Abstract
Background and methods
An efficacy population of 245 patients with vertigo of peripheral vestibular origin was recruited in Romania as part of a 3-month multinational, post-marketing surveillance study of open-label betahistine 48 mg/day (OSVaLD). Endpoints were changes in the Dizziness Handicap Index (primary endpoint), Medical Outcome Study Short-Form 36 (SF-36v2®), and the Hospital Anxiety and Depression Scale.
Results
During treatment, the total Dizziness Handicap Index score improved by 41 points (on a 100-point scale). Statistically significant improvements of 12–14 points were recorded in all three domains of the Dizziness Handicap Index scale (P<0.0001). Betahistine therapy was also accompanied by progressive improvements in mean Hospital Anxiety and Depression anxiety and depression scores (P<0.0001) and significant improvements in both the physical and mental component summary of the SF-36v2 (P<0.0001). Betahistine was well tolerated, with only one suspected adverse drug reaction recorded in the Romanian safety population (n=259).
Conclusion
Betahistine 48 mg/day was associated with improvements in multiple measures of health-related quality of life and had a good tolerability profile in these Romanian patients with recurrent peripheral vestibular vertigo.
Introduction
OSVaLD (Observational Study in patients suffering from recurrent peripheral vestibular Vertigo to assess the effect of betahistine 48 mg/day on quality of Life and Dizziness symptoms) documented a positive effect of betahistine therapy on health-related quality of life (HRQoL) in a multinational population of patients with recurrent peripheral vestibular vertigo.Citation1,Citation2 OSVaLD was conducted in 13 countries, with the single largest patient contingent recruited in Romania. This supplementary report of findings from OSVaLD examines HRQoL trends in the Romanian participants.
Materials and methods
Details of the methodology of OSVaLD have been published previously,Citation1,Citation2 and readers are referred to those sources for particulars of statistical methods and sample size calculations. OSVaLD was a post-marketing surveillance study of open-label betahistine in patients with vertigo of peripheral vestibular origin, and was undertaken in primary care centers in 13 countries with a planned duration of 3 months. The daily dose of betahistine was 48 mg/day, administered as 24 mg twice daily or 16 mg three daily as specified by the relevant product information for each country.
Scores on the Dizziness Handicap Index (DHI), the Medical Outcome Study Short-Form 36 (SF-36v2®), and the Hospital Anxiety and Depression Scale (HADS) were to be measured at baseline, at regular intervals during the study, and at study completion in the efficacy population (ie, patients who were prescribed betahistine at baseline and who attended at least one subsequent clinic visit and generated at least one score for at least one endpoint scale at baseline and at one or more later visits). The primary efficacy outcome was the change from baseline in total DHI score at 3 months. Statistical treatments of all three endpoints, including provisions for missing data, have been reported in detail elsewhere.Citation1,Citation2 All the endpoint indices used have been extensively evaluated and validated.Citation3–Citation12
The safety population, from which reports of adverse drug reactions were collated, consisted of all patients who received a prescription of betahistine at baseline and who made at least one clinic visit after baseline.
The design and conduct of OSVaLD conformed to international principles of Good Clinical Practice and the Declaration of Helsinki. The study protocol was submitted to independent institutional review as required by local regulatory provisions, for approval prior to starting the study. Informed consent was obtained from each patient as stipulated by local laws and regulations before they participated in the study. Patients were advised that they could withdraw from the study at any time if they wished to, that they could do so for any reason, and that they were not obliged to explain their reason. They were also assured that withdrawing from the study would have no effect on other treatments they might receive. The FOVEA Group (Rueil Malmaison, France) was responsible for data management and statistical analysis.
Results
In Romania, a total of 262 patients were recruited at 84 centers. Participating practitioners are named in Supplementary material. From this initial contingent was derived a safety population of 259 patients and an efficacy population of 245 patients; these patients were almost exclusively (>99%) white/Caucasian. Other salient demographic features of each contingent are shown in .
