Abstract
Background and objectives
Antibiotic resistance has emerged as one of the most important determinants of outcome in patients with serious infections, along with the virulence of the underlying pathogen. Photodynamic antimicrobial chemotherapy (PACT) has been proposed as an alternative approach for the inactivation of bacteria. This study aims to evaluate the antibacterial effect of sinoporphyrin sodium (DVDMS)-mediated PACT on Staphylococcus aureus and multidrug resistant S. aureus in vitro and in vivo.
Materials and methods
Bacteria were incubated with DVDMS and exposed to treatment with light. After PACT treatment, colony-forming units were counted to estimate the bactericidal effect. Intracellular reactive oxygen-species production was detected by flow cytometry. Flow cytometry and fluorescence-microscopy detection of bacterial cell-membrane permeability. Enzyme-linked immunosorbent assays were used to determine expression of VEGF, TGFβ1, TNFα, IL6, and bFGF factors in burn infection.
Results
DVDMS-PACT effectively killed bacterial proliferation. Intracellular ROS levels were enhanced obviously in the PACT-treatment group. SYTO 9 and propidium iodide staining showed a decrease in the ratio of green:red fluorescence intensity in the PACT-treatment group in comparison to the control group. Enzyme-linked immunosorbent-assay results revealed that in the healing process, the expression of bFGF, TGFβ1, and VEGF in the treatment group were higher than in the control group, which inhibited inflammation-factor secretion. In addition, skin-tissue bacteria were reduced after treatment.
Conclusion
These results indicate that DVDMS-PACT presents significant bactericidal activity and promotes wound healing after burn infections.
Introduction
Burns are a global public health problem, especially in undeveloped countries that lack adequate medical facilities, in terms of morbidity, long-term disability, and mortality.Citation1,Citation2 Wound infection is one of the most common complications after severe trauma, burn, or surgery, and it prolongs hospitalization, causes significant morbidity, and expends a considerable amount of medical resources. A previous study reported that a reduction in overall wound-infection rate from 4.42% to 2.5% at a single medical center over a 10-year period saved approximately $3 million in hospital costs.Citation3–Citation5 Exposure to hot water is one of the most frequent causes of burns.Citation6 All wounds will have some bacterial colonization. Staphylococcus aureus bacteria are early colonizers, and account for the majority of burn-wound infections; moreover, they are responsible for many other skin and soft-tissue infections in humans, including impetigo, folliculitis, cellulitis, and infected ulcers.Citation7–Citation9 More concerning is the fact that S. aureus skin infection can progress to invasive and life-threatening infections, such as bacteremia, abscesses, pneumonia, and sepsis.Citation10,Citation11 Several therapeutic strategies are used to combat infection by S. aureus, including antibiotic-based treatment, antibiotic-free treatments, immunotherapy, therapeutic vaccines, and occasionally combinations of these options.Citation12
The widespread use of antibiotics has resulted in a growing problem of antimicrobial resistance in community and hospital settings. Antimicrobial classes for which resistance have become a worrisome problem include the β-lactams, the glycopeptides, and the fluoroquinolones.Citation13 Therefore, novel antimicrobial drugs are continuously needed to counteract bacterial resistance development.Citation14 To keep up the pace of antibiotic resistance, new antibiotics, including vancomycin, linezolid, tedizolid, daptomycin, ceftaroline, and tigecycline, have been developed and introduced in recent years.Citation15,Citation16 However, the outbreak of various multidrug-resistant (MDR) S. aureus strains globally at an alarming rate resulted in treatment difficulties, which have imposed a burden on health-care systems and simultaneously intensified the need for new antimicrobial agents.Citation17 In particular, the use of topical antibiotics is controversial, since it has been suggested that such an approach induces antibiotic resistance faster than the use of oral antibiotics.Citation18,Citation19
It has been reported that silver nanoparticles are strong bactericidal agents, but they are also cytotoxic. Embedding them in a polymer matrix may reduce their cytotoxicity.Citation20 Antimicrobial nanomaterials are also available nanoporous bioglass containing silver (Z-)-4-bromo-5-(bromomethylene)-2(5H)-furanone-loaded poly(L-lactic acid) nanoparticles on microarc-oxidized titanium. These materials have good antibacterial efficiency.Citation21–Citation24 Photodynamic antimicrobial chemotherapy (PACT) is a promising method to eradicate pathogenic bacteria, because it kills these via cytotoxic reactive oxygen species (ROS). ROS are produced by the photosensitive drug after light irradiation, and inflict aspecific damage to bacteria.Citation25,Citation26 Much is already known about the photodynamic inactivation of microorganisms: both antibiotic-sensitive and -resistant strains can be successfully photoinactivated, and there is the additional advantage that repeated photosensitization of bacterial cells does not induce a selection of resistant strains.Citation27–Citation30
In our previous study, we investigated the photodynamic activity of a new photosensitive (sinoporphyrin sodium [DVDMS]). We observed that light-activated DVDMS enhanced intracellular ROS levels, which significantly induced cell death and markedly damaged S. aureus.Citation31 In this study we investigated in vivo PACT of second-degree thermal burn wounds in mice with S. aureus and MDR S. aureus infections (). We hypothesized that PACT could significantly downregulate inflammation of the infected tissues in the burns. The possible mechanism of action and clinical application were also investigated.
Figure 1 Diagram of in vivo DVDMS-PACT treatment protocol.
Notes: Burn-infected mice were randomly divided into four groups (eight wounds per group): model, 2 μM PACT, 5 μM PACT, and 10 μM PACT. The model mice did not receive any treatment. The laser was utilized with a power intensity of 300 mW/cm2. Finally, concentrations of TNFα, TGFβ1, VEGF, bFGF, IL6, and Hyp were detected using ELISA at different time points.
Abbreviations: DVDMS, sinoporphyrin sodium; ELISA, enzyme-linked immunosorbent assay; Hyp, hydroxyproline; PACT, photodynamic antimicrobial chemotherapy; ROS, reactive oxygen species.
Materials and methods
Bacterial growth
S. aureus (CMCC 26003) and MDR S. aureus (ATCC 29213) were provided by the Shaanxi Provincial Institute of Microbiology (Xi’an, China). The strains were stored at −80°C as glycerol stocks. For experiments, cultures of S. aureus were grown on trypticase soy agar (TSA; Aobox Biotechnology, Beijing, China) for 24 hours. A colony was subcultured on tryptic soy broth (Aobox Biotechnology) at 37°C overnight on a shaker incubator at 200 rpm (TS-200B; Tensuc Laboratory Instrument Manufacturing, Shanghai, China), then centrifuged for 5 minutes at 8,000 rpm and diluted with sterile 0.85% saline (pH 7.5) to a concentration of 108 CFU/mL.
