Abstract
Purpose
Rapid development of nanotechnology has recently brought significant attention to the extraordinary biological features of nanomaterials. The objective of the present investigation was to evaluate morphological characteristics of the assembles of gold and platinum nanoparticles (nano-Au and nano-Pt respectively), with Salmonella Enteritidis (Gram-negative) and Listeria monocytogenes (Gram-positive), to reveal possibilities of constructing bacteria-nanoparticle vehicles.
Methods
Hydrocolloids of nano-Au or nano-Pt were added to two bacteria suspensions in the following order: nano-Au + Salmonella Enteritidis; nano-Au + Listeria monocytogenes; nano-Pt + Salmonella Enteritidis; nano-Pt + Listeria monocytogenes. Samples were inspected by transmission electron microscope.
Results
Visualization of morphological interaction between nano-Au and Salmonella Enteritidis and Listeria monocytogenes, showed that nano-Au were aggregated within flagella or biofilm network and did not penetrate the bacterial cell. The analysis of morphological effects of interaction of nano-Pt with bacteria revealed that nano-Pt entered cells of Listeria monocytogenes and were removed from the cells. In the case of Salmonella Enteritidis, nano-Pt were seen inside bacteria cells, probably bound to DNA and partly left bacterial cells. After washing and centrifugation, some of the nano-Pt-DNA complexes were observed within Salmonella Enteritidis.
Conclusion
The results indicate that the bacteria could be used as a vehicle to deliver nano-Pt to specific points in the body.
Introduction
There is an urgent need for new, effective drug delivery systems. In the last few decades, nanotechnology has been increasingly employed in searching for methods to transport drugs to certain organs within the human body. Transporting systems able to transmit cell-insoluble drugs into cells; non-toxic for the surroundings; and with time-prolonged releasing of active substances, have remarkable potential for improving cancer therapy efficacy.Citation1
Several bacteria isolates may be applied for possible new drug delivery mechanisms. Their unique features including: potential to move; intracellular survival; and ability to settle in precisely programmed points of the body, allow the use of bacteria as specific medicine carriers.Citation2 However, the following has still to be elucidated: how can bacteria be enforced to ingest medicines; how can uncontrolled proliferation and distribution of bacteria be avoided within a host organism; and can nanoparticles be used as drugs, or links between drugs and bacteria?
Nanoparticles derived from noble metals are recognized as being non-genotoxic for the organism.Citation3 Moreover, gold and silver nanoparticles did not reveal proinflammatory activityCitation4 and even antiangiogenic properties of Au nanoparticles (nano-Au) were observed.Citation5 Nano-Au have been identified as promising candidates for many biological and biomedical applications.Citation6 They have been widely used as an inert agent for cell imaging, targeted drug delivery, cancer diagnostics, and various therapeutic applications, due to their small sizes, convenience in bioconjugation, and strong absorbing and scattering properties.Citation7–Citation10 They entrap small drugs, or biopharmaceutical agents such as therapeutic proteins and DNA, and can be designed to release these agents at the target site.
Platinum nanoparticles (nano-Pt) are of great interest due to their exceptional catalytic activity, which is affected by the ligands at their external surface. Nano-Pt may display multiple functional groups at the surface, which can be hydrophilic, lipophilic, and chemically reactive.Citation11 Some of the Pt compounds are used as very effective anticancer agents. This property is associated with the inhibition of DNA replication and mitosis by the addition of nano-Pt to DNA strand.Citation12 On the other side, research of Hikosaka et alCitation13 has revealed that nano-Pt have a nicotinamide adenine dinucleotide (NADH): ubiquinone oxidoreductase-like activity. This finding suggests that nano-Pt can be used as a remedy for oxidative stress diseases with suppressed mitochondrial complex I.Citation13 Affinity of nano-Pt to DNA strand can also support their activity as carriers locating medicine within bacteria.
