Abstract
Introduction
Pain control is an important first step in the treatment of shoulder impingement syndrome (SIS) because fear of pain must be removed as an obstacle to participation in an appropriate physical therapy program.
Methods
Adult patients with SIS-associated pain of at least 2 weeks’ duration and who had an average pain score of ≥4 on the zero- to ten-point Numeric Pain Rating Scale were eligible to enroll in this 2-week pilot study. Patients were treated with the heated lidocaine/tetracaine (70 mg/70 mg) patch (HLT patch) placed over the site of shoulder tenderness each morning and evening for a period of 2 to 4 hours. Average and worst pain during the previous 24 hours and shoulder range of motion were assessed at baseline and on Day 14. Results were expressed as change and percent change from baseline to Day 14. This pilot study was not powered for rigorous statistical analysis.
Results
Twenty patients (seven male, 13 female; average age 51.2 ± 10.8 years [mean ± standard deviation]) enrolled in this study, and 18 patients completed the protocol. The mean average pain score at baseline was 5.5 ± 1.1 (range 4 to 8). In the per-protocol population, average and worst pain scores declined by 2.4 ± 2.0 and 3.7 ± 2.7 points, respectively. Two-thirds of the patients demonstrated a clinically meaningful ≥30% decline in average pain score, and half of the patients demonstrated a ≥50% decline in average pain score. Shoulder internal rotation increased by 29.7° ± 21.8° and abduction increased by 40.0° ± 44.2°. Application-site erythema was reported by ten patients at some time during the study.
Conclusion
Patients treated with the HLT patch for 14 days demonstrated clinically meaningful improvement in pain intensity and range of motion. Further controlled research is necessary to characterize the efficacy and tolerability of the HLT patch in the treatment of SIS.
Introduction
Persistent shoulder pain or stiffness is a common reason for consulting with primary care physicians. In the Netherlands, shoulder complaints in general practice have been estimated at 12 to 25 per 1000 visits per year, and in England and Wales the estimate is 6.6 per 1000 visits.Citation1 A systematic review of the medical literature found that the point prevalence of shoulder pain in adults aged <70 years was between 7% and 27%, and the lifetime prevalence was 7% to 67%.Citation2 Direct health care costs attributable to shoulder pain and dysfunction are high. In the USA, an examination of the Medical Expenditure Panel Survey for the year 2000 revealed a total direct-cost estimate of US$7 billion with a mean cost per care episode of US$1667 and US$3011 for outpatient and hospital-based settings, respectively.Citation3
Shoulder impingement syndrome (SIS) is a common cause of shoulder pain and dysfunction and is typically caused by an impingement of the rotator cuff tendon under the acromion,Citation4,Citation5 although other pathologies may contribute.Citation6,Citation7 Initial treatment of SIS is conservative and consists of oral nonsteroidal anti-inflammatory drugs (NSAIDs) and supervised physical therapy regimens,Citation5,Citation6 with the goal of reducing pain and inflammation, healing the rotator cuff, and improving shoulder function.Citation8 It has been estimated that about two-thirds of subjects with SIS will demonstrate a satisfactory response to NSAIDs and physical therapy.Citation8 If symptoms persist, subacromial injection of lidocaine and a corticosteroid may be indicated to relieve pain and allow physical therapy to continue.Citation5,Citation6,Citation9 However, despite their frequent use, a meta-analysis of randomized clinical trials evaluating subacromial injection of corticosteroids for SIS concluded that these injections may provide a limited and short-term benefit.Citation10
Pain control is an important first step in the treatment of SIS because fear of pain may contribute to the original pathology as a result of fear of movement, avoidance of movement, and eventual disuse.Citation11,Citation12 Thus, pain and fear of pain must be removed as obstacles to participation in appropriate physical therapy and exercise programs. One possible adjunct in the management of SIS-associated pain is the heated lidocaine/tetracaine (70 mg/70 mg) patch (HLT patch) that contains a eutectic mixture of the two drugs and has an integrated oxygen-activated heating component that enhances the dermal delivery of the anesthetic agents.Citation13,Citation14 The HLT patch is approved in the USA and Europe and is indicated for use on intact skin to provide local dermal analgesia for superficial venous access and superficial dermatological procedures.Citation14 The depth and duration of anesthesia observed in a controlled study of the patchCitation15 suggest that it may be useful in the relief of pain associated with musculoskeletal structures lying close to the surface of the skin, as has been reported for other patches for the dermal delivery of local anesthetics.Citation16–Citation19 Thus, here we report an open-label pilot study that was designed to investigate the utility of the HLT patch in reducing pain and improving shoulder function in patients with SIS.