Table 1 Demographic features of the Romanian efficacy and safety subpopulations of the OSVaLD study
Betahistine was most often introduced in response to a new diagnosis (n=135, 55.1%) or inefficacy of existing therapy, which accounted for most of the remainder of the patients (n=97, 39.6%). The single most often recorded diagnosis associated with use of betahistine was peripheral vestibular vertigo of unknown pathophysiology (PVVP; n=102, 41.6% of cases); other prominent diagnoses included benign paroxysmal positional vertigo (BPPV; n=42, 17.1%) and Ménière’s disease (n=38, 15.5%). Across these three diagnoses, the proportions of cases attributed to a new diagnosis or inefficacy of current therapy were similar to those for the Romanian contingent as a whole. Multiple diagnoses were recorded in 21 cases (8.6%).
Almost 12% of patients in the efficacy contingent (n=29) had a diagnosis of ear infection and 14.7% had records of psychosomatic or psychiatric disorders, such as hyperventilation or panic disorder (n=36). Cerebrovascular diseases (≈30%), cardiac diseases (≈25%), and metabolic disorders (≈14%) were widely recorded.
Betahistine was almost exclusively prescribed at baseline at a dosage of 24 mg twice daily (n=240, 98%) in the Romanian contingent, and there was almost no change in betahistine dosage distribution during the study. Mean treatment duration was 90±14.5 days.
Combination antivertiginous therapy (meaning betahistine plus at least one other drug) was prescribed at baseline for 77 patients (31.4%), with gingko biloba being the single most widely used additional drug recorded (n=23). Use of combination therapy had declined to 24.1% (n=59) by the end of the study, with gingko biloba still the most frequently recorded agent used in this context (n=21, 8.6%). Among the single-diagnosis categories, combination therapy was more likely to be associated with Ménière’s disease (≈37%) than with PVVP or BPPV (both ≈29%). Forty-seven of the patients prescribed combination therapy were also taking cardiovascular drugs judged capable of having an impact on vertigo and 27 were taking psychotropic drugs judged capable of having an impact on vertigo.
Efficacy outcomes
DHI score
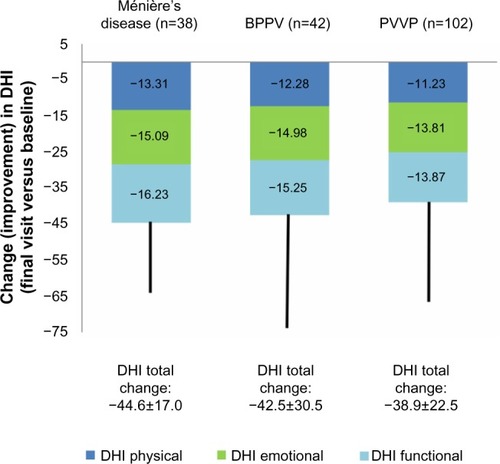
Net changes in the total DHI score and the three dimensions of that score are illustrated in . All those indices changed significantly from baseline (P<0.0001). Changes in all elements of the DHI score were similar among the three major diagnostic categories of PVVP, BPPV, and Ménière’s disease, as shown in . DHI responses were similar in both sexes. DHI responses were slightly larger in patients (n=168) prescribed betahistine monotherapy than in those (n=77) prescribed combination therapy, but these differences were small. In both these categories, the reductions in overall and dimension-specific DHI scores from baseline were highly statistically significant (P<0.0001).
Figure 1 Changes from baseline in components of the DHI and in total DHI score in the Romanian efficacy population of the OSVaLD study.
Abbreviations: DHI, Dizziness Handicap Index; OSVaLD, Observational Study in patients suffering from recurrent peripheral vestibular Vertigo to Assess the effect of betahistine 48 mg/day on quality of Life and Dizziness symptoms.
