Abstract
Neuropeptide Y (NPY), colocalized with norepinephrine neuron, is known to modulate sympathetic activity and feeding behavior. Although experimental type 1 diabetes has increased sympathetic activity at the early part of the disease process, little effort was made so far to understand the correlation between NPY level in the hypothalamus and sympathetic activity in diabetes. Male Sprague Dawley rats were made diabetic by a single injection of streptozotocin (65 mg/kg body weight, IV). The animals were then studied after 2, 4, and 8 weeks. Control animals received only citrate vehicle. In an effort to clarify the modulatory effect of NPY at the early stage of diabetes, the paraventricular nucleus (PVN) of hypothalamus was sampled by microdialysis for NPY and norepinephrine level. While NPY level was increased immediately within 2 weeks (along with feeding behavior), norepinephrine level was increased only after 8 weeks following injection of streptozotocin. The animals lost significant weight. These results are interpreted to mean that a strong correlation exists between the feeding behavior and NPY level in PVN. Since NPY is known to inhibit sympathetic activity it is possible that NPY receptors are down-regulated following diabetes. The higher level of norepinephrine indicating higher sympathetic activity did not allow the animals to gain weight. In addition, controversy exists regarding pleiotropic activities of NPY related to the feeding behavior of these animals.
Introduction
The hypothalamus controls many organs and bodily functions in health and disease, including diabetes.Citation1,Citation2 The paraventricular nucleus (PVN) produces neuropeptide Y (NPY) which is colocalized with catecholamines.Citation3,Citation4 NPY is a peptide involved in the regulation of body weight, energy balance, and sympathetic tone and its action is believed to be mediated through another peptide, leptin.Citation5–Citation7 Leptin is a circulating hormone which is produced by adipocytes and its plasma level is increased in obese individuals and type 2 diabetes. It has been shown earlier that normally NPY activity is suppressed by leptin, and the NPY-leptin axis is involved in the regulation of food intake, energy expenditure, and the development of obesity in type 2 diabetes via decreased sympathetic activity.Citation5–Citation7
In type 1 diabetes, in which the defect lies in the availability of insulin, the exact role of NPY on sympathetic activity is not known. Apparently in experimental type 1 diabetes the animals failed to gain weight in spite of the fact that the NPY concentration in the hypothalamic regions was increased.Citation8–Citation10 The question therefore arises as to whether or not NPY-leptin axis or NPY-sympathetic activity may provide some insight into overall regulation of food intake. Since insulin (or leptin) is known to inhibit NPY synthesis and increase sympathetic activity,Citation7 PVN appears to be overactive in insulin-deficient diabetic rats and could contribute to the compensatory hyperphagia and reduced energy expenditure. However, such hypothesis is unlikely to be true due to the presence of increased sympathetic activity observed in type 1 diabetic animals.Citation2,Citation9 As indicated early, the animals fail to gain weight although NPY levels are significantly increased in PVN. We therefore, wanted to study the levels of NPY and norepinephrine in PVN during the progression of diabetes following injection of streptozotocin which is known to produce type 1 diabetes. The aim was to correlate body weight and feeding behavior of these animals in relation to the levels of NPY and sympathetic activity. We did not attempt to evaluate the NPY-leptin axis since sympathetic activity has been shown to increase (and not decrease) after 8 weeks of streptozotocin injection.
Methods
Male Sprague-Dawley rats (175–200 g) of the same age were rendered diabetic by a single intrafemoral vein injection of streptozotocin (65 mg/kg body weight) dissolved in a citrate- buffered vehicle (pH 4.5). Age-matched normal rats injected with citrate buffer alone were used as controls. All rats were provided with food and water ad libitum during the entire experiment.
The diabetic animals were subdivided randomly into three groups. The first group of diabetic animals was sacrificed 2, 4 and 8 weeks after streptozotocin injection; the control animals were matched accordingly. The animals in the second group were injected with 3 U protamine zinc insulin/day subcutaneously for the last 4 weeks before sacrifice at 8 weeks.
Plasma samples were analyzed for blood glucose and insulin. The research protocol was approved by the Animal Care Committee at the Arabian Gulf University and the entire research was carried out according to the guidelines for ethical use/handling of small animals.
To collect the extracellular fluid from PVN, microdialysis experiments were carried out as described earlier.Citation11 The animals were anesthetized by intramuscular injection of ketamine xylazine mixture (40 mg ketamine and 4 mg of xylazine) and a microdialysis probe was inserted under the guidance of a stereotaxic instrument. The microdialysates were collected after one hour and then sent to clinical chemistry for the levels of NPY and norepinephrine to be measured by a high pressure liquid chromatography system as described in detail earlier.Citation11,Citation12 The animals were sacrificed after the microdialysis experiments.
