL-C, hypertension, diabetes, stroke, coronary heart disease, and lipid-lowering drugs were further adjusted. Subgroups and interaction analysis were performed based on gender (male or female), current smoking (yes or no), BMI (≥25.0 kg/m2 or <25 kg/m2), combined with diabetes (yes or no) and hypertension (yes or no). A two-sided P < 0.05 was considered statistically significant. All statistical analyses were performed using R version 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria) and SPSS version 19.0 (SPSS, Chicago, Illinois, USA).
Results
Characteristics of Participants by Early Life Stage of Exposure to the Great Chinese Famine
As demonstrated in , there were total of 19,658 individuals (7412 men and 12,246 women) with the average age was 59.31 ± 3.68 years participated in the present study. Among them, 3219 participants were with kidney stone and 16,439 participants were without kidney stone. The baseline parameters of age, gender, BMI, HDL-C, along with hypertension, coronary heart disease, and diabetes, as well as current smoking, drinking, and drug use, were shown to be significantly different between the kidney stone and non-kidney stone groups (all P<0.05). However, no significant changes in blood pressure, total cholesterol, triglyceride, LDL-C, fasting blood glucose, stroke, education, or income levels were found between the two groups (all P>0.05).
Table 1 Baseline Characteristics Between Subjects with and without Kidney Stone Group
In addition, as shown in , the prevalence of kidney stone in no-exposed, fetal-exposed, early-childhood-exposed, mid-childhood-exposed, and late-childhood-exposed groups were 645 (14.9%), 437 (15.9%), 676 (16.3%), 743 (17.0%), and 718 (17.6%), respectively (P<0.001). Age, gender distribution, systolic blood pressure, BMI, total cholesterol, HDL-C, LDL-C, combined with hypertension, coronary heart disease and stroke, education level, current smoking and drinking, taking lipid-lowering medication, antihypertensive medication, and hypoglycemic medication were all significant subgroup differences (all P<0.05).
Table 2 Baseline Characteristics Among Different Famine Exposure Groups
Association of Famine Exposure in Early Life Stage with Kidney Stone in Adulthood
As demonstrated in , the relationship between famine exposure and kidney stone was explored by multivariate logistic regression analysis. In model I, with none of the variables adjusted, the ORs for kidney stone from fetal-exposed, early-childhood-exposed, mid-childhood-exposed to late-childhood-exposed group were 0.92 (95% CI: 0.80, 1.04, P=0.178), 0.89 (95% CI: 0.79, 0.99, P=0.039), 0.97 (95% CI: 0.87, 1.09, P=0.641), and 0.94 (95% CI: 0.84, 1.05, P=0.295) (P for trend was 0.560), respectively, compared to none-exposed group. In model II, age and gender were adjusted, and the ORs for kidney stone from fetal-exposed, early-childhood-exposed, mid-childhood-exposed to late-childhood-exposed group were 1.39 (95% CI: 1.15, 1.68, P=0.001), 1.97 (95% CI: 1.46, 2.65, P<0.001), 2.83 (95% CI: 1.93, 4.15, P<0.001), and 3.50 (95% CI: 2.19, 5.58, P<0.001) (P for trend was <0.001), respectively, compared to none-exposed group. In model III when compared with the unexposed group, the fully adjusted ORs for kidney stone from fetal-exposed, early-childhood exposed, and mid-childhood-exposed groups were 1.37 (95% CI: 1.13, 1.68, P=0.002), 1.98 (95% CI: 1.45, 2.72, P<0.001), 2.94 (95% CI: 1.96, 4.42, P<0.001), and 3.48 (95% CI: 2.11, 5.72, P<0.001), respectively (P for trend <0.001).