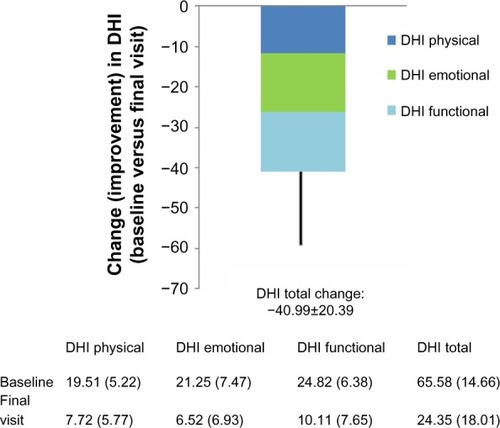
Figure 2 Mean changes in elements of the DHI score in patients with diagnoses of Ménière’s disease, BPPV and PVVP. Total change (improvement) in DHI score is shown at the bottom of each column.
HADS score
Baseline, end of study, and in-study changes in HADS scores are summarized in . Changes in mean HADS scores for both anxiety and depression between the baseline and final visits were highly statistically significant (P<0.0001). These findings were observed consistently in men and women and in patients with different diagnoses for the origins of vertigo.
Table 2 Trends in HADS-anxiety and HADS-depression scores and distributions in the Romanian efficacy population of the OSVaLD study
SF-36v2 score
At the baseline visit, the mean physical component summary (PCS) score was 40.2±7.9 and the mean mental component summary (MCS) score was 35±11. These scores were indicative of reduced HRQoL status (below the norm of the general US population). Improvements in both scores were recorded during the treatment period (P<0.0001 versus baseline, ). As shown in , there were consistent improvements in all domains of the SF-36v2 instrument. Some numerical differences were noted between men and women in some domain responses, but these were small and were not subjected to statistical comparison. PCS and MCS scores improved to a similar extent in patients stratified by diagnostic category, as shown in .
Figure 3 Changes from baseline in the domains of the SF-36v2 instrument in the Romanian efficacy population of the OSVaLD study (n=245).
Abbreviations: PF, physical functioning; RP, role limitation physical; BP, bodily pain; GH, general health perception; VT, vitality; SF, social functioning; RE, role limitation emotional; MH, mental health; OSVaLD, Observational Study in patients suffering from recurrent peripheral vestibular Vertigo to Assess the effect of betahistine 48 mg/day on quality of Life and Dizziness symptoms; SD, standard deviation; SF-36v2, Medical Outcome Study Short-Form 36.
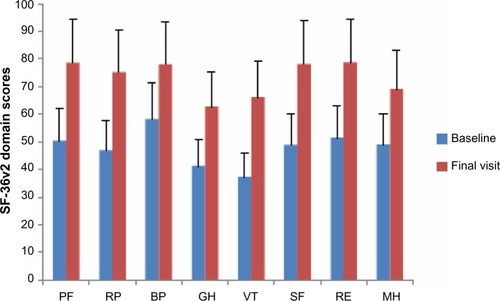
Table 3 On-study trends in the PCS and MCS subscales of the SF-36v2 in the Romanian efficacy population of the OSVaLD study
Body weight
The mean ± standard deviation change in weight between baseline and the final visit was 0.4±3.7 kg in the efficacy population, with identical mean changes between men and women. The average weight gain was smaller in patients with PVVP (n=102, 0.2±5.2 kg) than in those with BPPV or Ménière’s disease (n=80, ≈0.6±2 kg).
Subjective assessment of efficacy
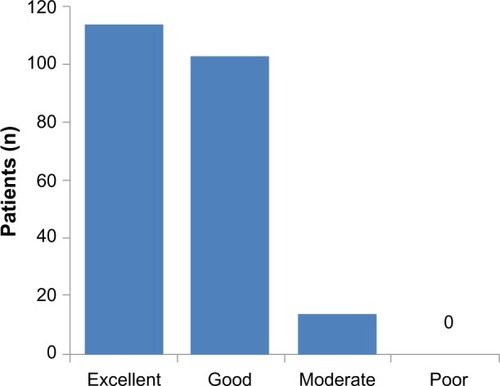
Patients’ assessments of treatment are illustrated in , stratified by recorded cause of vertigo. Regardless of diagnostic category, almost all patients rated betahistine therapy as “excellent” or “good”. No material differences in assessment levels were seen between men and women. Physicians’ and patients’ impressions of treatment showed close alignment (r=0.76, P<0.0001).