Methods
Ethics
The study protocol was reviewed and approved by the Quorum Review Institutional Review Board (Seattle, WA, USA). All study subjects provided signed informed consent prior to engaging in any study activities.
Patient population
Adult patients (≥18 years old) with pain associated with SIS of at least a 2-week duration were eligible to enroll in the study. Additional inclusion criteria included tenderness at the site of rotator cuff tendons, positive Hawkins’ and Neer’s signs, and an average pain score of ≥4 on the zero- to ten-point Numeric Pain Rating ScaleCitation20 over the past 24 hours.
Exclusion criteria included topical pain medication applied to the treatment area within 3 days of the screening visit, use of injected pain medication within 14 days of the screening visit, allergies or contraindications to any of the study drug components, clinically significant illness within 14 days of the screening visit, use of any class I antiarrhythmic agent, history of severe hepatic disease, and participation in a study of an unapproved drug within 30 days of the screening visit. Patients who had filed a SIS-associated disability claim or were receiving disability benefits due to SIS were ineligible for the study, as were women who were pregnant, breastfeeding, or not using appropriate birth control.
Study design
This open-label study was conducted at the Injury Care Medical Center (Boise, ID, USA) and was carried out in accordance with the Good Clinical Practice guidelines. At the screening visit, patients underwent a problem-based physical examination, and medical history and concomitant medication use were recorded. Enrolled patients were instructed to apply the HLT patch to the site of tenderness twice daily (about every 12 hours) for periods of 2 to 4 hours. Each evening, patients were instructed to record average and worst pain intensity during the previous 24 hours in their study diary using the Numeric Pain Rating Scale,Citation20 an eleven-point rating scale in which a score of 0 indicates no pain and a score of 10 represents the worst pain imaginable. Pain interference with general activity, normal work, and sleep during the previous 24 hours was also recorded using a similar eleven-point scale in which a score of 0 represents no interference and a score of 10 represents complete interference. In addition, they were instructed to record the use of any concomitant medications during the previous 24-hour period. Limits of internal rotation and abduction of the shoulder were assessed with a goniometer at baseline and on Day 14. On Day 14, patients were asked to grade their satisfaction of treatment by choosing one of the following categories: very dissatisfied, dissatisfied, no preference, satisfied, or very satisfied.
Patients were allowed to use acetaminophen for acute SIS pain that was not effectively treated by the HLT patch or for other minor pain (eg, headache). However, patients who used acetaminophen for SIS pain on two consecutive days were not included in the per-protocol population. NSAIDs were not allowed during the study, nor were any topically applied medications for the treatment of SIS pain.
Safety
All 20 patients who enrolled in the study received at least one treatment with the HLT patch and were included in the safety population. Adverse events (AEs) were monitored throughout the study. Erythema at the application site was evaluated at the final study visit (Day 14) by medical personnel using a five-point scale (0 = no erythema, 1 = very slight erythema, 2 = well-defined erythema, 3 = moderate to severe erythema, 4 = severe erythema [beet redness] to slight eschar formations [injuries in depth]).
Data analysis
This pilot study was designed to provide information to guide further research into the use of the HLT patch in the treatment of pain associated with SIS and was not powered for rigorous statistical analysis. Therefore, no hypothesis testing was performed. All results are presented using descriptive statistics.
Results
A total of 20 patients were enrolled in the study and 19 patients completed it. One patient was lost to follow-up due to incarceration. All patients were Caucasian. Seven patients were male and 13 were female. The average age of the patients was 51.2 ± 10.8 years (range 31 to 75 years). The mean baseline average pain score was 5.5 ± 1.1 (range 4 to 8). The per-protocol population included 18 subjects; one subject was lost to follow-up and provided no efficacy data, and one subject used acetaminophen on two or more consecutive days for SIS pain.