Salmonella Enteritidis is a motile, gram-negative (G−), rod-shaped bacterium belonging to the Enterobacteriaceae family. It is adapted to a wide range of hosts. Through flagella, this bacterium can spread from the intestine to the blood and lymph. It proliferates within eukaryotic cells. Recent work has shown that Salmonella Enteritidis reside within macrophages.Citation14
Listeria monocytogenes is a gram-positive (G+), food-borne pathogenic bacterium, which possesses the ability to be internalized by macrophages and non-phagocytic cells, including epithelial cells, endothelial cells, and hepatocytes. It enters the cell using advanced mechanisms, proliferates intracellularly and spreads without contact with the extracellular medium, using elongated protrusions.Citation15
Methods to deliver functional nano-molecules to specific targets within the body still remain to be investigated. In addition, the impact of noble metals’ nanoparticles on bacteria’s cells is still unclear. Considering physical and chemical properties of gold (Au) and platinum (Pt) nanoparticles and biological properties of Salmonella Enteritidis and Listeria monocytogenes, it is important to evaluate results of their self-organization. Consequently, the objective of the present investigation was to evaluate morphological characteristics of the assembles of nano-Au and nano-Pt with G+ and G− bacteria, to reveal possibilities of constructing bacteria- nanoparticle vehicles.
Methods
Nanoparticles
Hydrocolloids of nano-Au and nano-Pt were obtained from Nano-Tech Polska (Warsaw, Poland). They were produced by a patented electric non-explosive method (Polish patent 380649) from high purity metals (99.9999%) and high purity demineralized water. Both hydrocolloids had a concentration of 50 ppm. Shape and size of nanoparticles were inspected using a JEM-1220 transmission electron microscope (TEM) at 80 KeV (JEOL, Tokyo, Japan), with a Morada 11 mega-pixel camera (Olympus Soft Imaging Solutions GmbH, Münster, Germany) (). Diameter of particles ranged from 2–29 nm for Au and from 2–19 nm for Pt. Samples of Au and Pt for TEM were prepared by placing droplets of hydrocolloids on to formvar-coated copper grids (Agar Scientific Ltd., Stansted, UK). Immediately after drying of the droplets in dry air, grids were inserted into the TEM.
Bacteria
Bacteria strains (Salmonella enterica subspecies enterica serovar Enteritidis ATCC® 13076™ and Listeria monocytogenes ATCC 19111) were obtained from LGC Standards (Lomianki, Poland). Salmonella Enteritidis were grown on nutritive 2.8% agar (Bio-Rad, Hercules, CA, USA). Listeria monocytogenes were grown on brain heart infusion agar (Bio-Rad, Hercules, CA, USA) for 24 hours at 37°C. Bacteria were gently washed out from the agar using sterile distilled water. To remove liquid remains of the medium, bacteria were centrifuged at 4000 rpm for 5 min on an Eppendorf MiniSpin® centrifuge (Eppendorf, Hamburg, Germany) and the sediment (pellet) was re-suspended in Milli-Q water (Millipore, Billerica, MA, USA). To calculate the exact number of bacteria in the suspension, the optical density at 600 nm (OD600) was estimated using a spectrophotometer Helios Epsilon unicam (USA) and serial dilutions of the suspension were prepared. One milliliter of each dilution was transferred to petri dishes with agar. After 24 hours incubation at 37°C, the number of colonies was estimated. Based on the results of measurements carried out in triplicate, the density of bacteria suspension OD600 = 0.130 corresponded to 2.2 × 108 cfu.mL−1; and OD600 = 0.133 corresponded to 4 × 108 cfu.mL−1, for Salmonella Enteritidis and Listeria monocytogenes respectively.
Preparation of samples and microscopy
Experiment 1
Hydrocolloids of nano-Au or nano-Pt (200 μL) were added to two bacteria suspensions (200 μL) in the following order: nano-Au + Salmonella Enteritidis; nano-Au + Listeria monocytogenes; nano-Pt + Salmonella Enteritidis; nano-Pt + Listeria monocytogenes. Control samples of bacteria were treated with Milli-Q water. The samples were gently mixed for 15 min and then droplets of the samples were put on formvar-coated 300 mesh copper grids (Agar Scientific Ltd., Stansted, UK). The samples were dried at room temperature, under sterile conditions, and inspected using a JEM-2000EX TEM (JEOL, Tokyo, Japan).