Sensitizers and irradiation
DVDMS was kindly provided by Professor Qicheng Fang from the Chinese Academy of Medical Sciences (Beijing, China). It has a purity of 98.5%. It was dissolved in sterile physiological saline solutions to a final storage concentration of 1 mM and stored in the dark at −20°C. A semiconductor laser (excitation wavelength 635 nm; Ningju Photoelectric Technology, Xi’an, China) was used for PACT. Laser irradiance was measured using a radiometry system (Ningju Photoelectric Technology).
Reagents
2′,7′-Dichlorodihydrofluorescein diacetate (H2-DCF-DA) and SYTO 9 green fluorescent nucleic acid stain were obtained from Thermo Fisher Scientific (Waltham, MA, USA). Propidium iodide (PI) was obtained from the Sigma-Aldrich (St Louis, MO, USA). Malondialdehyde (MDA) and hydroxyproline (Hyp) assay kits were provided by Jiancheng Bioengineering Institute (Nanjing, China). IL6, bFGF, VEGF, TGFβ1, and TNFα enzyme-linked immunosorbent assay (ELISA) kits were purchased from Calvin Biotechnology (Suzhou, China).
Animals
Female BALB/c mice (age 5–6 weeks, 18–20 g body weight) were supplied by the Experimental Animal Center of the Fourth Military Medical University (Xi’an, China). They were housed in an air-conditioned room at 23°C±2°C with free access to food and water and maintained on a 12-hour light–dark cycle. All experiments using mice were approved by the animal care and use committee of Shaanxi Normal University (Xi’an, China). The mice were anesthetized by pentobarbital sodium (30 mg/kg of body weight) intraperitoneally for surgery and for subsequent PACT. Their back hair was removed using depilatory cream.
Uptake by S. aureus/MDR S. aureus
To substantiate the enrichment of DVDMS in S. aureus/MDR S. aureus, we examined the uptake of DVDMS by microscopy. Bacteria suspensions and DVDMS were incubated in the dark, and 500 μL of each sample was removed at every 15-minute interval. All samples were rinsed twice with PBS and then visualized under fluorescence microscopy (Axio Imager M2; Carl Zeiss Meditec, Jena, Germany).
Photodynamic treatment protocol
Suspensions of bacteria (108 CFU/mL) were incubated with 2 μM or 5 μM DVDMS in the dark for 75 minutes at 37°C, and then added to a 24-well flat-bottom plate. This 24-well plate containing bacterial suspensions was washed once by PBS before illumination, then irradiated with laser light at different light doses (10, 30, and 50 J/cm2). The temperature was kept at 37°C during irradiation.
CFU assay
CFU assays were used to measure the cytotoxicity of PACT. After photodynamic treatment, bacteria were spread on the TSA in tenfold serial dilutions. After 24-hour incubation at 37°C, bacteria were counted.
Intracellular reactive oxygen-species production
H2-DCF-DA, a nonfluorescent cell-permeant compound, is hydrolyzed by endogenous esterases within cells, and the de-esterified product can be converted into the fluorescent compound DCF upon oxidation by intracellular ROS. Fluorescence intensity is proportional to ROS production. Bacteria were coincubated with 10 μM H2-DCF-DA and 5 μM DVDMS for 75 minutes prior to PACT treatment. After PACT treatment, bacteria were washed with 0.85% saline, and the fluorescence intensity of DCF in each group was immediately analyzed using flow cytometry (NovoCyte; ACEA Biosciences, San Diego, CA, USA).
Bacteria-viability assay
The antibacterial activity of DVDMS-PACT was determined after incubation with bacteria suspensions for 75 minutes in the dark at 37°C. Then, the mixture solutions were exposed to 300 mW/cm2 light for different periods. Dyes (1:1 ratio) were added to the samples and kept in the dark for 15 minutes. The bacteria stains used were SYTO 9 and PI, which are a green fluorescent nucleic acid stain and a red fluorescent nucleic acid stain, respectively. Stained bacterial cells were visualized under fluorescence microscopy with 488–530 nm dual-band excitation-filter combination for SYTO 9 and PI simultaneously. Meanwhile, fluorescence intensity of the stained bacteria was measured at excitation/emission with fluorescein isothiocyanate for SYTO 9 and tetramethylrhodamine for PI using flow cytometry. The percentage of live cells in each group was represented by dividing the fluorescence intensity at emission 1 by the fluorescence intensity at emission 2.
DNA-fragmentation assay
DNA damage was evaluated using an easy and quantitative method that is based on flow-cytometry detection of DNA hypoploidy after adding PI to dying cells and permeabilizing them by freeze–thaw cycles.Citation32,Citation33 To investigate the effect of DVDMS-PACT on DNA damage to S. aureus, oligonucleosomal DNA fragmentation by flow cytometry was performed. Briefly, bacteria were incubated with 5 μM DVDMS for 75 minutes in the dark at 37°C, then irradiated with 50 J/cm2 of light. After treatment, bacteria were stained with 5 μg/mL PI and freeze–thawed for 30 seconds. Samples were immediately analyzed by flow cytometry.
Model establishment and material processing
Animal experiments were performed in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and approved by the animal care and use committee of Shaanxi Normal University (Xi’an, China). The mice were anesthetized with an intraperitoneal injection of pentobarbital sodium before burn creation. Burn wounds were induced by a tube with 75°C contact with the skin. The scald area was 1.5 cm-diameter circle (1.5%–2.2% of body-surface area calculated according to Meeh’s formula).Citation34 Tube contact with the skin was for 3, 6, and 9 seconds. After burn administration, the mice were resuscitated with an intraperitoneal injection of 1 mL sterile saline immediately. After 24 hours, skin-scald areas of different groups were fixed using 10% formalin for at least 24 hours. Samples were then paraffin-embedded, sectioned, and stained with H&E. Histopathological changes were observed using light microscopy (E600; Nikon, Tokyo, Japan). Bacteria (100 μL, 109/mL) were injected subcutaneously into the skin after scalding, the wound-infection model was ready after 48 hours, and the next experiment was conducted.
DVDMS-PACT in the burn-infected model
The burn-infected mice were randomly divided into four groups (eight wounds per group): control, 0.1 mL PBS; 10 μM DVDMS, 50 J/cm2 light; 5 μM DVDMS, 50 J/cm2 light; and 2 μM DVDMS, 50 J/cm2 light. Injections were administered into the scalded skin, and at 75 minutes postinjection, the mice were exposed to the indicated dose of light. Treatments were repeated the following day.
Tissue homogenates
The mice were killed at 1, 2, 3, 7, and 14 days after PACT treatment, and skin was excised immediately, rinsed in physiological saline, blotted dry, and weighted. Skin tissue (0.1 g) was homogenized in 1 mL physiological saline using a tissue grinder, followed by centrifugation at 3,000 rpm at 4°C for 10 minutes. Supernatant was prepared for detection. Levels of MDA and Hyp were determined with different kits.
