Abstract
Among all the cellular partners involved in inflammatory processes, monocytes and macrophages are the master regulators of inflammation. They are found in almost all the tissues and are nearly the only cells capable of performing each step of inflammation. Consequently, they stand as major relevant therapeutic targets to treat inflammatory disorders and diseases. The physiological phagocytic activity of macrophages prompts them to detect, to recognize, and eventually to engulf any nanosystem cruising in their neighborhood. Interestingly, nanosystems can be rationally engineered to afford multivalent, and multifunctional if needed, entities with multiplexed and/or reinforced biological activities. Indeed, engineered nanosystems bearing moieties specifically targeting macrophages, and loaded with or bound to drugs are promising candidates to modulate, or even eradicate, deleterious macrophages in vivo. In this review we highlight recent articles and concepts of multivalent nanosystems targeting monocytes and macrophages to treat inflammatory disorders.
Introduction
What do flu, acne, frostbite, atherosclerosis, multiple sclerosis, depression, and cancer have in common? These disorders all involve a more or less pronounced inflammatory reaction. Inflammation is the group of actions rapidly set up by the vascularized tissues following any type of aggression or injury. This immediate innate immune response involves a set of dynamic and highly regulated defense mechanisms, whose objectives are, first, to recognize and to eliminate the causal agent, then to repair and to regenerate the damaged tissue.Citation1 The cardinal clinical signs of inflammation are: redness (rubor), swelling (tumor), heat (calor), and pain (dolor). Redness and heat are due to local vasodilatation, whose role is to increase blood flow in order to recruit circulating leukocytes and plasma proteins. The swelling is triggered by the diffusion of plasma water into the tissue, this edema in turn compresses the nerves and causes pain. Signals recognized as a danger are either:
exogenous: an infectious microorganism, a physical aggression (trauma, heat, cold,…), a chemical agent (toxins, venoms,…), an inert foreign substance, or
endogenous: a defect of vascularization (secondary inflammation to ischemia-induced necrosis), a dysimmune aggression (allergy, auto-immunity) or a modified self-protein.
The cellular and molecular responses induced after this recognition step are called acute inflammation. It is short-lived (days to weeks), well-orchestrated and, whatever the triggering agent and/or the tissue damaged, its course is similar and involves intense vasculo-exudative mechanisms. This acute inflammatory process consists of a highly coordinated sequence of events: 1) the recruitment of blood leukocytes and plasma proteins; 2) the production by effectors cells of a huge number of inflammatory mediators in order to neutralize and to wipe out the triggering element and finally; 3) the resolution phase which allows healing and repair necessary to restore homeostasis and tissue function.Citation2 Of note, the resolution step is not a passive phenomenon. On the contrary, this inflammation end-process is also complex and highly regulated.Citation3 In case of infection, the inflammation further supports the onset and development of an adaptive immune response, necessary to induce immune memory that will allow a faster reply during the next infection.Citation4 Acute inflammation is therefore a beneficial protective process that ensures the maintenance of integrity and homeostasis of the body. Nevertheless, in addition to this clearly valuable role, inflammation, like the roman god Janus, offers, if deregulated, a second more devious and detrimental face. This can be explained by the fact that, in order to eliminate the triggering factor, the inflammatory effector cells produce huge amounts of molecular mediators (in particular, inflammatory cytokines, proteases and reactive species of oxygen and nitrogen). Although necessary, these compounds can become toxic and induce damages if they are produced in too high quantity or for too long. Thus, when the inflammatory process persists over time (months to years) and is self-sustained then it becomes chronic, harmful, and even pathogenic. The reasons why inflammation becomes chronic have not yet been fully elucidated but several hypotheses have been put forward: the persistence of the triggering agent, a poor quantitative or qualitative regulation of the effector cells, auto-immunity or a defect in the resolution phase. Anyway, it is now clearly established that chronic inflammation contributes to the etiology of a myriad of pathologies, such as cancer, metabolic diseases, inflammatory bowel diseases, cardiovascular diseases, neurological diseases, and autoimmune diseases. These chronic inflammatory diseases are a group of painful, sometimes debilitating, pathologies. They have a major impact on the patients’ quality of life and create both societal and economic burden. They are an outstanding and alarming global health problem, especially because their prevalence keeps growing both in industrialized and developing countries. It should be noted that the strength of inflammation does not need to be sustained to generate damage. Indeed, it is now established that low-grade inflammation (called meta-inflammation) is also at the root of many disorders.Citation5–Citation7 This is a critical point since this low-grade inflammation is induced, among other things, by obesity,Citation8 which is a global scourge affecting more than 650 million adults and 340 million children/teens worldwide.Citation75
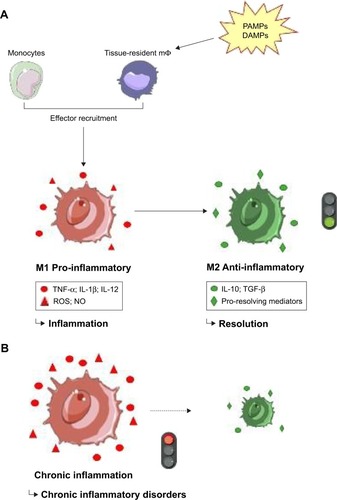