Figure 4 Patients’ impression of betahistine therapy in the Romanian efficacy population of OSVaLD (n=245).
Safety and tolerability
The only suspected adverse drug reaction (ADR) recorded in the Romanian contingent was a case of dyspepsia in a 46-year-old male patient. The relationship to study medication was recorded as “probable” but the event was not classified as either severe or serious.
In the study as a whole, 49 patients were reported as having a total of 76 ADRs. Twenty of the patients affected by suspected ADRs were in the Brazilian contingent and 12 in Slovenia and Spain. More patients experienced a suspected ADR at a dosage of 24 mg twice daily than at 16 mg three times daily (25 versus seven; dosage data not recorded for 28 suspected ADRs).
The most frequently reported suspected ADRs were gastrointestinal disorders (33 events in 27 patients, principally abdominal pain upper, nausea or dyspepsia) and nervous system disorders (14 events in 13 patients, principally headaches). The majority of suspected ADRs were characterized as mild (47 events in 33 patients) or moderate (28 events in 19 patients). No deaths were reported during the study.
Discussion
This secondary analysis, based on the Romanian contingent of the OSVaLD population, replicates the demonstration of improved HRQoL with betahistine that was recorded in the primary results.
Diseases of the peripheral vestibular system can have a significant adverse impact on the lives of patients.Citation13,Citation14 Data from Romanian participants in OSVaLD provided some corroboration of that view, with relatively high baseline mean scores on the HADS.Citation15–Citation17 The baseline SF-36v2 data are also consistent with the premise of low HRQoL in this subpopulation.Citation18
The primary efficacy criterion evaluated was the absolute change in mean total DHI score between the baseline and final (3-month) visits. Substantial improvements were registered on this measure, with a 41-point reduction in the mean total score and reductions of 12, 14, and 14 points, respectively, in the physical, emotional, and functional domains of the scale. These improvements were statistically robust (P<0.0001) and were broadly consistent across various subgroups, including sex and disease status at baseline. The improvement in total DHI score greatly exceeded the threshold for a minimally important change.Citation19,Citation20
Statistically significant (P<0.0001) and clinically meaningful improvements were also recorded with the HADS questionnaire and the PCS and MCS components of the SF-36v2. This consistency of effect across scales gives confidence that the responses seen are authentic, although the exploratory nature of the analysis must be borne in mind.
Our findings are compatible with evidence from a meta-analysis supporting the beneficial effects of betahistine in Ménière’s disease and vestibular vertigoCitation21 and with observations and findings from trials in which betahistine has in several instances been used as the reference therapy.Citation22–Citation25 The potential for use of betahistine in conjunction with vestibular rehabilitation maneuvers has recently been the subject of a local case reportCitation26 and should be noted, although the OSVaLD data offer no direct experiences on that point.
The finding of improved HRQoL among patients with BPPV who received betahistine during this study was considered at some length in the report of primary findings of this study,Citation1 and some further remarks on this subject may be offered. Particle repositioning is the preferred and recommended treatment for BPPV,Citation27 and nothing in our present data or the overall findings of OSVaLD contradict that advice. However, in OSVaLD as a whole, in our subpopulation and in the REVERT registry,Citation28 a high percentage of patients with a diagnosis of BPPV were treated with betahistine. Hence, the reality of primary care as revealed in these data appears to be that physicians make extensive use of betahistine in this situation, regardless of expert recommendations. Whether or not betahistine is effective in this indication cannot be confirmed from our data, although the fact that the impact of betahistine on HRQoL in BPPV patients was similar to that in patients with other diagnoses suggests to us that it confers some benefits; an alternative explanation of this similarity would require an extensive placebo effect operating across all diagnostic categories. As noted in the report of the primary data, the scale of the patient database for HRQoL-related effects of betahistine in BPPV generated by OSVaLD compares favorably with the dataset for canalith repositioning.Citation1 That in itself does not prove benefit from betahistine but, combined with our own observations, it leads us to believe that there is perhaps some benefit from this intervention in at least a proportion of patients with BPPV. Another possibility is that the prescription of betahistine in this indicationCitation28 is indicative of a lack of confidence on the part of primary care doctors in their ability to successfully perform repositioning maneuvers; such a conclusion is not per se incompatible with a favorable effect of betahistine, although it would imply other primary motives for using drug therapy. The lack of robust direct comparisons between repositioning and drug therapy has been noted elsewhereCitation29 and remains deserving of attention.