Enzyme-linked immunosorbent assay
ELISA was performed as described previously.Citation35 Briefly, the stop solution changes the color from blue to yellow, and the intensity of the color is measured at 450 nm using spectrophotometry (SpectraMax M5; Molecular Devices, Sunnyvale, CA, USA). In order to measure the concentration of cytokines in the sample, this ELISA kit includes a set of calibration standards. Calibration standards are assayed at the same time as the samples, and allow the operator to produce a standard curve of optical density versus cytokine concentration. The concentration of cytokines in the samples is then determined by comparing the optical density of the samples to the standard curve.
Wound-tissue Hyp and MDA content determination
Wound-tissue Hyp and MDA in mice were evaluated by alkaline and colorimetric methods, respectively. Hyp and MDA levels were determined by Hyp and MDA kits.
Bacterial loads in skin
To determine bacterial counts in the tissue samples, 10% of the tissue homogenates were then serially diluted in PBS (1:10, 1:100, 1:1,000, 1:10,000, 1:100,000, 1:1,000,000) and plated on TSA in triplicate. Plates were then incubated for at least 18 hours at 37°C under a humidified atmosphere. All colony counts were expressed as log10 CFU per gram tissue or milliliter wound fluid. Bacterial counts of >105 were considered to indicate bacterial infection.
Wound observation
To monitor the wound-healing process, we observed the leakage quantity of burn wounds, presence of secretions, healing range, and scabbing at different times after PACT treatment. Pictures were taken at different time points.
Histological assessment
Major organs were fixed using 10% formalin for at least 24 hours. Samples were then paraffin-embedded, sectioned, and stained with H&E. Histopathological changes were observed using light microscopy.
Statistical analysis
SPSS 19.0 software (SPSS Inc, Chicago, IL, USA) was used for statistical analysis. Values are expressed as means ± standard deviation of three samples obtained from three independent experiments. Statistical comparisons were made using one-way analysis of variance, and multiple comparisons between groups were performed using Tukey’s test, with P<0.05 considered statistically significant and P<0.01 highly significant.
Results
Uptake of DVDMS in S. aureus/MDR S. aureus
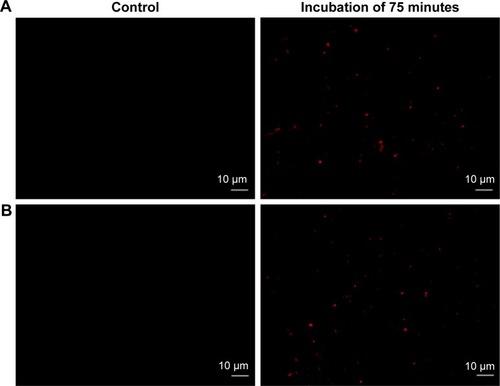
To substantiate the PACT effect in S. aureus/MDR S. aureus, we examined uptake of DVDMS by microscopy. As shown in , the vast majority of DVDMS fluorescence can be seen on the cell wall and in the cytoplasm of S. aureus after 75 minutes of incubation. Similar DVDMS uptake was also found in MDR S. aureus.
Figure 2 Uptake of DVDMS by Staphylococcus aureus/MDR S. aureus.
Notes: DVDMS uptake in S. aureus/MDR S. aureus. Bacteria were loaded with 5 μM DVDMS (right panels) and PBS (negative control, left panels) for 75 minutes. (A) S. aureus; (B) MDR S. aureus. The peak was reached at 75 minutes. Magnification is 63×.
Abbreviations: DVDMS, sinoporphyrin sodium; MDR, multidrug-resistant.
Colony-forming units
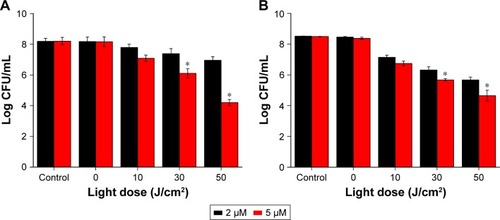
After photodynamic treatment with DVDMS, S. aureus and MDR S. aureus CFU was counted. shows significant bacterial activity of S. aureus/MDR S. aureus. Photodynamic treatment with DVDMS decreased bacteria growth in a DVDMS dose- and light dose-dependent manner. Antibacterial activity on S. aureus was observed while DVDMS was incubated at a concentration of 2 μM, with a 4.2-log reduction in CFU with DVDMS at a concentration of 5 μM (). Photodynamic action of DVDMS showed antibacterial activity on MDR S. aureus: 3.85-log reduction in CFU at an incubation concentration of 5 μM ().
Figure 3 CFU assay of Staphylococcus aureus/MDR S. aureus after DVDMS-PACT treatment.
Notes: Bacterial cells were incubated with 2 μM and 5 μM DVDMS for 75 minutes and irradiated by different light doses. (A) S. aureus: control, negative control; 2 μM, 5 μM, DVDMS treatment alone; 10 J/cm2, 30 J/cm2, 50 J/cm2, PACT treatment of 5 μM DVDMS and different light doses. (B) MDR S. aureus: variables as per A. Data expressed as means ± SD of three experiments. *P<0.05 vs control.
Abbreviations: CFU, colony-forming unit; DVDMS, sinoporphyrin sodium; MDR, multidrug-resistant; PACT, photodynamic antimicrobial chemotherapy.
Measurement of reactive oxygen species
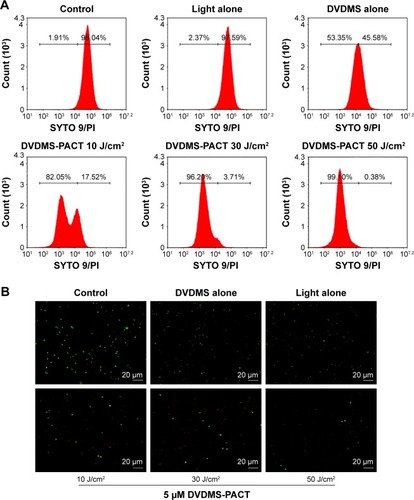
Flow-cytometry analyses indicated that exposure of S. aureus/MDR S. aureus to DVDMS-PACT treatment significantly enhanced intracellular ROS levels (). Relative ROS production was significantly higher (P<0.01) in the PACT groups than in the control groups in both bacteria, and ROS production was positively related to laser dose. Further, in the PACT groups, S. aureus showed considerably higher ROS production than MDR S. aureus (P<0.05) ().
Figure 4 ROS detection by flow cytometry.
Notes: ROS production in Staphylococcus aureus/MDR S. aureus was measured after DVDMS-PACT treatment. Bacteria were preincubated with H2-DCF-DA (10 μM), followed by illumination exposure at different light doses of 10, 30, and 50 J/cm2 in the presence of DVDMS (5 μM). Cytofluorometric profiles represent the distribution of bacterial cells after staining with H2-DCF-DA (S. aureus [A], MDR S. aureus [B]). Control, negative control; light alone, only irradiation 50 J/cm2 light dose; DVDMS alone, bacteria treated with 5 μM DVDMS alone. **P<0.01 vs untreated controls; #P<0.05 for S. aureus vs MDR S. aureus. (C) Distribution of the intensity of DCF + bacteria in different groups.