The effects of the HLT patch on measurement of SIS pain are presented in . In the per-protocol population, average and worst pain scores decreased by 2.4 ± 2.0 and 3.7 ± 2.7 points, respectively, from baseline to Day 14. Similar changes were seen for the pain interference scores (). The cumulative response rate for percent decrease in average pain score (ie, percent improvement in average pain intensity) is shown in . Two-thirds of the per-protocol population demonstrated at least a 30% decrease in average pain scores, and 50% of this population demonstrated at least a 50% reduction in average pain.
Figure 1 Response distribution of percent improvement of final average pain (N = 18). Two subjects had 0% improvement: one patient demonstrated no change from baseline and one patient demonstrated an increase in average pain intensity from baseline to final visit.
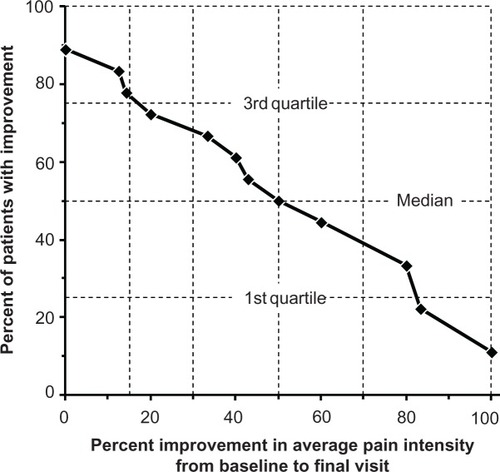
Table 1 Changes in pain scores and pain interference scores in the per-protocol population
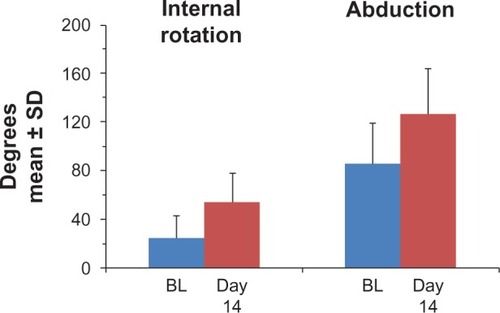
The effect of treatment on shoulder range of motion is shown in . In the per-protocol population, internal rotation increased by a mean 29.7° ± 21.8° during the study. Similarly, abduction increased by a mean 40.0° ± 44.2°.
Figure 2 Shoulder range of motion at baseline and after 14 days of treatment with the heated lidocaine/tetracaine patch.
On Day 14 in the per-protocol population, eight patients (44.4%) indicated they were “very satisfied” with their HLT patch experience and two patients (11.1%) were “satisfied.” Two patients (11.1%) were “very dissatisfied” and three patients (16.7%) were “dissatisfied.” Three patients (16.7%) expressed “no preference.”
No serious AEs were reported during the study and no patients withdrew from the study due to AEs. A total of 36 AEs were reported by 14 patients (70%) during the study; all were mild or moderate in severity. Ten subjects reported application-site erythema at some time during the study. On Day 14, Grade 2 and Grade 1 erythema was observed in three and six patients, respectively. Other application-site AEs included irritation and pruritus in two patients each and dermatitis, induration, pain, rash, swelling, and urticaria in one patient each.
Discussion
In this pilot study of the initial treatment of patients with SIS, 2 weeks of treatment with the HLT patch resulted in improvement in pain scores, pain interference scores, and range of motion ( and ). The majority of patients were “satisfied” or “very satisfied” with the HLT patch. The HLT patch was generally well tolerated.
The magnitude of the pain response in the present study is within the range considered clinically meaningful. For example, Farrar et alCitation20 analyzed the results of ten double-blind studies of patients with pain that used an eleven-point numerical pain rating scale. In their analysis, a change of −1.74 points or −27.9% was associated with “much or very much” improvement. As shown in , two-thirds of the patients in the present study demonstrated at least a 30% decrease in average pain score, suggesting that the HLT patch produced clinically meaningful improvement in most patients.