Table 3 Multivariate Logistic Regression Analysis the Relationship Between Famine Exposure and Kidney Stone Among Different Groups
Association of Famine Exposure with Kidney Stone by Subgroup
As shown in , we observed that childhood exposure to famine at any stage was associated with kidney stone in females, hypertensive patients, subjects without diabetes, none current smoking individuals and people with BMI <25 kg/m2. In addition, we also found that famine exposure in fetal period was significant relationship with kidney stone in adulthood in females, none current smoking individuals, people with BMI <25 kg/m2. Similar results were found when exposed to the fetal period in hypertension and diabetes subgroup, regardless of hypertension and diabetes status. However, no significant interactions were observed from all the subgroup analyses (all P-interaction >0.05).
Table 4 Subgroup Analysis on the Association of Famine Exposure with Kidney Stone
Discussion
In the present population-based study, we found that individuals who were exposed to the Great Chinese Famine in early life stage had higher kidney stone prevalence in adults. Gender differences existed in the effects of early exposure to famine on the development of kidney stone in adulthood. The independent effects of early exposure to famine on kidney stone in adulthood were not influenced by some traditional chronic disease risk factors including smoking, overweight or obesity, hypertension and diabetes.
Famine exposure was linked to kidney diseases. We discovered a higher likelihood of self-reported kidney stones in adults who had been exposed to the Great Chinese Famine during fetus and infancy. WangCitation7 found prenatal exposure to Great Chinese Famine may have long-term effects on declined glomerular filtration rate and the development of chronic kidney diseases in adult humans. The China Health and Retirement Longitudinal Study also indicated famine exposure in fetus might increase the risk of chronic kidney disease in adults.Citation8 AbateCitation10 discovered that prenatal exposure to the Great Ethiopian Famine was linked with decreased estimated glomerular filtration rate and higher risk of developing chronic kidney disease among survivors. These findings suggested that early exposure to famine may be crucial for the development of metabolic abnormalities and dysfunction in the kidneys in adults. Reduced glomerular filtration rate and chronic renal disease were risk factors for the development of chronic metabolic abnormalities, and chronic metabolic abnormalities were observed to accelerate the course of frequent stone production and recurrence.Citation20 They may also suggest that the fetus, infant, and childhood years may be a crucial window of opportunity for the prevention of chronic kidney disease, including kidney stones, in later life.
Subgroup analysis indicated that the relationship between famine exposure during early life and kidney stone in adulthood differed in gender, more pronounced in women. A previous study also found that early life malnutrition had a more significant effect on kidney dysfunction in adult women.Citation21 The gender difference of early life famine exposure and kidney stone remains unknown. There may be some reasons to explain this phenomenon. On the one hand, there was a phenomenon of “preference of sons to daughters” in some parts of China, which may cause more females to experience more nutritional deficiencies in infancy and early childhood stage. On the other hand, due to the relatively early development of female,Citation22 lack of nutrition in the early life has a great impact on the development of the neuroendocrine system of female, and the neuroendocrine system also played an important role in the development of kidney structure and function.Citation23 In a stratified analysis, we also showed that the effect of famine exposure on kidney stone was greater in the non-diabetic population. However, a study from China found that malnutrition during young adulthood significantly increased the risk of renal dysfunction in adult subjects with diabetes.Citation21 The main reasons for the different results may include differences in study endpoints, study populations and adjustment for covariates. In addition, we also found that the independent relationship between famine exposure and kidney stone did not interact with obesity, smoking status, diabetes and hypertension. These findings suggest that malnutrition in early life may have an important effect on the onset of kidney disease in adults, and prevention of kidney stone should begin in the fetus, infancy and early childhood.