Abbreviations: DA, diacetate; DCF, dichlorodihydrofluorescein; DVDMS, sinoporphyrin sodium; MDR, multidrug-resistant; PACT, photodynamic antimicrobial chemotherapy; ROS, reactive oxygen species.
![Figure 4 ROS detection by flow cytometry.Notes: ROS production in Staphylococcus aureus/MDR S. aureus was measured after DVDMS-PACT treatment. Bacteria were preincubated with H2-DCF-DA (10 μM), followed by illumination exposure at different light doses of 10, 30, and 50 J/cm2 in the presence of DVDMS (5 μM). Cytofluorometric profiles represent the distribution of bacterial cells after staining with H2-DCF-DA (S. aureus [A], MDR S. aureus [B]). Control, negative control; light alone, only irradiation 50 J/cm2 light dose; DVDMS alone, bacteria treated with 5 μM DVDMS alone. **P<0.01 vs untreated controls; #P<0.05 for S. aureus vs MDR S. aureus. (C) Distribution of the intensity of DCF + bacteria in different groups.Abbreviations: DA, diacetate; DCF, dichlorodihydrofluorescein; DVDMS, sinoporphyrin sodium; MDR, multidrug-resistant; PACT, photodynamic antimicrobial chemotherapy; ROS, reactive oxygen species.](/cms/asset/d0b60b13-430c-4a46-a5f4-e698fec21639/dijn_a_12193667_f0004_c.jpg)
![Figure 4 ROS detection by flow cytometry.Notes: ROS production in Staphylococcus aureus/MDR S. aureus was measured after DVDMS-PACT treatment. Bacteria were preincubated with H2-DCF-DA (10 μM), followed by illumination exposure at different light doses of 10, 30, and 50 J/cm2 in the presence of DVDMS (5 μM). Cytofluorometric profiles represent the distribution of bacterial cells after staining with H2-DCF-DA (S. aureus [A], MDR S. aureus [B]). Control, negative control; light alone, only irradiation 50 J/cm2 light dose; DVDMS alone, bacteria treated with 5 μM DVDMS alone. **P<0.01 vs untreated controls; #P<0.05 for S. aureus vs MDR S. aureus. (C) Distribution of the intensity of DCF + bacteria in different groups.Abbreviations: DA, diacetate; DCF, dichlorodihydrofluorescein; DVDMS, sinoporphyrin sodium; MDR, multidrug-resistant; PACT, photodynamic antimicrobial chemotherapy; ROS, reactive oxygen species.](/cms/asset/bfc12ea6-1d6d-4232-bc45-49579ab4cba0/dijn_a_12193667_f0004b_c.jpg)
Bacteria-viability assay
Fluorescence intensity was measured by flow cytometry. As shows, there was a decrease in the ratio of green:red fluorescence intensity in the combined-treatment group in comparison with the control group. Furthermore, the fluorescence micrography showed the number of live cells (green) decreased, whereas the number of dead (red) cells increased in the combined-treatment group compared with other groups ().
Figure 5 Flow cytometry and fluorescence microscopy to illustrate bacterial viability.
Notes: (A) Membrane permeability of Staphylococcus aureus was measured using flow cytometry after DVDMS-mediated photodynamic action. Data shown as means ± SD. (B) Cells were SYTO 9 (green)–PI (red) double-stained and viewed under fluorescence microscopy. (C, D) Same as A and B; bacteria MDR S. aureus. Magnification is 40×.
Abbreviations: DVDMS, sinoporphyrin sodium; MDR, multidrug-resistant; PACT, photodynamic antimicrobial chemotherapy; PI, propidium iodide.
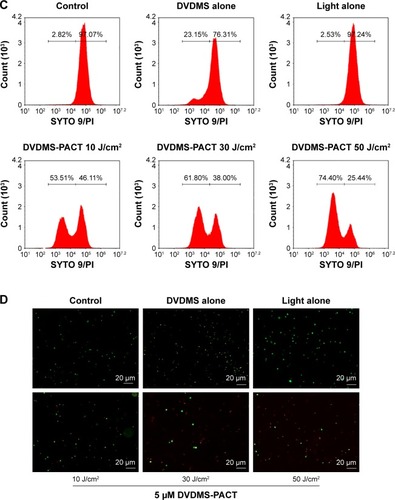
DVDMS-PACT-induced DNA fragmentation
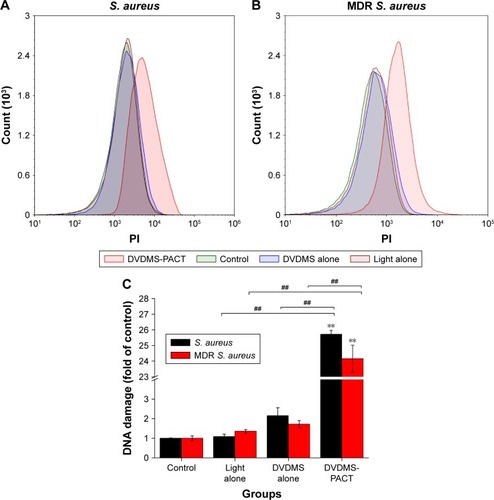
We performed PI staining with flow cytometry to evaluate DVDMS-PACT-induced DNA damage in S. aureus/MDR S. aureus (). As shown in , PACT treatment induced a 25.72-fold increase in DNA damage to S. aureus, and the damage level increased to 24.15-fold over control for MDR S. aureus.
Figure 6 Effects on DNA fragmentation of Staphylococcus aureus.
Notes: Bacterial cells were treated with light alone, DVDMS alone, and PACT, then stained with PI and analyzed by flow cytometry. (A, B) DNA damage vs PI fluorescence. Data expressed as means ± SD of three independent experiments. **P<0.01 vs control; ##P<0.01 vs DVDM alone and light alone.
Abbreviations: DVDMS, sinoporphyrin sodium; MDR, multidrug-resistant; PACT, photodynamic antimicrobial chemotherapy; PI, propidium iodide.
Enzyme-linked immunosorbent assay
Using ELISA to detect bFGF and IL6 contents in burn tissues, by day 7 bFGF in the 10 μM PACT-treatment group had reached its highest level, IL6 had reduced to its minimum level after 3 days (). bFGF level was high in the PACT-treatment group, and IL6 in the PACT-treatment group was lower than the control group (P<0.05).
Figure 7 Different factors determined by ELISA.