Among all the cellular components involved in the inflammatory reaction, monocytes/macrophages are the master regulators of inflammation. Indeed, these innate immune cells are found in all tissues and, due to their versatility property, are nearly the only cells capable of performing each steps of the acute inflammation listed above.Citation1 Monocytes, which arose from a myeloid hematopoietic progenitor of the bone marrow, are mononuclear phagocytes of the innate immune system that account for around 4% and 10% of nucleated peripheral blood cells in mice and humans respectively. They are divided into circulating and marginating pools (in the spleen and lungs), the latter being mobilized when needed, for instance in case of inflammation.Citation9 The marginal pool, defined as the fraction of cells interacting with the endothelium, would include up to 60% of the peripheral reservoir of monocytes.Citation10 Macrophages are the tissue counterparts of circulating monocytes. Although historically known mainly for their phagocytic and immune functions, it is now recognized that these cells also play a major role in tissue development, remodeling and homeostasis.Citation11,Citation12 Macrophages are highly plastic cells, perhaps the most versatile of the human body. They tailor their phenotype according to the microenvironment. Each cue identified induces a specific program of activation, called polarization, which results in a signal-specific functionality, and thereby, gives rise to an adapted and efficient immunological response. This functional diversity makes macrophages essential cells for the immune reactions. In the time course of inflammatory reaction, the main source of macrophages at the site of injury is circulating monocytes, which, once on site, differentiate into macrophages ().Citation13 As soon as a danger signal is detected and in response to a chemotactic gradient (mainly the chemokine CCL2), monocytes migrate from the blood to the injured site. These infiltrating monocytes are CD14++/CD16− in humans, and Ly6Chigh/CCR2high/CX3CR1-low in mice.Citation14–Citation16 They adhere to the vascular endothelium through adhesion molecules (selectins, cadherins, and integrins), enter the tissue by diapedesis, and once on site, they differentiate into inflammatory macrophages (the so-called “monocyte-derived macrophages”), ie, the effector cells of the defense response, strictly speaking.Citation17 Macrophages express several types of receptors that allow them to react to their environment: cytokine receptors (CSF-1R, IFNγR, IL-4R,…), phagocytic receptors (CD36, Fcγ receptors, MARCO, CD206,…)Citation18 and, above all, innate immune receptors (pattern recognition receptor, PRR).Citation19 These extra- or intracellular highly conserved receptors recognize both exogenous danger signals, ie, the molecular patterns associated with pathogens (PAMPs: pathogen-associated molecular patterns), and endogenous ones, ie, the tissue damage-associated molecules, also called alarmins (DAMPs: damage-associated molecular patterns). To name a few, the lipopolysaccharide (LPS), a component of the bacterial membrane, and the microbial nucleic acids belong to the group of PAMPs while the alarmins IL-33, HSP (heat shock protein), HMGB1 (high-motility group box 1) protein, and the S100 proteins family (all produced as a result of non-programmed cell death or tissue injury) belong to the group of DAMPs. The binding of these danger signals to the PRR activates numerous intracellular signaling pathways, which lead to the development of a specific pro-inflammatory activation program, setting up effector functions ensuring an efficient inflammatory response. This phenotype is characterized by 1) a high expression of antigen presenting and costimulatory molecules (HLA-DR, HLA-ABC, CD80, CD86,…); 2) the production of inflammatory mediators such as reactive oxygen species (ROS) (including superoxide anion O2·−), nitric oxide (NO), and inflammatory cytokines and chemokines (TNFα, IL1-β, IL-12, CXCL9, CXCL10, CCL2,…); and 3) a significant pro-inflammatory activity, which can be microbicide or anti-tumoral. However, macrophages are also strongly involved in the last phase of the inflammatory process: the resolution and repair step. To antagonize the pro-inflammatory function, macrophages then adopt an anti-inflammatory and immuno-regulatory phenotype. This pro-resolving phenotype is defined by 1) the expression of several markers, such as the mannose receptor (CD206), the antagonist to the IL-1 receptor (IL-1 RA), Ym1 and Fizz1; 2) the production of anti-inflammatory cytokines and chemokines (IL-10, TGF-β, CCL18, CCL22, CCL24,…) and pro-resolving lipid mediators such as maresins, protectins and resolvins;Citation3,Citation20 and 3) a shift in the metabolism of l-arginine which is catabolized by arginase 1, instead of iNOS, to synthetize l-ornithine and proline (the latter being involved in tissue repair), rather than NO. Thus these immuno-regulatory macrophages dampen inflammation and favor resolution and wound healing. The induction of these different phenotypes can be mimicked in vitro. The inflammatory phenotype is elicited by Th1 cytokines, such as IFN-γ, with or without LPS.Citation21 This is called classical activation or M1 polarization. Conversely, the anti-inflammatory phenotype is induced, inter alia, by stimulation with Th2 cytokines such as IL-4.Citation22 This alternative or M2 polarization encompasses, in fact, several phenotypes that share the immuno-regulation feature. It is important to emphasize that this old vision of a dichotomous M1/M2 activation modelCitation23,Citation24 is definitely inaccurate, in light of the recent high-resolution and high-dimensional studiesCitation25–Citation27 and because this model does not capture the complexity of microenvironmental stimuli. Nevertheless, this denomination allows us to understand the link between activation, phenotype, and functionality of the different subsets of macrophages. The scientific community has now agreed to apply a stimulus-specific nomenclature for in vitro and ex vivo studies.Citation28
Figure 1 The inflammatory response.