Also untested in OSVaLD was the possible effect of higher doses of betahistine in Ménière’s disease. It is widely held (see, for example, Strupp et alCitation30) that the appropriate dosage of betahistine in this condition is 48 mg three times daily, ie, three times the dose examined in OSVaLD. Even higher doses (up to 480 mg/day) have been used with benefit in severe cases.Citation31 These data suggest the possibility that the beneficial effects of betahistine on HRQoL seen in patients with Ménière’s disease in the Romanian contingent of OSVaLD may underrepresent the full effect that might be achieved with a larger dose.
OSVaLD was an open-label observational study; there are acknowledged limitations to studies of this type,Citation32 but it was a practical and appropriate format for a multinational trial conducted within the framework of routine care and compliant with the provisions and principles of the Strengthening the Reporting of Observational Studies in Epidemiology methodology.Citation33 Adrion and MansmannCitation34 have recently discussed the merits of Bayesian methodologies for the analysis of clinical trials with longitudinal count data as the primary endpoint, specifically vertigo. This is an approach that may find application in future studies.
Safety experience with betahistine in OSVaLD was generally satisfactory, with ADRs affecting <2.5% of the study population. Many of these suspected ADRs were predominance of gastrointestinal and nervous system disorders, which is compatible with other reports about betahistine;Citation35 the drug appears to retain a good tolerability profile even when administered for Ménière’s disease at doses ten times higher than those used in OSVaLD.Citation31 There was distinct national variation in rates of suspected ADR reporting, with only one suspected ADR being reported in the Romanian contingent, compared with reports of suspected ADRs in 20 patients in Brazil.
Conclusion
In 245 Romanian patients diagnosed with recurrent peripheral vestibular vertigo, betahistine 48 mg/day for 3 months was associated with sustained and statistically significant improvements in multiple indices of HRQoL. The safety and tolerability of the treatment were good in this cohort, with only one reported suspected ADR.
Author contributions
All the named authors made substantial contributions to the acquisition of data, and to its analysis and interpretation. They also contributed to drafting the article and/or revising it critically for important intellectual content. All named authors provided final approval of the version to be published and were accountable for ensuring that questions related to the accuracy or integrity of any part of the work were appropriately investigated and resolved.
Acknowledgments
The authors wish to thank the physicians and patients who participated in the OSVaLD survey. This report is published on behalf of the OSVaLD investigators, a full list of whom is provided in Appendix 1.
Supplementary material
Participating investigators from 88 centers in Romania
Alaicescu M (Bucharest), Augustin A (Bucharest), Bădescu A (Bucharest), Baltag D (Iaşi), Bărbos C (Timişoara), Becuş T (Târgu-Mureş), Bucan L (Bucharest), Călăraşu R (Bucharest), Cămpeanu A (Bucharest), Chirileanu RD (Timişoara), Comşa GI (Constanţa), Constantinescu D (Bucharest), Cotulbea S (Timişoara), Cozma S (Iaşi), Cucoş L (Iaşi), Docu AA (Constanţa), Dulămea A (Bucharest), Enache N (Bucharest), Ene A (Bucharest), Fischer TS (Cluj Napoca), Floare L (Cluj Napoca), Frăsineanu A (Bucharest), Geană I (Bucharest), Georgescu E (Bucharest), Georgescu M (Bucharest), Georgescu M-J (Bucharest), Gherman E (Bucharest), Hâncu A (Constanţa), Iliescu I (Constanţa), Ionescu-Mihăiţă ER (Bucharest), Ionita E (Craiova), Ionita I (Craiova), Iovănescu D (Craiova), Ladea M (Bucharest), Loghin V (Constanţa), Marceanu L (Braşov), Mărginean I (Cluj Napoca), Marian G (Bucharest), Marin M (Constanţa), Mariş C (Bucharest), Mârţu D (Iaşi), Matcău L (Timişoara), Muhlfay G (Târgu-Mureş), Muică L (Târgu-Mureş), Naconecinîi D (Iaşi), Niretean A (Târgu-Mureş), Niţă A (Constanţa), Niţu L (Bucharest), Oană N (Cluj Napoca), Oancea A (Bucharest), Oşanu M (Bucharest), Panea N (Cluj Napoca), Pascu A (Bucharest), Pastia M (Bucharest), Pavel R (Cluj Napoca), Pendefunda L (Iaşi), Petruţiu S (Târgu-Mureş), Plăviţu I (Bucharest), Poenaru M (Timişoara), Popa GC (Cluj Napoca), Popa G (Bucharest), Popi S (Constanţa), Popovici A (Timişoara), Prelipceanu D (Bucharest), Radu L (Bucharest), Rădulescu L (Iaşi), Roceanu A (Bucharest), Rusu A (Cluj Napoca), Sabău MS (Târgu-Mureş), Safta D (Bucharest), Sarafoleanu D (Bucharest), Stanciu M (Bucharest), Stănciulescu R (Bucharest), Ştefanache F (Iaşi), Stefanescu EH (Timişoara), Szatmari S (Târgu-Mureş), Szocs M (Târgu-Mureş), Tomescu L (Cluj Napoca), Tudorache B (Bucharest), Tudose C (Bucharest), Ursu C (Iaşi), Vasilescu L (Bucharest), Vasu I (Cluj Napoca), Vioreanu M (Constanţa), Zaboş D (Timişoara), Zaharia C (Craiova), Zainea V (Bucharest), Zarie G (Timişoara).
Disclosure
Preparation of this report was assisted by Hughes associates, Oxford, UK. OSVaLD is supported financially by Abbott Products Operations AG, Allschwil, Switzerland. The authors report no other conflicts of interest in this work.
References
- BenneckeHPérez-GarriguesHBin SidekDEffects of betahistine on patient-reported outcomes in routine practice in patients with vestibular vertigo and appraisal of tolerability: experience in the OSVaLD studyInt Tinnitus J201016142421609908
- Pérez-GarriguesHKuessnerDBeneckeHPatient baseline characteristics in a multinational study of betahistine in recurrent peripheral vestibular vertigo: the OSVaLD studyCurr Med Res Opin2007232753276117910803
- JacobsonGPNewmanCWThe development of the Dizziness Handicap InventoryArch Otolaryngol Head Neck Surg19901164244272317323
- EnloeLJShieldsRKEvaluation of health-related quality of life in individuals with vestibular disease using disease-specific and general outcome measuresPhys Ther1997778909039291947
- JarlsäterSMattsonETest of reliability of the dizziness handicap inventory and the activities-specific balance confidence scale for use in SwedenAdv Physiother20035137144
- KammerlindASLedinTESkargrenEIOdkvistLMReliability of clinical balance tests and subjective ratings in dizziness and disequilibriumAdv Physiother2005796107
- WareJEJrSherbourneCDThe MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selectionMed Care1992304734831593914
- HorneyCAWareJEJrRaczekAEThe MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructsMed Care1993312472638450681
- McHorneyCAWareJEJrLuJFSherbourneCDThe MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groupsMed Care19943240668277801
- Saris-BaglamaRNDeweyCJChisholmGBSF Health Outcomes™ Scoring Software User’s GuideLincoln, RI, USAQualityMetric Inc2004
- SF-36v2™Scoring SF-36 ScalesLincoln, RI, USAQualityMetric Inc2000
- ZigmondASSnaithRPThe Hospital Anxiety and Depression ScaleActa Psychiatr Scand1983673613706880820
- YardleyLDibbBOsborneGFactors associated with quality of life in Menière’s diseaseClin Otolaryngol Allied Sci20032843644112969347
- KinneySESandridgeSANewmanCWLong-term effects of Ménière’s disease on hearing and quality of lifeAm J Otol19971867738989954