Pain or fear of pain may directly contribute to the pathophysiology of SIS.Citation12 This concept, which has been described as the “fear-avoidance model” of musculoskeletal pain, is defined as a catastrophic misinterpretation of pain resulting in increased fear of pain, fear of movement, avoidance, and hypervigilance.Citation12 This behavior initiates and perpetuates a vicious cycle that leads to an elevation of chronic pain and long-term disability. Lentz et alCitation12 studied 142 subjects with unilateral shoulder disorders and assessed pain-related fear with the Tampa Scale of Kinesiophobia. They found that pain-related fear was statistically significantly associated with self-reported disability after controlling for demographics, pain intensity, and other physical impairment measures. Based on the fear-avoidance model theory, the reduction in pain observed in the present study may have had the direct effect of improvement in range of motion ().
Conservative therapy for SIS involves supervised physical therapy including stretching and gradual strengthening of the shoulder musculature.Citation21 However, pain control is an essential component of a successful physical therapy program.Citation9,Citation21 Initial pain management typically involves NSAIDs or acetaminophen,Citation9 and if symptoms persist, a subacromial injection of corticosteroids can be administered in combination with a local anesthetic.Citation9
The term “shoulder impingement syndrome” is often used indiscriminately to describe various conditions of chronic shoulder pain or discomfort. The disease it describes is part of a continuum of rotator cuff tendinopathy characterized by mucoid degeneration, collagen loss, and disorganization.Citation22,Citation23 Histopathologic examinations of symptomatic rotator cuff tendons have inconsistently shown evidence of inflammation despite substantial degenerative changes in the tendons.Citation22 Khan et alCitation22 failed to show histopathological evidence of inflammation, while Murphy and coworkersCitation7 found significant elevation of CD45, a marker of inflammation, in biopsies of the supraspinatus tendon from patients with early stage rotator cuff tendinopathy but not in samples taken from patients with more advanced disease. In addition, immunohistochemical examination of subacromial bursa biopsy specimens from patients with rotator cuff disease shows a clear increase in inflammatory cytokines, as well as in cyclooxygenase 1 and 2, compared with specimens from subjects without SIS.Citation24,Citation25
The pathophysiological processes that contribute to the pain of rotator cuff disease are many, and this variation may influence the response to treatment. The less-than-certain role of inflammation in the pathology of SIS calls into question the routine use of systemic NSAIDs, especially in view of their AE profile, and points to the need to explore alternative treatments that target different proposed mechanisms of pain. Other biochemical mediators that may influence nociceptors include glutamate and substance P.Citation26 Local anesthetics, such as lidocaine and tetracaine, are known to relieve pain by inhibiting the depolarization and firing of sensory nerve fibers through sodium channel blockade and possibly targeting substance P/neurokinin-1 signaling via sodium channel blockade.Citation27,Citation28 Additionally, high levels of the neurotransmitter glutamate have been identified by in situ microdialysis in patellar and Achilles tendinopathies,Citation29 and elevated expression of the glutamate receptor N-methyl-D-aspartate (NMDA) receptor type 1 has been associated with tendinopathies.Citation30,Citation31 The NMDA receptor is reported to be involved in chronic pain disorders, and both lidocaine and tetracaine inhibit this receptor.Citation32 Thus, it is possible that the HLT patch might attenuate pain in part by blockade of the NMDA receptor. However, without data regarding receptor expression in SIS, this mechanism of action must remain speculative.
The HLT patch is designed to have its primary effect on the skin and underlying subcutaneous tissue, but the present results suggest a drug effect within deeper structures with increased duration of application and twice-daily dosing. Wallace et alCitation15 reported that a 30-minute application of a single HLT patch resulted in elimination of pain to a mean depth of 8.22 mm, with this peak effect occurring 60 minutes after HLT patch removal. Longer application times, as were used in the present study, might be expected to result in deeper penetration of the lidocaine and tetracaine. Other investigators have shown that topically applied agents can be detected in structures beneath the skin following application. For example, Sekiya et alCitation33 found similar concentrations of ketoprofen in semitendinosus muscle and tendon at 14 hours following the administration of either 40 mg by dermal patch or 150 mg administered as a single, sustained-release oral dose, even though mean ketoprofen plasma concentrations were 17-fold higher in patients who received oral doses.