Notes: (A) Physiological acute inflammatory process. The detection of PAMPs or DAMPs triggers the inflammatory response. Thus, some circulating monocytes are recruited at the inflammatory site and resident macrophages are also requisitioned. This defense response, when it consists of an inflammatory phase and then of a resolution step is beneficial. (B) Pathological chronic inflammation. When the inflammatory phase persists in time and is self-maintained, it becomes chronic and paves the way to many chronic inflammatory disorders.
Abbreviations: PAMPs, pathogen-associated molecular patterns; DAMPs, damage-associated molecular patterns; mΦ, macrophage; ROS, reactive oxygen species; NO, nitric oxide.
The valency of an entity, either a chemical or a biological one, is the number of separate specific connections that this entity can engage with other entities through the so-called ligand–receptor interaction. Consequently, multivalent ligand–receptor interactions (ie, multivalency) are characterized by the simultaneous binding of multiple ligands (at least two) on one entity to multiple receptors on another. Multivalency is a common concept in biology and is very familiar to biologists. Indeed, a lot of biological processes occur through multivalent interactions: antigen–antibody interactions, transcription factors-DNA binding, virus- and bacterium-cell binding, cell–cell cross-talk and signaling.Citation29 In fact, multivalency has a lot of biological, biochemical, and biophysical features that monovalency has not. The strength of a single cognate interaction between a ligand and a receptor is called “affinity.” Natural ligands with multiple receptor binding sites (multivalent ligands) or multivalent engineered nano-devices interact through polyvalent interactions with their partner receptors. The strength of these polyvalent cognate interactions is called “avidity” (also “functional affinity”) and is much higher than the simple sum of the strengths of the single interactions. Therefore, from monovalent to oligovalent and then multivalent ligands, there is a strong enhancement in the intensity and duration of the stimulating signal which is delivered to a cell through a ligand–receptor interaction. In nature, the principle of multivalent ligands allows either the reinforcement of a monovalent activator signal already sufficient to induce a biological response by itself, or to overcome a low binding affinity with the specific receptor(s). The multivalent complexes of major histocompatibility complex (MHC) molecules associated with antigenic peptides allowing the transduction of an activating signal in T lymphocytes are examples of the first case.Citation30 The oligosaccharides, generally conjugated to other families of biomolecules, which will use the advantages of multivalence to bind to their protein receptor(s) and trigger a biological response are rather examples of the second case.Citation31
Multivalent ligands are potent inhibitors or effectors of biological processes.Citation32 Beyond the avidity parameter of such entities, their ability to cluster receptors leading to signal transduction, and therefore to a biological outcome, is another unique feature thereof.Citation33 Recently, it has been shown how nanoparticles (NPs) derived from active biological compounds allow the aggregation of membrane receptors, leading to the control of the physiology of stem cells in vitro and in vivo.Citation34 For theorists, researchers have used cell-free systems to understand fundamentally the molecular processes involved in receptor aggregation phenomena by multivalent ligands.Citation35,Citation36
Displaying ligands in multicopy at the surface of a given structure generally implies the implementation of nanosized objects. Indeed, several types of nano-architectures can be reliably prepared, with sizes spanning from that of small proteins, like insulin, to that of viruses. Today, nanosystems can be rationally engineered with predictable, controlled, and tunable properties dedicated to specific applications, including biomedical ones. We have recently written about NPs, tracing back a scientific perspective since the famous lecture of Pr Richard P. Feynman entitled There’s Plenty of Room at the Bottom in 1959 to the advent of nanotechnology in the 1990s.Citation37 Engineered nanosystems (ENSs) include polymers and dendrimers, organic and metallic NPs, micellar and liposomal supramolecular assemblies.Citation38 Thanks to the wide variety of PRRs they express, monocytes and macrophages are prompted to detect, to scan, and eventually to engulf any ENS cruising in their environment. Therefore they are a relevant target for biomedical applications based on ENSs.Citation39
Engineered nanosystems rationally designed to target monocytes/macrophages
As evoked above, carbohydrate moieties of biomolecules are the paradigm of ligands of which low affinity is naturally overcome by multivalency,Citation31 and carbohydrate receptors such as C-type lectins are promising targets to modulate immune responses with multivalent carbohydrate.Citation40 One of these C-type lectin receptors, the mannose receptor (CD206), is highly expressed on macrophages, including pro-inflammatory M1 macrophages. Therefore, a lot of studies have engineered mannose-functionalized nanosystems to efficiently target macrophages. These ENSs are intended as drug carriers and delivery systems ().
Table 1 Compilation of the studies selected for the current review
Mannosylated solid lipid nanoparticles (SLNs) to target alveolar macrophages
SLNs based on different solid lipids commonly used for pulmonary drug delivery have been designed for targeting alveolar macrophages.Citation41 These SLNs have been reinforced with a low percentage of stearylamine. The primary amine groups of the latter were used to covalently bind mannose moieties and to obtain mannosylated SLNs. In this preliminary study, only the non-mannosylated SLNs have been loaded with isoniazid, an antibiotic used to fight against tuberculosis. The authors have shown that incorporating isoniazid in SLNs decreases its intrinsic toxicity toward epithelial cells and macrophages. They have also shown that the multivalent capping of SLNs with mannose significantly enhances the uptake of the ENSs by macrophages. In vivo studies are ongoing to proof the efficacy of these ENSs against tuberculosis in mice by targeting alveolar macrophages infected by Mycobacterium tuberculosis.