The results are expressed as mean ± SEM and analyzed statistically by factorial analysis of variance. All groups were analyzed statistically with post hoc testing using Scheffe’s procedure. A P value of less than 0.05 was taken to reflect a significant difference between two groups.
Results
shows the general characteristic features of the different experimental groups. As expected, following streptozotocin injection, the mean body weight of this group was significantly lower after 8 weeks as compared to the control group. Severe hyperglycemia in the presence of significantly depressed plasma insulin levels was also observed. Food consumption on the other hand, was increased in diabetic animals. These characteristics are similar to those reported earlier.Citation1,Citation2 Daily injection for 2 weeks with 3 U insulin to the 4-week diabetic animals partly reversed the observed changes ().
Table 1 General characteristics of experimental animals (8 weeks study)
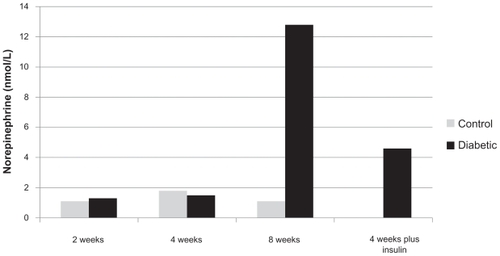
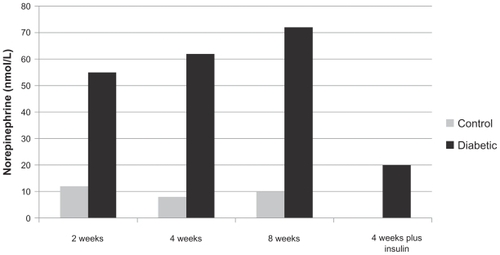
and show the extracellular content of norepinephrine and NPY in PVN of the hypothalamus measured after microdialysis sampling at different stages of diabetes, respectively. While NPY level was significantly increased following the development of diabetes (), norepinephrine concentration did not increase till 8 weeks following streptozotocin injection (). Norepineprine concentration (nmol/L) was 12.8 ± 1.5 after 8 weeks of diabetes as compared to 1.1 ± 0.4 in control animals. Such increase was statistically significant (P value was less than 0.05). Four weeks of diabetes plus insulin treatment reduced the norepinephrine level to 4.6 ± 0.7. The increased level of norepinephrine strengthens the view that increased sympathetic activity occurs (including cardiomyopathy) in diabetes after 8 weeks of study.Citation1,Citation2 On the other hand, the NPY level was immediately increased following diabetes (control 12 ± 1; two weeks after diabetes, 55 ± 8). The results were also statistically significant as the P value was less than 0.05. further indicates that eight weeks following diabetes, the NPY level continued to increase (72 ± 12). These results are unlikely to be artifactual in nature as insulin treatment partly reversed the levels (20 ± 3).
Discussion
It has been shown earlier that NPY is involved in the regulation of body weight, energy balance and sympathetic tone. Egawa et alCitation13 have shown that central administration of NPY affects the sympathetic activity to interscapular brown adipose tissue and the suppressive effect of NPY is quite predominant. It is therefore generally agreed that NPY is a neurochemical modulator of the sympathetic nervous system which controls energy expenditure. NPY exerts its major feeding effect in PVN of the hypothalamus which receives a dense NPY/agouti-related peptide projection from the arcuate nucleus. Since insulin (and leptin) inhibits the synthesis of NPY, it is believed that in obesity and noninsulin diabetes (type 2 diabetes), the hypothalamus is resistant to inhibition by insulin (and leptin) and peptide projection in arcuate nucleus-PVN is stimulated leading to a rise in NPY level, hyperphagia, sympathetic inhibition, reduced energy expenditure, and enhanced weight gain.Citation8
Our results are significant because the animals (type 1 diabetes) behaved in a completely different way to previous studies.Citation14–Citation16 Whereas NPY level was increased immediately, the sympathetic activity did not decrease after injection of streptozotocin. As a matter of fact sympathetic activity as measured by the level of NE in PVN was increased significantly at a later stage of diabetes.