Study limitations
This study was open-label and of short duration. As no placebo-treated control group was included, it cannot be determined to what degree placebo response contributed to the observed outcomes in this study. Further controlled research is warranted before any firm conclusions can be drawn.
Conclusion
The latest consensus is that SIS most likely represents a rotator cuff tendinopathy. Given our understanding of the underlying pathophysiology relating to tendinopathies and pain, use of anti-inflammatory agents may not optimally target two recently implicated components of SIS pain: glutamate-NMDA signaling and substance P/neurokinin-1 signaling via sodium channel blockade. In this pilot study, patients with SIS demonstrated clinically meaningful improvement in pain intensity, pain interference scores, and range of motion after 14 days of treatment with the HLT patch, suggesting a potential role in this patient population. Further controlled research is warranted to fully characterize the efficacy and benefit–risk profile of the HLT patch for the relief of pain associated with SIS.
Acknowledgments
The authors thank ZARS Pharma and Nuvo Research for the support provided for the development of this article. The authors also thank Arnold R Gammaitoni, PharmD, of Nuvo Research, for his review for medical accuracy. Professional medical writing and editing assistance was paid for by Nuvo Research and was provided by Edward Weselcouch, PhD, and Diana Talag, MS, ELS, of PharmaWrite (Princeton, NJ, USA). This article was prepared according to the International Society for Medical Publication Professionals’ Good Publication Practice for Communicating Company-Sponsored Medical Research: The GPP2 Guidelines.
Disclosure
RR has been a consultant for and has received research funding from Nuvo Research. TBM is a full-time employee of ZARS Pharma, a Nuvo Research company, which markets Synera in the USA. The authors declare no other conflicts of interest in this work.
References
- van der WindtDAKoesBWde JongBABouterLMShoulder disorders in general practice: incidence, patient characteristics, and managementAnn Rheum Dis199554129599648546527
- LuimeJJKoesBWHendriksenIJPrevalence and incidence of shoulder pain in the general population; a systematic reviewScand Cardiovasc J20043327381
- JohnsonMPCrossleyKLO’NeilMEAl-ZakwaniISEstimates of direct health care expenditures among individuals with shoulder dysfunction in the United StatesPaper presented at the 2004 Annual Meeting of the American Society of Shoulder and Elbow TherapistsSeptember 29–October 2, 2004New York, NY
- NeerCSAnterior acromioplasty for the chronic impingement syndrome in the shoulder: a preliminary reportJ Bone Joint Surg AM197254141505054450
- BussDDFreehillMQMarraGTypical and atypical shoulder impingement syndrome: diagnosis, treatment, and pitfallsInstr Course Lect20095844745719385554
- KoesterMCGeorgeMSKuhnJEShoulder impingement syndromeAm J Med2005118545245515866244
- MurphyRJKliskeyKWhewayKWatkinsEBBeardDJCarrAJRotator cuff tendinopathy: immunohistochemical changes across the spectrum of pathologyBr J Sports Med2013479e2
- MorrisonDSFrogameniADWoodworthPNon-operative treatment of subacromial impingement syndromeJ Bone Joint Surg AM19977957327379160946
- BurbankKMStevensonJHCzarneckiGRDorfmanJChronic shoulder pain: part II. TreatmentAm Fam Physician200877449349718326169
- BuchbinderRGreenSYoudJMCorticosteroid injections for shoulder painCochrane Database Syst Rev20031CD00401612535501
- LeeuwMGoossensMELintonSJCrombezGBoersmaKVlaeyenJWThe fear-avoidance model of musculoskeletal pain: current state of scientific evidenceJ Behav Med2007301779417180640
- LentzTABarabasJADayTBishopMDGeorgeSZThe relationship of pain intensity, physical impairment, and pain-related fear to function in patients with shoulder pathologyJ Orthop Sports Phys Ther200939427027719346624