Mannosylated thiolated chitosan and chitosan-polyethyleneimine NPs to enhance infected macrophage uptake
Compounds containing antimony (such as meglumine-antimoniate, the so-called glucantime) are used to fight against leishmaniasis. Leishmania protozoa are inoculated in humans by sandflies during their blood meals. These parasites infect macrophages to differentiate and to undergo multiplication. However, the therapeutic efficacy of these compounds is dampened due to the development of resistance mechanisms by the parasites, such as an increased activity of the P-gp efflux pump. Thiolated NPs of chitosan and chitosan-polyethyleneimine have been designed to inhibit both the trypanothione reductase and the P-gp efflux pump.Citation42 The trypanothione reductase is responsible for the synthesis of trypanothione, an essential glutathione-derived anti-oxidant molecule which is specific to some parasites, including Leishmania. Efflux pumps are responsible for the expulsion of drugs once they have been internalized by the targeted cells. The thiolated NPs loaded with glucantime were further capped with mannose residues to enhance their uptake by macrophages. Indeed, the authors have shown that the uptake of glucantime by macrophages through phagocytosis of thiolated ENSs is increased by 18.9- (for chitosan-based NP) and 33.7-fold (for chitosan-polyethyleneimine-based one), as compared with free glucantime. On the whole, and according to their enhanced uptake by infected macrophages, the anti-leishmanial activity of these chitosan-containing ENSs in vitro is increased by 7.4- (for chitosan-based NPs) and 14.4-fold (for chitosan-polyethyleneimine based ones), as compared with free glucantime.
N-acetyl-mannosylated gold NPs to elicit the activation of macrophages
In the same way that lipid-based and chitosan-based NPs, metallic NPs can be functionalized with saccharides. Gold NPs have been coated with non-immunoactive mono- and disaccharides based on synthetic N-acetyl-mannose residues, modeled after the capsular polysaccharide of the bacterium Neisseria meningitidis.Citation43 The glyco-gold NPs are taken up by both a mouse cell line of macrophages and primary human monocytes, and elicit the activation thereof. In particular, these metallic ENSs increase the antigen presenting capabilities of human primary monocytes, leading to the subsequent activation of autologous adaptive immune cells such as CD4+ T lymphocytes (proliferation, secretion of IL-2) in co-cultures. In this study, the importance of multivalency to induce the immuno-activation of monocytes is clearly evidenced as monovalent, non-conjugated mono- and disaccharides are not immuno-active. Moreover, authors have shown that the largest 5 nm gold NP (ie, with enhanced multivalency) perform far better than the smallest 2 nm ones.Citation43 However, due to renal clearance issues, a gold core of 5 nm in diameter is the largest that can be used in human health.
Mannosylated polyamidoamine (PAMAM) dendrimers as drug carriers to target inflammatory macrophages in atherosclerotic plaques
Dendrimers and dendrons are soft matter NPs that have turned out to be very attractive to biologists promptly after their pioneering synthesis.Citation44 They are multivalent hyper-branched (arborescent) non-linear polymers whose synthesis affords isomolecular batches of perfectly defined molecules. They are built from a core on which successive series of branches are linked according to iterative reactions. At each step of the dendritic synthesis, the presence of a point of divergence at the end of each branch enables the grafting of a number of branches equal to two or three times the number of branches at the previous step.Citation44 Therefore, dendrimers and dendrons are arborescent molecules. The number of series of branches determines the generation of the dendrimer (ie, a dendrimer bearing one series of branches is a generation one dendrimer, a dendrimer bearing two series of branches is a generation two dendrimer, and so on). The synthesis ends by the grafting of the desired surface functions which will be eventually displayed in multicopy. Primarily, dendrimers have been envisaged as drug carriers and vectors. Like several other NPs reviewed above, the commercially available polyamidoamine (PAMAM) dendrimers have been functionalized with mannose residues to target macrophages in atherosclerotic plaques.Citation45 Indeed, resident macrophages of these plaques are regulators of both the progression and the stability thereof. The aim of the study was to efficiently address anti-inflammatory ligands of the liver-x-receptor (LXR) to macrophages of atherosclerotic plaques as the systemic administration of these anti-inflammatory compounds precludes their therapeutic efficacy through hepatic uptake. Both mannose residues and LXR ligands have been covalently bound to the terminal amine groups of the PAMAM dendritic scaffold. When administered intravenously to mouse models of atherosclerosis, the ENSs are significantly accumulated in atherosclerotic plaque-associated macrophages. After four weekly injections, the progression and necrosis as well as the inflammation of plaques (assessed by the expression of genes targeted by the pro-inflammatory nuclear factor κB) are significantly decreased.
Simian virus (SV) 40-like NP expressing targeting and drug peptides to target inflammatory macrophages in atherosclerotic plaques
Saccharide residues such as mannose are not the only moieties used to rationally design ENSs expected to target macrophages. Peptides can be targeting moieties when incorporated in ENSs, as illustrated in the two next articles that we have chosen for this review. In the first one,Citation46 the authors have designed a multifunctional virus-like NP of SV40. This smart ENS expresses a peptide that specifically targets macrophages. This peptide has been genetically inserted in a viral protein used to prepare the ENS. An anticoagulant peptide drug (hirulog, a thrombin inhibitor) has been genetically fused at the N-terminus of the same viral protein. Once produced and purified, the fusion proteins undergo self-assembly in 12 pentamers packaging one single near infra-red quantum dot (fluorescent probe). This trifunctional ENS (targeting peptide, therapeutic peptide, fluorescent probe) has been shown to target and to image macrophages in atherosclerotic plaques in diseased mice. Moreover, the therapeutic peptide hirulog inserted in the viral protein retains its anti-thrombin activity.