We have also reported earlierCitation1,Citation2 that 8-weeks diabetic rats had higher sympathetic tone based on NE content, turnover, synthesis, and release. Although higher sympathetic activity supports increased energy expenditure and weight loss in our model, it failed to resolve the issue as to why the sympathetic activity would be increased in the presence of higher NPY level. However, based on our previous reports it is very likely that NPY receptors are down-regulated following the development of diabetes and it failed to elicit inhibition as is often found in hypertension and congestive heart failure.Citation12
There is now compelling evidence supporting a role of insulin in regulation of the sympathetic nervous system. For example, infusion of insulin during euglycemic clamping significantly increases plasma catecholamine concentrations and regional ‘spillover’.Citation17,Citation18 Such behavior in sympathetic activity is likely to happen in type 2 diabetes where the availability or production of insulin is usually unaffected. However, the data presented here support the view that the central mechanism involved in appetite, body weight, and sympathetic regulation differs in type 1 (insulin dependent) diabetes. The sympathetic system is increased after a brief period and is directly correlated with the NPY level in the hypothalamus, with body weight, and feeding behavior. It is certainly interesting to know if there is a down-regulation of NPY receptors following streptozotocin-induced diabetes mellitus. As indicated earlier, we have shown that NPY receptors are down-regulated in situations where overproduction of norepinephrine exists such as in congestive heart failure.Citation12
In conclusion, we strongly believe that the role of NPY in different types of diabetes varies. In the presence of a high level of NPY, type 1 (insulin dependent) diabetes is associated with increased sympathetic tone, increased energy expenditure, and weight loss. Type 2 (insulin independent) diabetes on the other hand may mimic obesity due to decreased sympathetic tone induced directly by a higher level of NPY. The role of leptin should also be considered when the hypothalamic regulation of energy balance is evaluated, particularly in situations such as type 2 diabetes.
Acknowledgment
The author acknowledges the Arabian Gulf University for providing all the facilities to carry out the experiments. Microdialysis instruments were kindly provided and donated by Prof Grant N Pierce, Executive Director, St. Boniface General Hospital Research Centre, Winnipeg, Canada.
Disclosure
The author discloses no conflicts of interest.
References
- GangulyPKPierceGNDhallaKSDhallaNSDefective sarcoplasmic reticular calcium transport in diabetesAm J Physiol1983224E528E5356134470
- GangulyPKDhallaKSInnesIRBeamishREDhallaNSAltered norepinephrine turnover and metabolism in diabetic cardiomyopathyCirc Res1986596846933815759
- WooNDMukherjeeKGangulyPKNorepinephrine levels in the paraventricular nucleus of spontaneously hypertensive rats: role of neuropeptide YAm J Physiol1993265H893H8988214124
- WooNDSahaiAAndersonWAGangulyPKModulation of sympathetic activity by brain neuropeptide in cardiac hypertrophyAm Heart J1991122102810341833961
- VelkoskaEColeTJMorrisMJEarly dietary intervention: long-term effects on blood pressure, brain neuropeptide Y, and adiposity markersAm J Physiol2005288E1236E1243
- ZukowskaZPonsJLeeEWLiLNeuropeptide Y: a mediator linking sympathetic nerves, blood vessels and immune system?Can J Physiol Pharmacol200381899412710520
- FrankishHMDrydenSHopkinsDWangQWilliamsGNeuropeptide Y, the hypothalamus, and diabetes: insights into the central control of metabolismPeptides1995167577717479313
- BerceloABarbeFLiompartENeuropeptide Y and leptin in patients with obstructive sleep apnea syndrome: role of obesityAm J Resp Crit Care Med200517118318715516536
- GangulyPKBeamishREDhallaKSInnesIRDhallaNSNorepinephrine storage, distribution and release in diabetic cardiomyopathyAm J Physiol1987252E734E7393296780
- SahuASninskyCAKalraPSKalraSPNeuropeptide-Y concentration in microdissected hypothalamic regions and in vitro release from the medial basal hypothalamus – preoptic area of streptozotocin-diabetic rats with or without insulin substitution therapyEndocrinology19901261921982136723
- KirouacGJGangulyPKCholecystokinin induced release of dopamine in the nucleus accumbens of the spontaneously hypertensive ratBrain Res19956892452537583328
- BasuSSinhaSKShaoQGangulyPKDhallaNSNeuropeptide Y modulation of sympathetic activity in myocardial infarctionJ Am Coll Cardiol199627179618038636570
- EgawaMYoshimatsuHBrayGANeuropeptide Y suppresses sympathetic activity to interscapular brown adipose tissue in ratsAm J Physiol1991260R328R3341996720
- GangulyPKPierceGNDhallaNSDiabetic cardiomyopathy: membrane dysfunction and therapeutic strategiesJ Appl Cardiol19872323338
- GangulyPKNeuropeptide receptors: future therapeutic target in congestive heart failureJ Health Sci200046430433
- GangulyPKRiceKMPanagiaVDhallaNSSarcolemmal phosphatidylethanolamine N-methylation in diabetic cardiomyopathyCirc Res1984555045126478554
- TackCJJLendersJWMWillemsenJJInsulin stimulates epinephrine release under euglycemic conditions in humanMetabolism1998472432499500557
- PatelJNCoppackSWGoldsteinDSMilesJMEisenhoferGNorepinephrine spillover in forearm and subcutaneous adipose tissue before and after eatingJ Clin Endocrin Metabol20028733733377