Notes: (A) bFGF levels were determined by ELISA in the four different groups at 1, 2, 3, and 7 days following full-thickness injury of mice. (B) IL6 levels were determined by ELISA in the four different groups at 1, 2, 3, and 7 days following full-thickness injury of rats. (C) TNFα levels were determined by ELISA in the four different groups at 1, 2, 3, and 7 days following full-thickness injury of rats. (D) VEGF levels were determined by ELISA in the five different groups at 1, 2, 3, 7, and 14 days following full-thickness injury of rats. *P<0.05, **P<0.01. Data shown as means ± SD from eight mice in each group.
Abbreviation: ELISA, enzyme-linked immunosorbent assay.
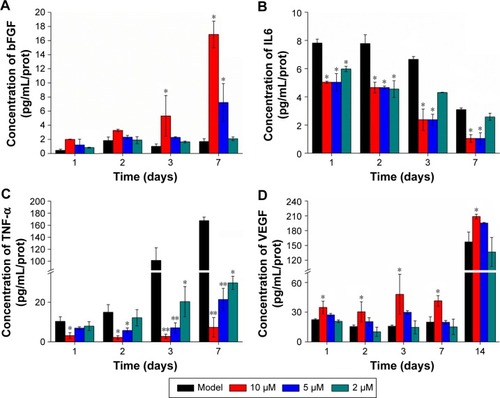
Throughout the healing process, TNFα expression in PACT-treatment groups was lower than the control group (, P<0.01), while at DVDMS concentration of 10 μM, TNFα expression in PACT was significantly lower than the control group. In the late period of treatment, TNFα expression in PACT groups was increasing gradually, but still much lower compared with the control group. Also, in PACT groups lower light doses induced higher TNFα expression. Compared with model group, VEGF content in each treatment group increased gradually with time after burn infection, and levels in each treatment group increased significantly compared with the model group (P<0.05, ).
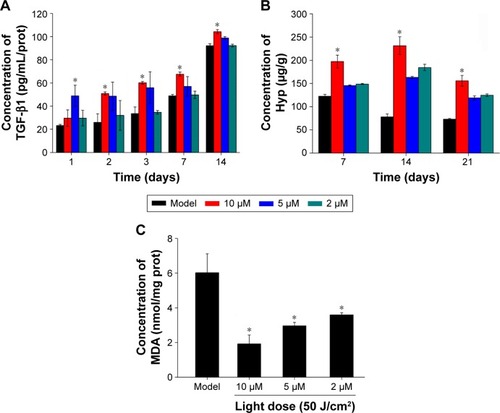
TGFβ1 is an important cell-growth factor that initiates and terminates tissue repair. TGFβ1 expression can promote fibroblast growth, capillary angiogenesis, collagen formation, granulation-tissue growth, and wound repair.Citation36 The results of this study showed that TGFβ1 expression in PACT groups was better than that in the model control group (P<0.05). The PACT group treated with high light doses was superior to the others (P<0.05, ). The high-dose PACT-treatment group significantly increased TGFβ1 content.
Figure 8 Factor levels of traumatic skin tissue at different times.
Notes: (A) TGFβ1 levels were determined by ELISA in the groups at 1, 2, 3, 7, and 14 days following full-thickness injury of rats. (B) Hyp levels were tested in the groups at 7, 14, 21 days following full-thickness injury of rats. (C) MDA levels were tested in the groups at 14 days following full-thickness injury of rats. *P<0.05. Data shown as means ± SD from eight mice in each group.
Abbreviations: ELISA, enzyme-linked immunosorbent assay; Hyp, hydroxyproline; MDA, malondialdehyde.
Wound-tissue MDAand Hyp content determination
Hyp content in PACT-treatment groups was obviously higher than the model group after 7, 14, and 21 days treatment (P<0.05, ). MDA content in the model group was obviously higher than PACT-treatment groups after 7, 14, and 21 days’ treatment (P<0.05, ).
Bacterial loads in skin
Antimicrobial activity of DVDMS-PACT against bacteria was evaluated in vivo by homogenizing infected burn wounds and quantifying CFU present in tissue on days 1, 3, 5, 7, and 14. Treatment with DVDMS-PACT significantly decreased the bacterial loads in skin compared with the control group (P<0.01). demonstrates that the density of MDR S. aureus bacterial growth decreased gradually in the DVDMS-PACT-treatment group. is a quantitative representation of . shows the results for S. aureus.
Figure 9 CFU assay of Staphylococcus aureus and MDR S. aureus of skin tissue at different times.
Notes: (A) Representative bacterial colonies on trypticase soy agar are shown. (B) MDR S. aureus CFU counts in PACT treatment and the model group (without any treatment) were assessed with 20 μM DVDMS at different times. (C) Bacteria counts of S. aureus with 10 μM DVDMS treatment at different times. Data expressed as means ± SD of three independent experiments. *P<0.05; **P<0.01. (D) Wound observation at different times after PACT treatment.
Abbreviations: DVDMS, sinoporphyrin sodium; MDR, multidrug-resistant; PACT, photodynamic antimicrobial chemotherapy.
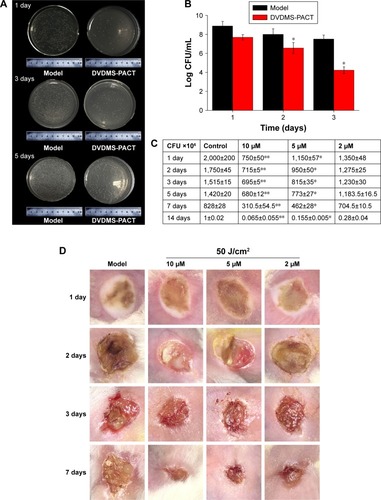
Wound observation
Topical treatment with DVDMS-PACT significantly accelerated wound healing in mice compares to the control group ().
Evaluation of side effects using DVDMS-PACT
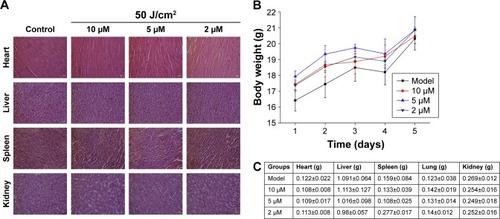
We examined the potential in vivo toxicity of DVDMS-PACT. We harvested major organs: heart, liver, spleen, and kidney. We were not in a position to detect any organ damage using H&E staining (). Furthermore, we did not detect any overt signs of toxic side effects or changes in body weight and organ weight () with DVDMS-PACT at 10 μM, suggesting that DVDMS had no adverse effect on the growth of mice. These results suggested there were no observable side effects of DVDMS at the treatment dose and that the treatment was relatively safe to administer. However, the present safety evaluation of DVDMS is somewhat limited, and should be expanded in a series of rigorous assessments before DVDMS is clinically used.
Figure 10 Evaluation of side effects using DVDMS-PACT.