Phospholipid-based and PEGylated NP expressing targeting peptides and carrying siRNA to suppress tumor-associated macrophages (TAMs)
In the second article, the authors implemented an immunotherapy approach against cancer by targeting TAMs. TAMs are resident, M2-like polarized macrophages of the tumor micro-environment. As such, they generate an immuno-suppressive background within the tumor, promoting thereby the growth of the latter. Indeed, they secrete immuno-suppressive mediators leading to the inhibition of cytotoxic infiltrating CD8+ T lymphocytes which are like disarmed anti-tumor soldiers. Therefore, TAMs are relevant therapeutic targets to fight against cancer. The aim of the study was to inhibit the TAMs, or even to deplete them from the tumor. Nevertheless, it is challenging to specifically address therapeutics to TAMs. For instance, it has been shown that NPs modified with mannose at their surface could target TAMs.Citation47 However, such mannose-capped NPs can also bind dendritic cells and even tumor cells. More recently it has been proposed to tackle the challenge of specifically targeting TAMs using a new ENS: the M2-like TAM dual-targeting NP (M2NP).Citation48 M2NP has structure and function controlled by both a peptide that targets the scavenger receptor B type 1 (SR-B1) and a peptide that has higher specificity to M2 polarized macrophages than to any other leukocyte.Citation49 These two peptides have been fused in one single entity and incorporated in phospholipid based, PEGylated NPs. These ENSs were further loaded with anti-colony stimulating factor-1 receptor (anti-CSF-1R) small interfering (si)-RNA. The CSF-1/CSF-1R pathway is crucial for the differentiation and survival of TAMs, and CSF-1R is restrictively expressed by TAMs in the area of the tumor. Therefore, the anti-CSF-1R siRNA is a promising drug to inhibit the deleterious activity of TAMs. When administered to tumor-bearing mice, the M2NP reaches the expectations of the authors. First of all, M2NP has higher affinity to TAMs than to resident macrophages in liver, spleen, and lungs. Then, mice treated with siRNA-loaded M2NP showed a dramatic decrease of TAMs (52%), with an inhibition of the production of the immuno-suppressive interleukin-10 (IL-10) and tumor growth factor β (TGF-β) within the tumor. On the contrary, the production of immuno-stimulatory cytokines (IL-12 and IFN-γ) and the infiltration of anti-tumor CD8+ T lymphocytes are enhanced. As a consequence, the size of the tumor is dramatically decreased (87%), and the survival of treated animals is significantly prolonged.
Adamantane-based dendrons capped with ibuprofen to reverse inflammatory activation of peritoneal macrophages
The fact that dendrimers and dendrons display multivalency by essence (see above) has been also harnessed to enhance the anti-inflammatory activity of drugs. Authors have grafted ibuprofen at the surface of adamantane-based dendrons (HYDRAmers).Citation50 Two HYDRAmers have been prepared: the generation 1 HYDRAmer that bears three ibuprofen moieties, and the generation 2 that bears nine ibuprofen moieties. In vitro assessment of the anti-inflammatory potential of the two dendritic ENSs has been performed with concentrations guaranteeing the incubation of cells with equal amounts of free (control) or conjugated ibuprofen. Two types of mouse macrophages have been used in this study: a cell line and primary peritoneal macrophages. Prior to exposure to free or to dendrimer-conjugated ibuprofen, macrophages were pre-stimulated with lipopolysaccharide (LPS), a pro-inflammatory bacterial compound. Analysis of the production of inflammatory cytokines (TNF-α and IL-6) by the treated macrophages shows that the two ibuprofen-HYDRAmers are more effective than free ibuprofen. Moreover, higher multivalency of the generation 2 ibuprofen-HYDRAmer allows a higher anti-inflammatory potential when compared to the generation 1 competitor. Although this proof of concept is encouraging regarding therapeutic applications of multivalent ENSs, however, it has to be mentioned that the ibuprofen-HYDRAmers exhibit enhanced cytotoxicity on macrophages when compared to the one of free ibuprofen.
Phosphorus-based dendrimers capped with azabisphosphonates to target monocytes to treat mouse inflammatory diseases
In some scarce cases, dendrimers have shown therapeutic effects by themselves, without any drug cargo or bound drug. In particular, several families of dendrimers have intrinsic anti-inflammatory properties, throughout different mechanisms of action.Citation44,Citation51 Among them, phosphorus-based dendrimers, also called polyphosphorhydrazone (PPH) dendrimers, are naturally targeting human monocytes when they bear anionic azabisphosphonate (ABP) groups at their surface.Citation52 Once internalized by monocytes, the generation 1 PPH dendrimer capped with 12 ABP groups triggers a M2-like anti-inflammatory activation of monocytes.Citation53 This unique dendrimer has shown therapeutic efficacy in mouse models of chronic inflammatory diseases of auto-immune origin such as experimental arthritis (relevant to human rheumatoid arthritis)Citation54 and experimental auto-immune encephalomyelitis (EAE, relevant to human multiple sclerosis).Citation55 In both cases, monocytes and macrophages activated by the PPH dendrimer are involved in the resolution of the inflammatory disorder in cooperation with other immune cells.Citation56 Besides these preclinical studies of the therapeutic efficacy of the PPH dendrimer, we have shown that ABP groups are crucial for the bioactivity of the molecule, insofar as analog dendrimers capped with the same number of isosteric functions of the ABP groups (such as sulfonate and carboxylate groups) are inactive.Citation57,Citation58 We have also shown that maximization of multivalency occurs in the active PPH dendrimer through the three-dimensional directionality of the moleculeCitation59 and a low flexibility of the phosphonate clusters at its surface.Citation60 Nevertheless, the specific receptors of the molecule at the surface of monocytes have to be uncovered.