Experiment 2
Hydrocolloids of nano-Pt (200 μL) were added to Salmonella Enteritidis suspension (200 μL). To remove unattached nanoparticles, the samples were gently mixed for 15 min and then centrifuged at 4000 rpm for 5 min, washed with ultrapure Milli-Q water, centrifuged at 4000 rpm for 5 min, and re-suspended in Milli-Q water. Samples for microscopic evaluation were prepared using the methods described above. Control samples of bacteria not treated with nanoparticles were prepared using the same methods.
Experiment 3
In the third experiment, we wanted to confirm that the substance realized from Salmonella Enteritidis, after nano-Pt treatment, was DNA. Furthermore, the viability of bacteria after incubation with nano-Pt was determined. Bacteria suspended in phosphate buffering solution (PBS) were divided into the control sample, to which 0.5 mL of Milli-Q water was added, and four samples, to which 0.5 mL of colloidal nano-Pt was added. Samples were incubated for 45, 60, and 90 min and then centrifuged at 5000 rpm for 5 min. The supernatant was discarded and the remaining pellet containing bacteria was suspended in 0.5 mL solution with the fluorescent marker DAPI (4′,6-diamidyno-2-fenyloindol) and PBS in the proportion 1 μL DAPI to 1 mL PBS. Bacteria with the fluorescent dye were incubated for 15 min. After this time, to remove overdose of the dye, samples were centrifuged (5000 rpm for 5 min) and the obtained pellet was suspended in 200 μL PBS. The control was a suspension of bacteria in 200 μL of PBS, subjected to all stages of the experiment, except the incubation with nano-Pt. The resulting suspension was thoroughly stirred and one drop was placed on a microscope slide and immediately covered with a coverslip. The resulting preparations were observed at 300x magnification with an Olympus FV1000 confocal microscope, with FV10-ASW software version 1.4 (Olympus Corporation, Tokyo, Japan). The preparations were observed at the excitation wave length of 405 nm and in the Nomarski contrast. The viability of Salmonella Enteritidis was also measured. Samples of Salmonella Enteritidis, prepared as in experiment 1, were incubated with hydrocolloids of nano-Pt (1:1) and with Milli-Q water as the control, for 45, 60, and 90 min. Samples of Salmonella Enteritidis were then spread and grown on nutritive 2.8% agar (Bio-Rad, Hercules, CA, USA), for 24 hours, at 37°C and bacterial colonies were counted.
Results
Experiment 1
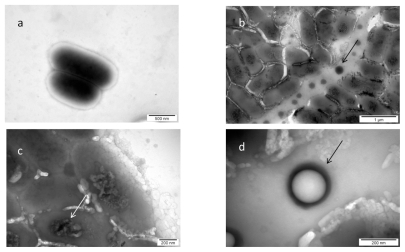
Examination of the control bacteria indicated some changes caused by the used methods (centrifugation, Milli-Q water, drying), but did not show any toxicological or destructive alteration of bacteria. Morphology of interaction between nano-Au and Salmonella Enteritidis is presented in . Almost all of nano-Au were aggregated and removed from bacterial cells. The aggregates were trapped within flagella network and biofilm. Moreover, some bacteria cells were destroyed and their cell walls were disrupted. The cells were malformed, had shrunk, and a loss of cytoplasm was observed. Interestingly, nano-Au was trapped within the flagella-biofilm network and kept at some distance from bacteria.
Figure 2 TEM image of Salmonella Enteritidis and gold (Au) nanoparticles: a) control; b, c, and d) Salmonella Enteritidis with Au nanoparticles. Arrows point to nano-Au.
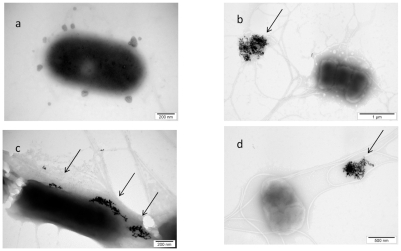
The results of the morphological interaction of gold nanoparticles with Listeria monocytogenes also showed that nano-Au were aggregated and non-specifically placed on the cell wall (). The cell wall was disintegrated and the cytoplasmic membrane was disrupted. Thereafter, nano-Au were entirely surrounded by biofilm and removed from bacteria.