Notes: (A) Effect of different treatments on structural changes in major organs in mice. Major organ sections were stained with H&E. Histopathological changes were observed under light microscopy. (B) Body weight versus number of days after different treatments. (C) Weight of major organs in the mice. Data shown as means ± SD from eight mice in each group.
Abbreviations: DVDMS, sinoporphyrin sodium; PACT, photodynamic antimicrobial chemotherapy.
Discussion
Burns, a form of skin wound, are a frequently occurring affliction in the clinic. Bacterial infection, the main complication of burn patients, is the dominant cause of death. As such, anti-infection treatment is an important link for burn patients.Citation37 In addition to this, the emergence of high antimicrobial resistance among bacterial pathogens has made management of treatment of postoperative wound infections difficult.Citation38,Citation39 However, MDR clinical pathogens cause dangerous, life-threatening invasive infections and are resistant to a wide range of broad-spectrum antibiotics. The mechanisms behind multidrug (MD) resistance are complex. Based on the frequent development of MDR bacterial strains from the hospital environment, there is an urgent need for the development of alternative medicines against MDR pathogens.Citation40 The development of strategies to combat bacteria growing in antibiotics is a challenging task, given that those bacteria are much more resistant to antimicrobial therapies.
PACT is a promising therapeutic option to control microbial growth effectively. The photosensitizer (PS) is the critical component of PACT, and can directly affect efficiency. DVDMS is an identified sensitizer based on Photofrine® (PF). Research has shown that DVDMS is a good sensitizer of antibacterial activity in vitro and can produce a large amount of ROS.Citation31 The clinical value of PACT as a topical antimicrobial treatment depends both on its bactericidal activity and its cytotoxicity toward host tissue.Citation18
In this study, we demonstrated that it is possible to photoinactivate S. aureus rapidly when present in a burn infected with DVDMS as PS in vitro and in vivo. Fluorescence microscopy showed DVDMS concentration in bacteria reached its maximum in 75 minutes (). We primarily investigated the bactericidal effect of PACT using DVDMS on S. aureus and MDR S. aureus. In our preliminary study, we found that DVDMS reached its maximum 75 minutes after incubation, and 50 J/cm2 light with DVDMS had a significant effect on S. aureus. Firstly, we optimized DVDMS-PACT parameters. A 4 log10 reduction in CFU was observed at 5 μM DVDMS combined with 50 J/cm2 light. Moreover, the same results were achieved in MDR S. aureus. The CFU assay showed that DVDMS-PACT decreased the survival of bacteria in a DVDMS dose- and light dose-dependent manner ().
It has been reported that ROS contribute to the microbicidal activity of phagocytes, regulation of signal transduction, and gene expression, and induce oxidative damage to nucleic acid, proteins, and lipids.Citation25 Our previous studyCitation31 demonstrated ROS in PACT treatment in antibacterial action. shows that abundant ROS were generated after PACT treatment. What is more, the amount of ROS produced occurred in a light dose-dependent manner (). ROS induce cell death through a variety of photochemical mechanisms.
There have been different opinions on the criteria for bacterial viability to define a bacterial cell as dead or alive.Citation41–Citation44 Cellular and membrane integrity is considered to be one criterion distinguishing between viable and dead bacterial cells. Viable cells are assumed to have intact and tight cell membranes that cannot be penetrated by some staining compounds, whereas dead cells are considered to have disrupted and/or broken membranes.Citation26,Citation45 The combined usage of SYTO 9 and PI in a commercially available kit was first described in 1996, and it is promoted as a rapid and reliable method for assessment of bacterial viability that gives quantitative results and can be applied to microplate-reader flow-cytometry combined staining with SYTO 9 and PI. Green fluorescent SYTO 9 is a cell membrane-permeable agent and red fluorescent PI is an impermeable reagent for nuclei staining. The fluorescent SYTO 9 binds only with viable bacterial cells, whereas the membrane-impermeable PI is commonly used to stain damaged or compromised cells and emits red fluorescence, which usually indicates dead cells.Citation46 Fluorescence intensity was measured with flow cytometry and fluorescence microscopy. Data in show that membrane permeability was changed after PACT treatment and permeability enhanced with PACT dose increased. Furthermore, the presence of condensed chromatin and DNA fragmented in S. aureus/MDR S. aureus confirmed that DVDMS-PACT can induce DNA damage, which might prove crucial for the role of ROS in PACT treatment ().
Despite continual advances in treatment of burns, wound infection still remains a huge threat to burn patients. Septic processes account for approximately 73% of all death within the initial 5 days of a burn.Citation44,Citation47 The presence of large amounts of necrotic tissue with protein-rich wound exudates at the burn site provides a highly nutritive medium for the proliferation of microbes, leading to increased rates of wound infection in these patients.Citation48,Citation49 In this study, we demonstrated that it is possible to photoinactivate S. aureus rapidly when it is present in a burn wound with DVDMS as PS. The fact that the bacteria had 48 hours to colonize the wound and multiply manyfold gives the experiments more clinical significance than if PACT was carried out shortly after bacterial contamination. The use of CFU counts from homogenized tissues removed from euthanized animals allows the progress of the infection to be followed over time in individual mice (). The necessity of using a much higher concentration of DVDMS in vivo (10 μM in burn wounds compared to 5 μM that efficiently killed bacteria in vitro) may be explained by the much higher concentration of biological material in the infected burn (host proteins and cells, in addition to bacteria) to which the PS can bind. MDR S. aureus bacteria is consistent with the results, and the concentration of the whole is greater than normal bacteria cause the drug-resistant bacteria itself to have a corresponding mechanism of drug resistance, such as efflux pump, the photosensitive is restricted to entrance, and the specific mechanisms needs to be further confirmed. This means that the vast majority of the singlet oxygen produced in the burn infection is wasted on nonbacterial matter, and it is thus necessary to produce much more singlet oxygen to achieve bacterial cell killing. Therefore, in order to optimize PACT in burn infections, selectivity of the PS for the bacteria over mammalian cells and proteins is vital. In addition, shows that mouse skin healed faster in the PACT-treatment group.