Poly-carboxylated NPs to derail monocytes to the spleen to treat mouse inflammatory diseases
Other anionic NPs have shown tropism toward macrophages. These are polystyrene (PS), nano-diamond (ND), and poly(lactic-co-glycolic acid) (PLGA) NPs.Citation61 When they are highly negatively charged with carboxylate functions, these ENSs have immuno-modulatory functions in vivo in several unrelated mouse models of inflammatory disorders. They are qualified as “immune-modifying microparticles” (IMPs) despite their size being around 500 nm. These poly-anionic IMPs are recognized and taken up by circulating inflammatory monocytes through a scavenger receptor, the macrophage receptor with collagenous structure (MARCO). This receptor-mediated up-take reduces the migration of inflammatory monocytes to the sites of inflammation. The cells are rather redirected to the spleen where they die by caspase 3-mediated apoptosis. The availability of clinical-grade PLGA should foster the rapid clinical translation of this innovative concept of derailing monocytes with fatal outcome.Citation62
Conclusion
Macrophages are present in most, if not all, organs of the human body, where they assume a myriad of functions ranging from defense mechanisms to development and maintenance of tissue homeostasis, wound healing, repair and remodeling following an injury.Citation11,Citation12 In addition, these cells are involved in a broad spectrum of pathologies. De facto, they represent a relevant therapeutic target. Nevertheless, winning a battle requires a thorough knowledge of one’s enemy. Accordingly, the manipulation of monocytes/macrophages for therapeutic purposes requires, beforehand, an accurate knowledge of the biology of these cells. The latter has evolved tremendously in recent years, leading to an unprecedented paradigm shift. Indeed, the dogma that tissue macrophages were perpetually renewed from circulating monocytes,Citation63,Citation64 derived from bone marrow progenitors, has had its day but is now clearly over. Thanks to the advent of new technologies, and in particular those of fate-mapping, it is now established that the great majority of tissue-resident macrophages have an embryonic origin. They are seeded before birth, during embryonic development and, in steady-state, they maintain themselves by self-renewal during adulthood, independently of an influx of circulating monocytes.Citation65–Citation68 The study of the ontogeny of macrophages is still in progress, but in light of recent work, several scenarios stand outCitation69–Citation71 ():
Table 2 Origin of tissue-resident macrophages
tissues in which the resident macrophages have only an embryonic origin. They originate from a progenitor either 1) in the yolk sac: cerebral parenchyma of the brain; or 2) in the fetal liver (primitive hematopoiesis): liver, lungs, spleen and kidneys; or 3) a mixture of both: epidermis,
tissues in which the resident macrophages have a dual origin, embryonic (primitive hematopoiesis in the fetal liver) and adult (definitive hematopoiesis in the bone marrow): liver and pancreas,
tissues in which the resident macrophages come solely from bone marrow myeloid progenitors derived from the adult hematopoiesis: intestine and dermis.
Hence, the majority of the resident macrophages comes from embryonic progenitors, with an exception made during pathophysiological episodes, such as under inflammatory conditions, where some particular infiltrating monocytes (Ly6Chigh/CCR2high/CX3CR1low) give rise to monocyte-derived macrophages. Nonetheless, one should kept in mind that these studies were carried out in the mouse and that the transferability of the discoveries to humans is only presumed. Another bottom line element is the knowledge of the functions of macrophages within tissues, both in a healthy and pathological context. Furthermore, although they may be ontogenetically identical, resident macrophages of distinct tissues, have significant differences, both in terms of gene expression profile, expressed markers, and/or assured functions.Citation11,Citation12,Citation72,Citation73
Our vision of the world of innate immune cells has dramatically changed: we must now consider macrophages as a heterogeneous population, both in terms of ontogeny, functionality and involvement in diseases. With these new insights into the biology of monocytes/macrophages brought about by the rise of brand-new advanced technologies, we can now rationally target these cells when they have undergone a deleterious pathway of activation or exploit their therapeutic potential. As reviewed herein, multivalent ENSs are relevant and promising therapeutic tools to modulate the immuno-activation of macrophages. Nevertheless, the up-coming challenge to tackle is the specific targeting of the different subsets of macrophages, differently involved in inflammatory diseases and disorders. Undoubtedly, thanks to their tunable and versatile properties, ENSs are the appropriate tools to reach this objective.Citation74 However, the long-term toxicity of ENSs has to be evaluated: general toxicity, neuro-toxicity, and immuno-toxicity in humans as well as environmental toxicity.Citation37
Disclosure
The authors report no conflicts of interest in this work.