Figure 3 TEM image of Listeria monocytogenes and gold (Au) nanoparticles: a) control; b, c, and d) Listeria monocytogenes with Au nanoparticles. Arrows point to nano-Au (b and d); and to aglomerate of nano-Au (c).
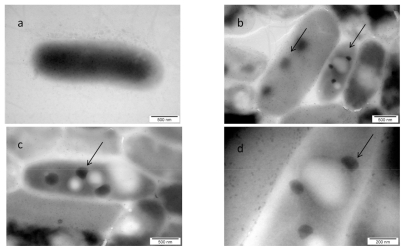
The visualization of the interaction between nano-Pt and Salmonella Enteritidis is shown in . The picture shows that nano-Pt disintegrated bacteria cell walls and cytoplasmatic membranes; moreover, they entered bacteria and were seen as intensively black spots within the cells. Nano-Pt were located specifically, probably attached to some intracellular compounds (DNA), but under microscope electron beam, these black spots evaporated becoming white ().
Figure 4 TEM image of Salmonella Enteritidis and platinum (Pt) nanoparticles: a) control; b, c, and d) the same cell of Salmonella Enteritidis with Pt nanoparticles. Arrows point to nano-Pt (b); and the spots observed with increasing magnification (b, c, and d). Black spots are seen evaporating under the microscope electron beam and are finally seen as white spots.
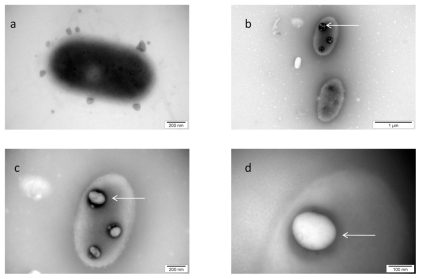
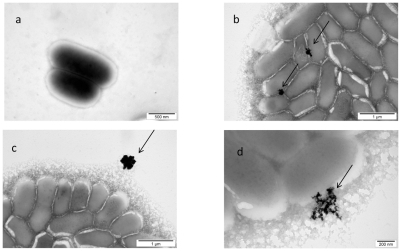
The image of interaction between nano-Pt and Listeria monocytogenes, shown in , indicated that nano-Pt are destructive to these bacteria. The influence of nano-Pt on Listeria monocytogenes clearly pointed out that nano-Pt were seen within bacteria, moreover, they were located close to DNA and probably bound to it. Interestingly, these new molecules (nano-Pt with DNA) were removed or escaped from the bacteria cells. The cell wall and cytoplasmatic membrane were disintegrated and cytoplasm was lost. As in the case of Salmonella Enteritidis, nano-Pt evaporated under the electron beam.
Experiment 2
This experiment was performed to elucidate to what extent nanoparticles, identified from morphological pictures in experiment one, as attached or being inside bacteria, were truly attached or were stably anchored bacteria cells. To remove unattached particles, mixtures with bacteria were centrifuged and washed. For further examination, Salmonella Enteritidis was chosen as a better candidate for bacteria-nanoparticle molecules construction. It was revealed that platinum complexes seen as intensively black spots were still within the bacteria ().
Experiment 3
This experiment was performed to confirm that nano-Pt is actually removing DNA from Salmonella Enteritidis, and whether this process is time-dependent. In this experiment, the average number of bacteria observed by the confocal microscope in the Nomar-ski contrast and the average number of bacteria labelled with DAPI (DNA-binding fluorochrome) were determined. The average number of bacteria cells () seen as a cell wall image observed with the Nomar- ski contrast (), after incubation for 45, 60, and 90 min with nano-Pt, did not change. However, the number of cells containing DNA (seen as a blue) was reduced after 60 and 90 min. Viability of Salmonella Enteritidis was also decreased and after 60 and 90 min of incubation, was about one quarter of that measured in the control condition ().