Although the present study demonstrated that famine exposure in fetuses and childhood may significantly increase the risk of kidney stone in adulthood, the mechanisms between them remain unclear. We hypothesized the possible mechanisms or roles as follows: first, the retention of related metabolites in the kidneys caused by renal insufficiency and abnormal metabolism was an important reason for the formation of kidney stone.Citation4,Citation24,Citation25 Malnutrition may result in incomplete development of renal structure and function, and ultimately affect renal function and renal metabolism.Citation25,Citation26 Previous researches have shown that body weight was closely related to the total number of glomeruli,Citation27–29 and nutrition restriction in uterus may lead to low birth weight, resulting in less glomeruli and increased risk of chronic kidney disease in later life.Citation8,Citation30 Second, the lack of trace elements caused by malnutrition was also an important cause of abnormal kidney development and metabolism.Citation26 It has been reported that trace elements were closely related to the occurrence of kidney diseases.Citation31,Citation32 Third, uric acid metabolism was associated with kidney stone,Citation33 and it has been proven that famine exposure in early life was an independent risk factor for hyperuricemia in adulthood.Citation34 Four, food shortages were inevitable during famine, so there may be a lot of food insecurity. A previous study showed that there existed a significant association between food insecurity and kidney stones.Citation35 In addition, it was now generally accepted that kidney stone was also associated with metabolic abnormalities in the body, such as hyperparathyroidism and hypercortisolism.Citation36 Both thyroid function and cortisol function were regulated by the brain and nerves, and studies have shown that nutritional deficiencies have a direct and severe impact on brain and nerve development.Citation37,Citation38 Finally, hypertension, hyperglycemia, insulin resistance and diabetes were all associated with kidney stone formation,Citation39,Citation40 and studies have found that famine exposure in early life was closely related to the development of diabetes and hypertension in adults.Citation41,Citation42
Although we found for the first time in a large population that exposure to the Great Chinese Famine in early life significantly increased the incidence of kidney stone in adulthood, some identified limitations should also be mentioned. First, the causal relationship between famine exposure and kidney stone could not be drawn due to the present study was a cross-sectional survey. Second, kidney stone was mostly self-reported from participants, and recall bias was unavoidable. Third, there were various types of kidney stone, and lacked of information on kidney stone severity, which were not classified in this study. Fourth, incomplete information on famine exposure, the exposure time to famine could not be accurately defined and some population may die due to famine. The current study only analyzed surviving subjects, so it may overestimate or underestimate the effects of famine. Fifth, this study could not distinguish the severity of famine, nor did it measurement for some nutrient elements or dietary habits, and only grouped famine exposure based on the date of birth. In addition, the results of this study may not be extrapolated due to the lack of assessments of renal function and uric acid, although we adjusted for numerous covariates. Finally, this study did not explore the specific mechanism of famine exposure and the occurrence of kidney stone, and more researches were still needed in the future.
Conclusions
In conclusion, early childhood exposure to the Great Chinese Famine was independently related with an increased incidence of kidney stone in adults, indicating that early childhood starvation may play a role in adult renal metabolism and malfunction. This research suggested that the nutritional health of the fetal, infancy, and childhood stages should be prioritized in the prevention of kidney stones. The fundamental processes behind the associations between famine exposure during childhood and an increased risk of kidney stone needed additional investigation.
Ethics Approval and Consent to Participate
The study protocol was approved by the Institutional Review Board of the Guangdong Provincial People’s Hospital (No. GDREC2016438H (R2)) and this study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All participants gave written informed consent.
Author Contributions
All authors made a significant contribution to the work reported, whether that was in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Data Sharing Statement
The datasets used in this analysis are available from the corresponding author.