- MichopoulosIDouzenisAKalkavouraCChristodoulouCMichalopoulouPKalemiGHospital Anxiety and Depression Scale (HADS): validation in a Greek general hospital sampleAnn Gen Psychiatry20087418325093
- MozaffariehMSacuSBeneschTWedrichAMental health measures of anxiety and depression in patients with retinal detachmentClin Pract Epidemiol Ment Health200731017640389
- PallantJFBaileyCMAssessment of the structure of the Hospital Anxiety and Depression Scale in musculoskeletal patientsHealth Qual Life Outcomes200538216364179
- FarivarSSCunninghamWEHaysRDCorrelated physical and mental health summary scores for the SF-36 and SF-12 Health Survey, V.IHealth Qual Life Outcomes200755417825096
- WhitneySLHudakMTMarchettiGFThe activities-specific balance confidence scale and the Dizziness Handicap Inventory: a comparisonJ Vestib Res1999925325910472037
- TamberALWilhelmsenKTStrandLIMeasurement properties of the Dizziness Handicap Inventory by cross-sectional and longitudinal designsHealth Qual Life Outcomes2009710120025754
- NautaJJMeta-analysis of clinical studies with betahistine in Ménière’s disease and vestibular vertigoEur Arch Otorhinolaryngol201427188789723778722
- ScholtzAWSteindlRBurchardiNBognar-SteinbergIBaumannWComparison of the therapeutic efficacy of a fixed low-dose combination of cinnarizine and dimenhydrinate with betahistine in vestibular neuritis: a randomized, double-blind, non-inferiority studyClin Drug Investig201232387399
- MaslovaraSSoldoSBPuksecMBalabanBPenavicIPBenign paroxysmal positional vertigo (BPPV): influence of pharmacotherapy and rehabilitation therapy on patients’ recovery rate and life qualityNeuroRehabilitation20123143544123232168
- Djelilovic-VranicJAlajbegovicATiric-CamparaMBetahistine or cinnarizine for treatment of Meniere’s diseaseMed Arch20126639639823409520
- LepchaAAmalanathanSAugustineAMTyagiAKBalrajAFlunarizine in the prophylaxis of migrainous vertigo: a randomized controlled trialEur Arch Otorhinolaryngol10292013 Epub ahead of print
- GeorgescuMStoianSMogoantăCACiubotaruGVVestibulary rehabilitation – election treatment method for compensating vestibular impairmentRom J Morphol Embryol20125365165622990562
- BhattacharyyaNBaughRFOrvidasLAmerican Academy of Otolaryngology-Head and Neck Surgery FoundationClinical practice guideline: benign paroxysmal positional vertigoOtolaryngol Head Neck Surg20081395 Suppl 4S47S8118973840
- AgusSBeneckeHThumCStruppMClinical and demographic features of vertigo: findings from the REVERT RegistryFront Neurol201344823675366
- HiltonMPinderDThe Epley (canalith repositioning) manoeuvre for benign paroxysmal positional vertigoCochrane Database Syst Rev20042CD00316215106194
- StruppMKremmydaOBrandtTPharmacotherapy of vestibular disorders and nystagmusSemin Neurol20133328629624057832
- LeziusFAdrionCMansmannUJahnKStruppMHigh-dosage betahistine dihydrochloride between 288 and 480 mg/day in patients with severe Menière’s disease: a case seriesEur Arch Otorhinolaryngol20112681237124021626121
- GauthierSJubyAMorelliLRehelBSchecterRA large, naturalistic, community-based study of rivastigmine in mild-to-moderate AD: the EXTEND StudyCurr Med Res Opin2006222251226517076986
- von ElmEAltmanDGEggerMPocockSJGøtzschePCVandenbrouckeJPThe Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studiesPLoS One20074e296
- AdrionCMansmannUBayesian model selection techniques as decision support for shaping a statistical analysis plan of a clinical trial: an example from a vertigo phase III study with longitudinal count data as primary endpointBMC Med Res Methodol20121213722962944
- Jeck-TholeSWagnerWBetahistine: a retrospective synopsis of safety dataDrug Saf2006291049105917061910