Figure 7 Confocal microscope image of Salmonella Enteritidis: 1) control; 2, 3, and 4) after incubation with platinum nanoparticles for 45, 60, and 90 min respectively. a) Nomarski contrast; b) stained with DAPI; and c) merged pictures.
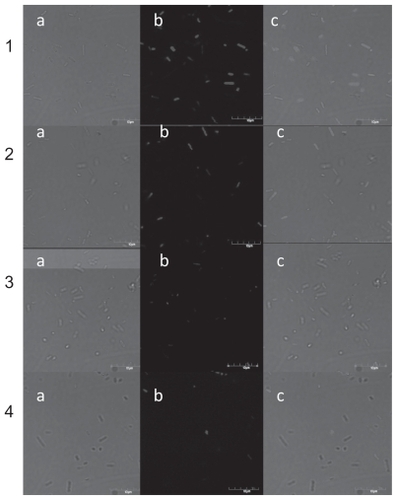
Table 1 Average number and viability of Salmonella Enteritidis incubated with nano-Pt for 45, 60, and 90 min. DNA visualization was performed by labelling bacteria with DAPI (DNA-binding fluorochrome)
Discussion
The purpose of the present study was to identify possibilities of using bacteria as a vehicle for transporting nanoparticles and/or nanoparticle-drug molecules. We focused on possibilities of placing nanoparticles of Au and Pt within bacteria, using a simple mechanism of self-organization, but without destroying cell walls of bacteria. The biological action of nanoparticles on mammalian and bacterial cells is highly dependent on their functional groups present on the surface of nanoparticles, which may determine their toxicity.Citation16,Citation17 To avoid influence of the additional chemical groups which surround nanoparticles and to determine chemical properties of nanoparticles, we used nano-Au and nano-Pt produced in high purity water by electric methods (the same for Au and Pt). Consequently, only hydrogen ion (H+) or hydroxide ion (OH−) groups could be present on the nanoparticle surface.
The structure of the cell wall of G+ Listeria monocytogenes and G- Salmonella Enteritidis is quite different. Listeria monocytogenes has a thick (20–80 nm), continuous cell wall composed of highly cross-linked layers of peptidoglycan, covalently bound to teichoic acids, while the Salmonella Enteritidis cell wall is thin (5–10 nm) but more complex. Peptidoglycan is surrounded by an outer membrane containing lipolysaccharide and also by some non-specific porins, which can potentially transport smaller nanoparticles (around 1 nm). Interestingly, in spite of significant differences in cell walls structure, the self-organization between nanoparticles and bacteria was affected by the type of bacteria to a lesser degree than by the type of nanoparticles.
In our studies, nano-Au formed aggregates trapped within flagella network, when examined with Salmonella Enteritidis (). The pictures might indicate that this process is initiated by fimbria network creation, which probably promotes the development of biofilm.Citation18 Formations of biofilm by Salmonella Enteritidis increase colonization, but also protect bacteria cells,Citation19 which in this case could be a system of protection against nanoparticles. The formation of nano-Au aggregates kept the nanoparticles away from the bacteria cells, which might be more safe for the bacteria but unsuitable for constructing a bacteria-nanoparticle drug delivery system. Listeria monocytogenes also produced biofilm, but mainly showed abilities to form colonies, making it impossible to observe single cells as it was in the case of Salmonella Enteritidis (). Probably, building a multi-cells structure was, for Listeria monocytogenes, a form of protection against nano-Au, but make it difficult to use Listeria monocytogenes as a drug delivery vehicle.
Nanoparticles of Au were mainly placed outside bacteria (), however, some aggregates, containing small (about 5–10 nm) particles of nano-Au, were non-specifically placed on the surface of both bacteria. Bacterial cell walls and cytoplasm membrane in both bacteria were disintegrated, but nano-Au were not seen inside the cells. Pan et alCitation20 observed that particles of 1–2 nm were highly toxic and that nanoparticles larger than 15 nm were not toxic. Consequently, the possibility that the smallest (about 1 nm) nanoparticles could enter the cells could be considered. However, in the present experiment, it was not possible to observe such small nanoparticles.