Additional information
Funding
References
- Peerapen P, Thongboonkerd V. Caffeine in kidney stone disease: risk or benefit? Adv Nutr. 2018;9:419–424. doi:10.1093/advances/nmy016
- Siener R. Nutrition and kidney stone disease. Nutrients. 2021;13(6):1917. doi:10.3390/nu13061917
- Thongprayoon C, Krambeck AE, Rule AD. Determining the true burden of kidney stone disease. Nat Rev Nephrol. 2020;16(12):736–746. doi:10.1038/s41581-020-0320-7
- Wang Z, Zhang Y, Zhang J, Deng Q, Liang H. Recent advances on the mechanisms of kidney stone formation (review). Int J Mol Med. 2021;48:1–10. doi:10.3892/ijmm.2021.4982
- Mahmoodpoor F, Rahbar SY, Barzegari A, Ardalan M, Zununi VS. The impact of gut microbiota on kidney function and pathogenesis. Biomed Pharmacother. 2017;93:412–419. doi:10.1016/j.biopha.2017.06.066
- Ticinesi A, Nouvenne A, Chiussi G, Castaldo G, Guerra A, Meschi T. Calcium oxalate nephrolithiasis and gut microbiota: not just a gut-kidney axis. A nutritional perspective. Nutrients. 2020;12(2):548. doi:10.3390/nu12020548
- Wang N, Ning Z, Xia F, et al. Exposure to famine in early life and chronic kidney diseases in adulthood. Nutr Diabetes. 2018;8(1):4. doi:10.1038/s41387-017-0014-9
- Lv S, Shen Z, Zhang H, et al. Association between exposure to the Chinese famine during early life and the risk of chronic kidney disease in adulthood. Environ Res. 2020;184:109312. doi:10.1016/j.envres.2020.109312
- Roseboom T, de Rooij S, Painter R. The Dutch famine and its long-term consequences for adult health. Early Hum Dev. 2006;82(8):485–491. doi:10.1016/j.earlhumdev.2006.07.001
- Abate KH, Abdulahi M, Abdulhay F, et al. Consequences of exposure to prenatal famine on estimated glomerular filtration rate and risk of chronic kidney disease among survivors of the great Ethiopian famine (1983-85): a historical cohort study. Nutr J. 2021;20:19. doi:10.1186/s12937-021-00675-8
- Jiang W, Han T, Duan W, et al. Prenatal famine exposure and estimated glomerular filtration rate across consecutive generations: association and epigenetic mediation in a population-based cohort study in Suihua China. Aging. 2020;12(12):12206–12221. doi:10.18632/aging.103397
- Cullen-Mcewen LA, van der Wolde J, Haruhara K, et al. Podocyte endowment and the impact of adult body size on kidney health. Am J Physiol Renal Physiol. 2021;321(3):F322–F334. doi:10.1152/ajprenal.00029.2021
- Lu J, Xuan S, Downing NS, et al. Protocol for the China PEACE (Patient-centered Evaluative Assessment of Cardiac Events) million persons project pilot. BMJ Open. 2016;6(1):e10200. doi:10.1136/bmjopen-2015-010200
- Huang YQ, Liu L, Yu YL, et al. The relationship between famine exposure in early life and left atrial enlargement in adulthood. J Hum Nutr Diet. 2021;34:356–364. doi:10.1111/jhn.12802
- Wang Z, Dong Y, Xu R, Wang X, Li Y, Zou Z. Early-life exposure to the Chinese great famine and later cardiovascular diseases. Int J Public Health. 2021;66:603859. doi:10.3389/ijph.2021.603859
- Huang YQ, Liu L, Yu YL, et al. The relationship between famine exposure during early life and carotid plaque in adulthood. Eur J Clin Nutr. 2021;75:546–554. doi:10.1038/s41430-020-00756-7
- Hollander D, Kampman E, van Herpen CM. Pretreatment body mass index and head and neck cancer outcome: a review of the literature. Crit Rev Oncol Hematol. 2015;96:328–338. doi:10.1016/j.critrevonc.2015.06.002
- Yang W, Lu J, Weng J, et al. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362(12):1090–1101. doi:10.1056/NEJMoa0908292
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–2219. doi:10.1093/eurheartj/eht151
- Siener R. Dietary treatment of metabolic acidosis in chronic kidney disease. Nutrients. 2018;10(4):512. doi:10.3390/nu10040512
- Qin Q, Chang K, Wu Q, et al. Undernutrition when young and the risk of poor renal function in adulthood in women with diabetes in Shanghai, China. J Int Med Res. 2021;49(5):675889697. doi:10.1177/03000605211016671