At infection sites, the release and accumulation of bacterial components, such as lipoteichoic acid, from Gram-positive bacteria are known to trigger various inflammatory mediators.Citation50 The experiment confirmed that PACT can significantly reduce the number of bacteria colonies under phase callus, increase the rate of wound healing, and promote resistance to infection and healing efficacy. In specific conditions of inflammation-mediated pathophysiology, TNFα is recognized as a crucial contributing factor.Citation51,Citation52 Impaired healing in animal models and age-related delayed healing of acute human wounds exhibit raised local and systemic levels of TNFα that may parallel the proinflammatory phenotype.Citation53,Citation54 The pleiotropic cytokine IL6 is produced by macrophages, dendritic cells, mast cells, and other innate immune cells; consequently, this cytokine has long been considered a marker of inflammation.Citation55 Using ELISA to detect the concentration of TNFα and IL6 at different times, results showed that PACT treatment inhibited inflammation-factor secretion, significantly reduce the scalded-tissue concentration of TNFα and IL6, and prevent further deepening of the wound. bFGF mediates angiogenic activity in early surgical wounds, and significantly accelerates granular tissue formation and reepithelialization.Citation56 TGFβ1 plays an important role in the process of differentiation and tissue formation, can regulate cell proliferation, differentiation, and mesenchymal cell protein expression, and participates in the epithelial regeneration in the process of wound healing, fibroblast fibrosis, interstitial proliferation, angiogenesis, and the important stimulating factor of granulation-tissue fibrosis.Citation57,Citation58 Results showed that in the healing process, PACT-treatment groups induced higher expression bFGF and TGFβ1 than the model control group. These results suggest that PACT treatment can inhibit wound deterioration and reduce inflammation. In addition, the concentration of VEGF rose almost at the same time in the PACT-treatment process. VEGF has been described as one of the most important stimulators of angiogenesis and vasopermeability, and is produced by macrophages as a result of the burn’s hypoxic environment to stimulate endothelial cell migration and proliferation.Citation41,Citation42 Indeed, this increase in VEGF levels coincided with the decrease in wound area, suggesting the importance of angiogenesis stimulation by DVDMS-PACT to the overall acceleration of wound healing, observed mainly at 7 and 14 days (). MDA levels showed us the extent of cell damage caused by free radicals.Citation59 Collagen not only confers strength and integrity to the tissue matrix but also plays an important role in homeostasis and epithelialization in wound healing. Collagen is composed of the amino acid Hyp, which has been used as a biochemical marker for tissue collagen.Citation60 MDA and Hyp levels demonstrated the promotion of wound healing after PACT treatment.
Finally, there were no detectable side effects using DVDMS-PACT at the therapeutic dose according to preliminary safety analysis, and the treatment was relatively safe to administer. Nanomaterials are used for antibacterial purposes, due to their unique advantages. Smaller particles facilitate penetration and enhance their antibacterial properties, higher entrapment efficiency of nanoparticles increases drug content at the site of action, and lower minimum inhibitory concentration and minimum bactericidal concentration achieved with nanoparticles indicates that better antibacterial activity is achieved with smaller amounts of drug. Further work is necessary on modification and loading of DVDMS to enhance PACT antibacterial efficiency.
Conclusion
Taken together, our results clearly demonstrate the advantages of DVDMS compared to other clinical sensitizers, as well as a synergistic effect between DVDMS and light. DVDMS-PACT can effectively suppress bacteria and MDR-bacteria proliferation, and produces a large amount of ROS to damage bacterial cell membranes. In addition, DVDMS-PACT can promote the healing of wounds in burn infection.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (81472846 and 81571834), Natural Science Foundation of Shaanxi Province (2017JM8004), and Fundamental Research Funds for Central Universities (GK201602003 and 2017CBY006).
Disclosure
The authors report no conflicts of interest in this work.
References
- PriyaKSGnanamaniARadhakrishnanNBabuMHealing potential of Datura alba on burn wounds in albino ratsJ Ethnopharmacol200283319319912426086
- UpadhyayNKumarRMandotraSSafety and healing efficacy of sea buckthorn (Hippophae rhamnoides L.) seed oil on burn wounds in ratsFood Chem Toxicol20094761146115319425187
- SenCKGordilloGMRoySHuman skin wounds: a major and snowballing threat to public health and the economyWound Repair Regen200917676377119903300
- AllisonRRMoghissiKPhotodynamic therapy (PDT): PDT mechanismsClin Endosc2013461242923422955
- SeilJTWebsterTJAntimicrobial applications of nanotechnology: methods and literatureInt J Nanomedicine201272767278122745541
- KhorasaniGHosseinimehrSJZamaniPGhasemiMAhmadiAThe effect of saffron (Crocus sativus) extract for healing of second-degree burn wounds in ratsKeio J Med200857419019519110531
- ChabyGSenetPVaneauMDressings for acute and chronic wounds: a systematic reviewArch Dermatol2007143101297130417938344
- WangFGaoWThamphiwatanaSHydrogel retaining toxin-absorbing nanosponges for local treatment of methicillin-resistant Staphylococcus aureus infectionAdv Mater201527223437344325931231
- MüllerPAlberDGTurnbullLSynergism between Medihoney and rifampicin against methicillin-resistant Staphylococcus aureus (MRSA)PLoS One201382e5767923469049
- WuGZhuBHongXLuoPXiaZRole of cytokines in host defense against Staphylococcus aureus skin infectionHistol Histopathol201732876176628078661
- GreenhalghDGTopical antimicrobial agents for burn woundsClin Plast Surg200936459760619793554
- PraphakarRAMunusamyMASadasivuniKKRajanMTargeted delivery of rifampicin to tuberculosis-infected macrophages: design, in-vitro, and in-vivo performance of rifampicin-loaded poly(ester amide)s nanocarriersInt J Pharm20165131–262863527693734
- RiceLBMechanisms of resistance and clinical relevance of resistance to β-lactams, glycopeptides, and fluoroquinolonesMayo Clinic Proc2012872198208
- KlahnPBrönstrupMBifunctional antimicrobial conjugates and hybrid antimicrobialsNat Prod Rep201734783288528530279
- MousaviMBehrouzBIrajianGMahdaviMKorpiFMotamedifarMPassive immunization against Pseudomonas aeruginosa recombinant PilA in a murine burn wound modelMicrob Pathog2016101838827836762
- FairRJTorYAntibiotics and bacterial resistance in the 21st centuryPerspect Med Chem201462564
- DudhagaraPRGhelaniADPatelRKPhenotypic characterization and antibiotics combination approach to control the methicillin-resistant Staphylococcus aureus (MRSA) strains isolated from the hospital derived fomitesAsian J Med Sci2014227278