In the present experiment, the visualization of the association between nano-Pt and bacteria indicated significant effects of nano-Pt on both Salmonella Enteritidis and Listeria monocytogenes. Cell wall and cytoplasmic membrane of both bacteria were disintegrated, however, Listeria monocytogenes, like in the case of nano-Au, created colonies, probably as a defence against nano-Pt. Interestingly, Salmonella Enteritidis did not demonstrate the mechanism of fimbria-biofilm production, which was seen as a remedy against nano-Au. Moreover, we noticed that the cell wall and cytoplasmic membrane of both microorganisms were permeable for nano-Pt, which could be seen as intensively black spots, being black even when microscopic pictures were lightened and not existing in the control bacteria. Moreover, the present pictures () showed that nano-Pt was bound to specific intracellular compounds; probably DNA. Our results are in agreement with previous studies,Citation21 revealing that platinum demonstrates effective affinity to DNA strand. These properties may be utilized for using nano-Pt conjugates to inhibit DNA replication, by binding to its strand and therefore acting as potential anticancer agents. The antioxidant activity of platinum complexes, which can penetrate the nucleus membrane and enter the nucleus, has recently gained attention due to its potential for the treatment of a variety of cancers.Citation22 For example, cisplatin and carboplatin are used as chemotherapeutic agents for ovarian and testicular cancer.Citation23 It is well known that in cancer therapy, tissue-unspecific drug application is a key problem. The possibility to locate nano-Pt inside bacteria ghost, being a locomotive force, could provide target-specific drug delivery. Previously, bacterial cells as ghosts were used as a very promising drug delivery system in a study with Escherichia coli.Citation2
In the case of Listeria monocytogenes, we observed that nano-Pt-DNA molecules (as intensively black spots) were seen inside the cells, but the majority was placed outside the cells. It is therefore likely that nano-Pt, after binding with DNA, was removed from Listeria monocytogenes (). In the case of Salmonella Enteritidis, the association of nano-Pt and DNA was also visualized as dark spots (), but seen rather within bacteria cells. Interestingly, these spots were disappearing under the microscope electron beam, especially when magnification was increasing. In Salmonella Enteritidis, in contrast to Listeria monocytogenes, the spots were mostly inside the bacterial cells and only a few were removed from bacteria cells.
Considering the results from the first experiment, only Salmonella Enteritidis and nano-Pt were chosen in the second experiment, because only nano-Pt was undoubtedly seen within Salmonella Enteritidis, and did not flow out from the cells. In this experiment, Salmonella Enteritidis were mixed with nano-Pt and then centrifuged and washed to remove nanoparticles being unbound or unattached to bacteria’s structures. As we supposed, dark spots placed within bacteria could be observed (). However, only a part of bacterial cells still had the spots while the other bacterial bodies were empty.
In experiment 3, we confirmed our hypothesis that after treatment with nano-Pt, DNA is removed from cells of Salmonella Enteritidis. However, after 60 min of incubation, only about half of the cells lost their DNA, moreover, this process did not increase in the following 30 min. Abilities of nano-Pt entering Salmonella cells and binding with DNA seem to be very promising for a drug delivery system, even when only part of bacterial cells contain nano-Pt-DNA molecules. A fundamental problem seems to be determination of optimal time and other conditions of incubation allowing location of nano-Pt within bacteria and binding with DNA. We have also examined viability of Salmonella Enteritidis after nano-Pt treatment, showing that after 90 min of incubation, about 25% of bacteria was still able to replicate. It can be suspected that if about half of bacteria after incubation for 60–90 min with nano-Pt still contained DNA, and about a quarter of bacteria was still alive, the other quarter of bacteria contains nano-Pt-DNA molecule, but it is not able to replicate.
Conclusion
Visualization of morphological interaction between nano-Au and Salmonella Enteritidis and Listeria monocytogenes, showed that nano-Au were aggregated within flagella or biofilm network, but were not specifically attached. In addition, nano-Au visible under the microscope did not penetrate inside the bacterial cell. The analysis of morphological effects of interaction between nano-Pt and bacteria revealed that nano-Pt entered cells of Listeria monocytogenes but were removed from the cells. In the case of Salmonella Enteritidis, nano-Pt were seen inside bacteria cells, indicating that the bacteria cells could be used as a vehicle to deliver nano-Pt, and active substances attached to Pt, to specific points in the body.