- Martin CL, Ruble DN. Patterns of gender development. Annu Rev Psychol. 2010;61(1):353–381. doi:10.1146/annurev.psych.093008.100511
- Feneberg R, Schaefer F, Veldhuis JD. Neuroendocrine adaptations in renal disease. Pediatr Nephrol. 2003;18(6):492–497. doi:10.1007/s00467-003-1160-y
- Evan AP, Worcester EM, Coe FL, Williams JJ, Lingeman JE. Mechanisms of human kidney stone formation. Urolithiasis. 2015;43(Suppl 1):19–32. doi:10.1007/s00240-014-0701-0
- Khan SR, Pearle MS, Robertson WG, et al. Kidney stones. Nat Rev Dis Primers. 2016;2(1):16008. doi:10.1038/nrdp.2016.8
- Taylor EN, Curhan GC. Role of nutrition in the formation of calcium-containing kidney stones. Nephron Physiol. 2004;98(2):55–63. doi:10.1159/000080265
- Hughson M, Farris AB, Douglas-Denton R, Hoy WE, Bertram JF. Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney Int. 2003;63(6):2113–2122. doi:10.1046/j.1523-1755.2003.00018.x
- Warnock DG. The fault is not in our stars but may be in our embryos: glomerular number in low birth weight babies. Nephron. 2017;136(1):1–02. doi:10.1159/000465509
- Schreuder MF, Nyengaard JR, Fodor M, van Wijk JA, Delemarre-Van DWH. Glomerular number and function are influenced by spontaneous and induced low birth weight in rats. J Am Soc Nephrol. 2005;16:2913–2919. doi:10.1681/ASN.2004100875
- Al Salmi I, Hannawi S. Birth weight and susceptibility to chronic kidney disease. Saudi J Kidney Dis Transpl. 2020;31:2913–2919. doi:10.4103/1319-2442.292305
- Mascarenhas S, Mutnuri S, Ganguly A. Deleterious role of trace elements - silica and lead in the development of chronic kidney disease. Chemosphere. 2017;177:239–249. doi:10.1016/j.chemosphere.2017.02.155
- Lindeman RD. Trace minerals and the kidney: an overview. J Am Coll Nutr. 1989;8:285–291. doi:10.1080/07315724.1989.10720303
- Tran T, Maalouf NM. Uric acid stone disease: lessons from recent human physiologic studies. Curr Opin Nephrol Hypertens. 2020;29(4):407–413. doi:10.1097/MNH.0000000000000610
- Zhang W, Luan R. Early-life exposure to the Chinese famine of 1959-61 and risk of hyperuricemia: results from the China health and retirement longitudinal study. Bmc Public Health. 2020;20:15. doi:10.1186/s12889-019-8017-1
- Wang W, Lu X, Shi Y, Wei X. Association between food insecurity and kidney stones in the United States: analysis of the national health and nutrition examination survey 2007–2014. Front Public Health. 2022;10:1015425. doi:10.3389/fpubh.2022.1015425
- Zisman AL. Effectiveness of treatment modalities on kidney stone recurrence. Clin J Am Soc Nephrol. 2017;12(10):1699–1708. doi:10.2215/CJN.11201016
- Black MM. Impact of nutrition on growth, brain, and cognition. Nestle Nutr Inst Workshop Ser. 2018;89:185–195. doi:10.1159/000486502
- Penido RC, Isaac ML, Penido AB. Influence of malnutrition on the development of the central nervous system of malnourished children. Nutr Neurosci. 2020;23(2):85–92. doi:10.1080/1028415X.2018.1472962
- Geraghty R, Abdi A, Somani B, Cook P, Roderick P. Does chronic hyperglycaemia increase the risk of kidney stone disease? Results from a systematic review and meta-analysis. BMJ Open. 2020;10(1):e32094. doi:10.1136/bmjopen-2019-032094
- Wong Y, Cook P, Roderick P, Somani BK. Metabolic syndrome and kidney stone disease: a systematic review of literature. J Endourol. 2016;30:246–253. doi:10.1089/end.2015.0567
- Xin X, Yao J, Yang F, Zhang D. Famine exposure during early life and risk of hypertension in adulthood: a meta-analysis. Crit Rev Food Sci Nutr. 2018;58:2306–2313. doi:10.1080/10408398.2017.1322551
- Liu H, Chen X, Shi T, et al. Association of famine exposure with the risk of type 2 diabetes: a meta-analysis. Clin Nutr. 2020;39(6):1717–1723. doi:10.1016/j.clnu.2019.08.002