- LambrechtsSADemidovaTNAaldersMCHasanTHamblinMRPhotodynamic therapy for Staphylococcus aureus infected burn wounds in micePhotochem Photobiol Sci20054750350915986057
- BeythNHouri-HaddadYDombAKhanWHazanRAlternative antimicrobial approach: nano-antimicrobial materialsEvid Based Complement Alternat Med2015201524601225861355
- LiuHLDaiSHFuKYHsuSHAntibacterial properties of silver nanoparticles in three different sizes and their nanocomposites with a new waterborne polyurethaneInt J Nanomedicine201051017102821187943
- HuGXiaoLTongPAntibacterial hemostatic dressings with nanoporous bioglass containing silverInt J Nanomedicine201272613262022745538
- ChengYWuJGaoBFabrication and in vitro release behavior of a novel antibacterial coating containing halogenated furanone-loaded poly(L-lactic acid) nanoparticles on microarc-oxidized titaniumInt J Nanomedicine201275641565223152682
- SaravananMNandaAExtracellular synthesis of silver bionanoparticles from Aspergillus clavatus and its antimicrobial activity against MRSA and MRSEColloids Surf B Biointerfaces201077221421820189360
- SaravananMVemuAKBarikSKRapid biosynthesis of silver nanoparticles from Bacillus megaterium (NCIM 2326) and their antibacterial activity on multi drug resistant clinical pathogensColloids Surf B Biointerfaces201188132533121798729
- PanJSHongMZRenJLReactive oxygen species: a double-edged sword in oncogenesisWorld J Gastroenterol200915141702170719360913
- StiefelPSchmidt-EmrichSManiura-WeberKRenQCritical aspects of using bacterial cell viability assays with the fluorophores SYTO9 and propidium iodideBMC Microbiol2015153625881030
- SperandioFFHuangYYHamblinMRAntimicrobial photodynamic therapy to kill Gram-negative bacteriaRecent Pat Antiinfect Drug Discov20138210812023550545
- WainwrightMPhotodynamic antimicrobial chemotherapy (PACT)J Antimicrob Chemother199842113289700525
- ZolfaghariPSPackerSSingerMIn vivo killing of Staphylococcus aureus using a light-activated antimicrobial agentBMC Microbiol200992719193212
- ZeinaBGreenmanJPurcellWDasBKilling of cutaneous microbial species by photodynamic therapyBr J Dermatol2001144227427811251558
- MaiBWangXLiuQThe antibacterial effect of sinoporphyrin sodium photodynamic therapy on Staphylococcus aureus planktonic and biofilm culturesLasers Surg Med201648440040826749227
- KryskoDVVandenBTD’HerdeKVandenabeelePApoptosis and necrosis: detection, discrimination and phagocytosisMethods200844320522118314051
- FanJWangYWangXThe antitumor activity of Meconopsis horridula Hook, a traditional Tibetan medical plant, in murine leukemia L1210 cellsCell Physiol Biochem20153731055106526401616
- GilpinDACalculation of a new Meeh constant and experimental determination of burn sizeBurns19962286076118982538
- ZhangYLiangDDongLAnti-inflammatory effects of novel curcumin analogs in experimental acute lung injuryRespir Res2015164325889862
- PakyariMFarrokhiAMaharlooeiMKGhaharyACritical role of transforming growth factor beta in different phases of wound healingAdv Wound Care (New Rochelle)20132521522424527344
- OlsonMMLeeJTContinuous, 10-year wound infection surveillance: results, advantages, and unanswered questionsArch Surg199012567948032346380
- AndhogaJMachariaAGMaikumaIRWanyonyiZSAyumbaBRKakaiRAerobic pathogenic bacteria in post-operative wounds at Moi Teaching and Referral HospitalEast Afr Med J2002791264064412678447
- NandaASaravananMBiosynthesis of silver nanoparticles from Staphylococcus aureus and its antimicrobial activity against MRSA and MRSENanomedicine20095445245619523420
- KasithevarMPeriakaruppanPMuthupandianSMohanMAntibacterial efficacy of silver nanoparticles against multi-drug resistant clinical isolates from post-surgical wound infectionsMicrob Pathog201710732733428411059
- KimCKKarauMJGreenwood-QuaintanceKESuperantigen-producing Staphylococcus aureus elicits systemic immune activation in a murine wound colonization modelToxins (Basel)20157125308531926670252
- KlevensRMMorrisonMANadleJInvasive methicillin-resistant Staphylococcus aureus infections in the United StatesJAMA2007298151763177117940231
- LiXGuoHTianQEffects of 5-aminolevulinic acid-mediated photodynamic therapy on antibiotic-resistant staphylococcal biofilm: an in vitro studyJ Surg Res201318421013102123622723
- LiLLiuYHaoPPEDOT nanocomposites mediated dual-modal photodynamic and photothermal targeted sterilization in both NIR I and II windowBiomaterials20154113214025522972
- GopinathVPriyadarshiniSLokeMFBiogenic synthesis, characterization of antibacterial silver nanoparticles and its cell cytotoxicityArab J Chem Epub20151210
- GopinathVPriyadarshiniSAl-MalekiARIn vitro toxicity, apoptosis and antimicrobial effects of phyto-mediated copper oxide nanoparticlesRSC Adv20166112110986110995
- DouJLJiangYWXieJQZhangXGNew is old, and old is new: recent advances in antibiotic-based, antibiotic-free and ethnomedical treatments against methicillin-resistant Staphylococcus aureus wound infectionsInt J Mol Sci2016175E61727120596
- VentolaCLThe antibiotic resistance crisis: part 1: causes and threatsP T201540427728325859123
- BowlerPGDuerdenBIArmstrongDGWound microbiology and associated approaches to wound managementClin Microbiol Rev200114224426911292638
- SongXYZengLJinWWSecretory leukocyte protease inhibitor suppresses the inflammation and joint damage of bacterial cell wall-induced arthritisJ Exp Med1999190453554210449524
- AshcroftGSJeongMJAshworthJJTumor necrosis factor-alpha (TNF-α) is a therapeutic target for impaired cutaneous wound healingWound Repair Regen2012201384922151742
- BrüünsgaardHPedersenBKAge-related inflammatory cytokines and diseaseImmunol Allergy Clin North Am2003231153912645876
- BruunsgaardHEffects of tumor necrosis factor-alpha and interleukin-6 in elderly populationsEur Cytokine Netw200213438939112517724
- WangJWangQHanTSoluble interleukin-6 receptor is elevated during influenza A virus infection and mediates the IL-6 and IL-32 inflammatory cytokine burstCell Mol Immunol201512563364425176527
- NissenNNPolveriniPJGamelliRLDipietroLABasic fibroblast growth factor mediates angiogenic activity in early surgical woundsSurgery199611944574658644013
- HerreraBSKantarciAZarroughAHasturkHLeungKPVan DykeTELXA4 actions direct fibroblast function and wound closureBiochem Biophys Res Commun201546441072107726188508
- FergusonMWJO’KaneSScar-free healing: from embryonic mechanisms to adult therapeutic interventionPhilos Trans R Soc Lond B Biol Sci2004359144583985015293811
- HonnegowdaTMUdupaEGRaoPKumarPSinghRSuperficial burn wound healing with intermittent negative pressure wound therapy under limited access and conventional dressingsWorld J Plast Surg20165326527327853690
- GhaffariAManafiAMoghimiHRClindamycin phosphate absorption from nanoliposomal formulations through third-degree burn escharWorld J Plast Surg20154214515226284183
- KashiTSEskandarionSEsfandyari-ManeshMImproved drug loading and antibacterial activity of minocycline-loaded PLGA nanoparticles prepared by solid/oil/water ion pairing methodInt J Nanomedicine2012722123422275837