Acknowledgments
This work has been supported by grant MNiSW N31104931/3849, Poland and grant 2106-08-0025 Danish Agency for Science, Technology and Innovation.
Disclosure
The authors declare that they have no competing interests in this work.
References
- OrtegaJVigilCEChodkiewiczCCurrent progress in targeted therapy for colorectal cancerCancer Control201017171520010514
- PauknerSKohlGJalavaKLubitzWSealed bacterial ghosts – novel targeting vehicles for advances drug delivery of water-soluble substancesJ Drug Target20031115116113129825
- SawoszEGrodzikMZielińskaMNiemiecTOlszańskaBChwalibogANanoparticles of silver do not affect growth, development and DNA oxidative damage in chicken embryosArch Geflügelk200973208213
- SawoszEGrodzikMLisowskiPHydrocolloids of Ag, Au and Ag/Cu alloy nanoparticles influence inflammatory state at transcriptional levelBull Vet Inst Pulawy2010548185
- MukherjeePBhattacharyaRWangPAntiangiogenic properties of gold nanoparticlesClin Cancer Res20051193530353415867256
- SternSTMcNeilSENanotechnology safety concerns revisitedToxicol Sci200810142117602205
- GuoWDaiJZhangDZhuQBianGRedox active gold nanoparticles modified with tetrathiafulvalene derivative via direct sulfur bridgeInorganic Chem Com20058994997
- HanGGhoshPDeMRotelloVMDrug and gene delivery using gold nanoparticlesNanobiotechnol2007314045
- Schroeder-ReiterEHoubenAWannerGImmunogold labeling of chromosomes for scanning electron microscopy: a closer look at phosphorylated histone H3 in mitotic metaphase chromosomes of Hordeum vulgareChromosome Res200311658559614516067
- WangWChenCQianMZhaoXSAptamer biosensor for protein detection using gold nanoparticlesAnal Biochem200837321322118054771
- NelAXiaTMädlerLLiNToxic potential of materials at the nano-levelScience200631162262716456071
- BrookMAPlatinum in silicone breast implantsBiomaterials2006273274328616483647
- HikosakaKKimJKaijtaMKanayamaAMiyamotoYPlatinum nanoparticles have an activity similar to mitochondrial NADH:ubiquinone oxidoreductaseColloids Surf. B: Biointerfaces200866219520018653320
- LuSKilloranPBRileyLWAssociation of Salmonella enterica serovar enteritidis yafD with resistance to chicken egg albumenInfect Immun2003126734674114638758
- BraunLCossartPInteractions between Listeria monocytogenes and host mammalian cellsMicrob Infect20002803811
- GoodmanCMMcCuskerCDYilmzaTRotelloVMToxicity of gold nanoparticles functionalized with cationic and anionic side chainsBioconjugate Chem200415897900
- ConnorEEMwamukaJGoleAMurphyCJWyattMDGold nanoparticles are taken up by human cells but do not cause acute cytotoxicitySmall2005132532717193451
- AustinJWSandersGKayWWCollinsonSKThin aggregative fimbriae enhance Salmonella Enteritidis biofilm formationFEMS Microbiol Lett200616222953019627964
- HelkeDMWongACLSurvival and growth characteristics of Listeria monocytogenes and Salmonella typhimurium on stainless steel and buna-N rubberJ Food Protect19945711963968
- PanYNeussSLeifertASize-dependent cytotoxicity of gold nanoparticlesSmall200731941194917963284
- HoppstockKSuresBMerianEAnkeMIhnatMStoepplerMPlatinum-group metalsElements and their Compounds in the Environment: Occurrence, Analysis and Biological RelevanceWeinheimWiley200410471086
- BeckmanKBAmesBNOxidative decay of DNAJ Biol Chem199727219633196369289489
- McBrienDCHSlaterTFBiochemical mechanisms of platinum antitumour drugsOxfordIRL Press1986