- SawyerJFebbraroSMasudSAshburnMACampbellJCHeated lidocaine/tetracaine patch (Synera, Rapydan) compared with lidocaine/prilocaine cream (EMLA) for topical anaesthesia before vascular accessBr J Anaesth2009102221021519151049
- Synera (lidocaine 70 mg and tetracaine 70 mg) topical patch [prescribing information]Salt Lake City, UTZARS Pharma2010
- WallaceMSKopeckyEAMaTBrophyFCampbellJCEvaluation of the depth and duration of anesthesia from heated lidocaine/tetracaine (Synera) patches compared with placebo patches applied to healthy adult volunteersReg Anesth Pain Med201035650751320975464
- AffaitatiGFabrizioASaviniAA randomized, controlled study comparing a lidocaine patch, a placebo patch, and anesthetic injection for treatment of trigger points in patients with myofascial pain syndrome: Evaluation of pain and somatic pain thresholdsClin Ther200931470572019446144
- NalamachuSCrockettRSGammaitoniARGouldEMA comparison of the lidocaine patch 5% vs naproxen 500 mg bid for the relief of pain associated with carpal tunnel syndrome: a 6-week, randomized, parallel-group studyMed Gen Med20068333
- BurchFCoddingCPatelNSheldonELidocaine patch 5% improves pain, stiffness, and physical function in osteoarthritis pain patients. A prospective, multicenter, open-label effectiveness trialOsteoarthritis Cartilage200412325325514972343
- KivitzAFairfaxMSheldonEAComparison of the effectiveness and tolerability of Lidocaine Patch 5% versus Celecoxib for osteoarthritis-related knee pain: post hoc analysis of a 12-week, propsective, randomized, active-controlled, open-label, parallel-group trial in adultsClin Ther200830122366237719167595
- FarrarJTYoungJPJrLaMoreauxLWerthJLPooleRMClinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scalePain200194214915811690728
- CodsiMJThe painful shoulder: when to inject and when to referCleve Clin J Med200774747347848017682625
- KhanKMCookJLBonarFHarcourtPAstromMHistopathology of common tendinopathies. Update and implications for clinical managementSports Med199927639340810418074
- LewisJSRotator cuff tendinopathyBr J Sports Med200943423624118801774
- BlaineTAKimYSVoloshinIThe molecular pathophysiology of subacromial bursitis in rotator cuff diseaseJ Shoulder Elbow Surg200514Suppl 184S89S15726092
- VoloshinIGelinasJMaloneyMDO’KeefeRJBiglianiLUBlaineTAProinflammatory cytokines and metalloproteases are expressed in the subacromial bursa in patients with rotator cuff diseaseArthroscopy20052191076.e11076.e916171632
- KhanKMCookJLMaffulliNKannusPWhere is the pain coming from in tendinopathy? It may be biochemical, not only structural, in originBr J Sports Med2000342818310786860
- WeiserTComparison of the effects of four Na+ channel analgesics on TTX-resistant Na+ currents in rat sensory neurons and recombinant Nav 1.2 channelsNeurosci Lett2006395317918416293367
- LiYMWingroveDETooHPLocal anesthetics inhibit substance P binding and evoked increases in intracellular Ca2+Anesthesiology19958211661737530414
- AlfredsonHForsgrenSThorsenKLorentzonRIn vivo microdialysis and immunohistochemical analyses of tendon tissue demonstrated high amounts of free glutamate and glutamate NMDAR1 receptors, but no signs of inflammation, in Jumper’s kneeJ Orthop Res200119588188611562137
- SchizasNLianOFrihagenFEngebretsenLBahrRAckermannPWCoexistence of up-regulated NMDA receptor 1 and glutamate on nerves, vessels and transformed tenocytes in tendinopathyScand J Med Sci Sports201020220821519422642
- SchizasNWeissRLianOFrihagenFBahrRAckermannPWGlutamate receptors in tendinopathic patientsJ Orthop Res20123091447145222354721
- SugimotoMUchidaIMashimoTLocal anaesthetics have different mechanisms and sites of action at the recombinant N-methyl-D-aspartate (NMDA) receptorsBr J Pharmacol2003138587688212642389
- SekiyaIMoritoTHaraKKetoprofen absorption by muscle and tendon after topical or oral administration in patients undergoing anterior cruciate ligament reconstructionAAPS PharmSciTech201011115415820087696