References
- MedzhitovROrigin and physiological roles of inflammationNature2008454720342843518650913
- FullertonJNGilroyDWResolution of inflammation: a new therapeutic frontierNat Rev Drug Discov201615855156727020098
- BuckleyCDGilroyDWSerhanCNProresolving lipid mediators and mechanisms in the resolution of acute inflammationImmunity201440331532724656045
- NewsonJStablesMKarraEResolution of acute inflammation bridges the gap between innate and adaptive immunityBlood2014124111748176425006125
- LiCXuMMWangKAdlerAJVellaATZhouBMacrophage polarization and meta-inflammationTransl Res2018191294429154757
- RobinsonWHLepusCMWangQLow-grade inflammation as a key mediator of the pathogenesis of osteoarthritisNat Rev Rheumatol2016121058059227539668
- Ruiz-NúñezBDijck-BrouwerDAMuskietFAThe relation of saturated fatty acids with low-grade inflammation and cardiovascular diseaseJ Nutr Biochem20163612027692243
- AsgharASheikhNRole of immune cells in obesity induced low grade inflammation and insulin resistanceCell Immunol2017315182628285710
- HamonPLoyherPLBaudesson de ChanvilleCLicataFCombadièreCBoissonnasACX3CR1-dependent endothelial margination modulates Ly6Chigh monocyte systemic deployment upon inflammation in miceBlood2017129101296130728011675
- van FurthRSluiterWDistribution of blood monocytes between a marginating and a circulating poolJ Exp Med198616324744793944542
- DaviesLCJenkinsSJAllenJETaylorPRTissue-resident macrophagesNat Immunol2013141098699524048120
- GordonSPlüddemannATissue macrophages: heterogeneity and functionsBMC Biol20171515328662662
- GinhouxFJungSMonocytes and macrophages: developmental pathways and tissue homeostasisNat Rev Immunol201414639240424854589
- GeissmannFJungSLittmanDRBlood monocytes consist of two principal subsets with distinct migratory propertiesImmunity2003191718212871640
- Grage-GriebenowEFladHDErnstMHeterogeneity of human peripheral blood monocyte subsetsJ Leukoc Biol2001691112011200054
- IngersollMAPlattAMPotteauxSRandolphGJMonocyte trafficking in acute and chronic inflammationTrends Immunol2011321047047721664185
- ShiCPamerEGMonocyte recruitment during infection and inflammationNat Rev Immunol2011111176277421984070
- GordonSPhagocytosis: an immunobiologic processImmunity201644346347526982354
- KawaiTAkiraSThe role of pattern-recognition receptors in innate immunity: update on Toll-like receptorsNat Immunol201011537338420404851
- WynnTAVannellaKMMacrophages in tissue repair, regeneration, and fibrosisImmunity201644345046226982353
- NathanCFMurrayHWWiebeMERubinBYIdentification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activityJ Exp Med198315836706896411853
- SteinMKeshavSHarrisNGordonSInterleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activationJ Exp Med199217612872921613462
- MartinezFOGordonSThe M1 and M2 paradigm of macrophage activation: time for reassessmentF1000Prime Rep201461324669294
- MillsCDKincaidKAltJMHeilmanMJHillAMM-1/M-2 macrophages and the Th1/Th2 paradigmJ Immunol2000164126166617310843666
- GinhouxFSchultzeJLMurrayPJOchandoJBiswasSKNew insights into the multidimensional concept of macrophage ontogeny, activation and functionNat Immunol2016171344026681460
- LavinYWinterDBlecher-GonenRTissue-resident macrophage enhancer landscapes are shaped by the local microenvironmentCell201415961312132625480296
- XueJSchmidtSVSanderJTranscriptome-based network analysis reveals a spectrum model of human macrophage activationImmunity201440227428824530056
- MurrayPJAllenJEBiswasSKMacrophage activation and polarization: nomenclature and experimental guidelinesImmunity2014411142025035950
- MammenMChoiSKWhitesidesGMPolyvalent interactions in biological systems: implications for design and use of multivalent ligands and inhibitorsAngew Chem Int Ed Engl199837202754279429711117
- CochranJRAivazianDCameronTOSternLJReceptor clustering and transmembrane signaling in T cellsTrends Biochem Sci200126530431011343923
- JayaramanNMultivalent ligand presentation as a central concept to study intricate carbohydrate-protein interactionsChem Soc Rev200938123463348320449063
- VarnerCTRosenTMartinJTKaneRSRecent advances in engineering polyvalent biological interactionsBiomacromolecules2015161435525426695
- GestwickiJECairoCWStrongLEOetjenKAKiesslingLLInfluencing receptor-ligand binding mechanisms with multivalent ligand architectureJ Am Chem Soc200212450149221493312475334
- ConwayAVazinTSpelkeDPMultivalent ligands control stem cell behaviour in vitro and in vivoNat Nanotechnol201381183183824141540
- PerlAGomez-CasadoAThompsonDGradient-driven motion of multivalent ligand molecules along a surface functionalized with multiple receptorsNat Chem20113431732221430692
- TomasSMilanesiLMutual modulation between membrane-embedded receptor clustering and ligand binding in lipid membranesNat Chem20102121077108321107373
- PoupotRBergozzaDFruchonSNanoparticle-based strategies to treat neuro-inflammationMaterials2018112270
- CupaioliFAZuccaFABoraschiDZeccaLEngineered nanoparticles. How brain friendly is this new guest?Prog Neurobiol2014119–1202038
- HeHGhoshSYangHNanomedicines for dysfunctional macrophage-associated diseasesJ Control Release201724710612628057522
- LepeniesBLeeJSonkariaSTargeting C-type lectin receptors with multivalent carbohydrate ligandsAdv Drug Deliv Rev20136591271128123727341
- CostaASarmentoBSeabraVMannose-functionalized solid lipid nanoparticles are effective in targeting alveolar macrophagesEur J Pharm Sci201811410311329229273
- SarwarHSAshrafSAkhtarSMannosylated thiolated polyethylenimine nanoparticles for the enhanced efficacy of antimonial drug against LeishmaniasisNanomedicine (Lond)2018131254129173059
- FallariniSPaolettiTBattagliniCOFactors affecting T cell responses induced by fully synthetic glyco-gold-nanoparticlesNanoscale20135139040023175231
- HayderMFruchonSFourniéJJPoupotMPoupotRAnti-inflammatory properties of dendrimers per seScientificWorldJournal2011111367138221789472
- HeHYuanQBieJDevelopment of mannose functionalized dendrimeric nanoparticles for targeted delivery to macrophages: use of this platform to modulate atherosclerosisTransl Res2018193133029172034
- SunXLiWZhangXIn vivo targeting and imaging of atherosclerosis using multifunctional virus-like particles of Simian Virus 40Nano Lett201616106164617127622963
- ZhuSNiuMO’MaryHCuiZTargeting of tumor-associated macrophages made possible by PEG-sheddable, mannose-modified nanoparticlesMol Pharm20131093525353023901887
- QianYQiaoSDaiYMolecular-targeted immunotherapeutic strategy for melanoma via dual-targeting nanoparticles delivering small interfering RNA to tumor-associated macrophagesACS Nano20171199536954928858473
- CieslewiczMTangJYuJLTargeted delivery of proapoptotic peptides to tumor-associated macrophages improves survivalProc Natl Acad Sci U S A201311040159191592424046373
- LamannaGRussierJDumortierHBiancoAEnhancement of anti-inflammatory drug activity by multivalent adamantane-based dendronsBiomaterials201233225610561722560196
- FruchonSPoupotRPro-inflammatory versus anti-inflammatory effects of dendrimers: the two faces of immuno-modulatory nanoparticlesNanomaterials201779251
- PoupotMGriffeLMarchandPDesign of phosphorylated dendritic architectures to promote human monocyte activationFaseb J200620132339235117077311
- FruchonSPoupotMMartinetLAnti-inflammatory and immunosuppressive activation of human monocytes by a bioactive dendrimerJ Leukoc Biol200985355356219047518
- HayderMPoupotMBaronMA phosphorus-based dendrimer targets inflammation and osteoclastogenesis in experimental arthritisSci Transl Med201138181ra35
- HayderMVarilhMTurrinCOPhosphorus-based dendrimer ABP treats neuroinflammation by promoting IL-10-producing CD4(+) T cellsBiomacromolecules201516113425343326397709
- FruchonSPoupotRThe ABP dendrimer, a drug-candidate against inflammatory diseases that triggers the activation of interleukin-10 producing immune cellsMolecules20182361272
- LedallJFruchonSGarzoniMInteraction studies reveal specific recognition of an anti-inflammatory polyphosphorhydrazone dendrimer by human monocytesNanoscale2015742176721768426335052
- RollandOTurrinCOBacquetGEfficient synthesis of phosphorus-containing dendrimers capped with isosteric functions of amino-bismethylene phosphonic acidsTetrahedron Lett2009501820782082
- CaminadeAMFruchonSTurrinCOThe key role of the scaffold on the efficiency of dendrimer nanodrugsNat Commun20156772226169490
- HayderMGarzoniMBochicchioDThree-dimensional directionality is a pivotal structural feature for the bioactivity of azabisphosphonate-capped poly(PhosphorHydrazone) nanodrug dendrimersBiomacromolecules201819371272029443507
- GettsDRTerryRLGettsMTTherapeutic inflammatory monocyte modulation using immune-modifying microparticlesSci Transl Med20146219219ra217
- HarrisonCInflammatory disorders: monocytes derailed by microparticlesNat Rev Drug Discov201413317524525780
- van FurthRCohnZAThe origin and kinetics of mononuclear phagocytesJ Exp Med196812834154355666958
- van FurthRCohnZAHirschJGHumphreyJHSpectorWGLangevoortHLThe mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cellsBull World Health Organ19724668458524538544
- GinhouxFGreterMLeboeufMFate mapping analysis reveals that adult microglia derive from primitive macrophagesScience2010330600584184520966214
- HashimotoDChowANoizatCTissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytesImmunity201338479280423601688
- HoeffelGWangYGreterMAdult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophagesJ Exp Med201220961167118122565823
- YonaSKimKWWolfYFate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasisImmunity2013381799123273845
- GinhouxFGuilliamsMTissue-resident macrophage ontogeny and homeostasisImmunity201644343944926982352
- LavinYMorthaARahmanAMeradMRegulation of macrophage development and function in peripheral tissuesNat Rev Immunol2015151273174426603899
- TussiwandRGautierELTranscriptional regulation of mononuclear phagocyte developmentFront Immunol2015653326539196
- GautierELShayTMillerJGene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophagesNat Immunol201213111118112823023392
- LavinYMeradMMacrophages: gatekeepers of tissue integrityCancer Immunol Res20131420120924777851
- MahonEBarboiuMSynthetic multivalency for biological applicationsOrg Biomol Chem20151343105901059926434808
- World Health Organization [webpage on the Internet]Obesity and overweight / Facts about overweight and obesity [February 16, 2018] Available from: http://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweightAccessed September 4, 2018
