Abstract
Due to their small particle size and large and modifiable surface, nanoparticles have unique advantages compared with other drug carriers. As a research focus in recent years, polyethylene glycol–polylactic acid (PEG–PLA) block copolymer and its end-group derivative nanoparticles can enhance the drug loading of hydrophobic drugs, reduce the burst effect, avoid being engulfed by phagocytes, increase the circulation time of drugs in blood, and improve bioavailability. Additionally, due to their smaller particle size and modified surface, these nanoparticles can accumulate in inflammation or target locations to enhance drug efficacy and reduce toxicity. Recent advances in PEG–PLA block copolymer nanoparticles, including the synthesis of PEG–PLA and the preparation of PEG–PLA nanoparticles, were introduced in this study, in particular the drug release and modifiable characteristics of PEG–PLA nanoparticles and their application in pharmaceutical preparations.
Introduction
Polylactic acid (PLA) is a synthetic biodegradable polymer. In the aquatic environment, it hydrolyzes into nontoxic hydroxyl-carboxylic acid through ester bond cleavage and then is metabolized into water and carbon dioxide through a citric acid cycle. Due to its suitable biodegradability, good security, low immunity, and good mechanical strength, PLA has been approved by the US Food and Drug Administration for application in tissue engineering, medical materials, drug carriers, for example,Citation1 and it has good prospects for peptides, proteins, vaccines, anticancer drugs, and other drug carriers. However, PLA applications are limited due to its weak hydrophilicity, excessively long degradation time, and low drug loading of polar drugs.Citation2 On the other hand, polyethylene glycol (PEG) has many advantages, such as good hydrophilicity, flexibility, antiphagocytosis against macrophages, resistance to immunological recognition, non-combination with proteins, and biocompatibility.Citation3–Citation6 Through copolymerization with PEG, PLA can be improved in hydrophilicity, degradation rate,Citation7 and crystallization,Citation8 showing great potential for development in drug delivery. The degradation products of PEG–PLA block copolymer can enter the tricarboxylic acid cycle or be eliminated by the kidney. Thus, in low concentration the copolymer is nontoxic and not accumulative in vivo.Citation9 The copolymerization of PLA and PEG can increase the drug loading, reduce the burst effect, and prolong the in vivo residence time of drugs and avoid them being engulfed by macrophages. The synthesis of PEG–PLA block copolymer and its end-group derivatives and the preparation of PEG–PLA nanoparticles will be discussed in this paper, in particular their drug release and modifiable characteristics and their application in pharmaceutical preparations.
Synthesis of PEG–PLA block copolymer and its end-group derivatives
Ring-opening polymerization of PEG and lactide
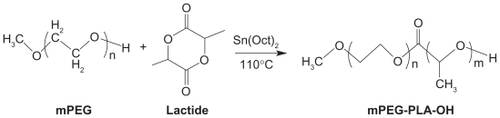
These polymers may be synthesized by ring-opening polymerization between PEG or its end-group derivatives (such as methoxy polyethylene glycol [mPEG]) and lactide ().Citation10 Tin salts are the commonly used catalysts, especially stannous compounds with a higher catalytic efficiency. However, due to the toxicity of these heavy metal compounds, acetic acid bismuth was used as an initiator by Kricheldorf et al.Citation11 It was found that in the copolymerization system of l-lactide and PEG tetramer, the length of polymer chain could be controlled by changing the proportion of monomer and initiator, and copolymers with different molecular structures could be synthesized, such as A-B stellate copolymer, A-B-A triblock copolymer, multiblock copolymer, and reticular copolymer.
Figure 1 Synthesis scheme of mPEG–PLA.Citation10
Copyright © 2010, Elsevier Limited. Reproduced with permission from Zhang X, Li Y, Chen X, et al. Synthesis and characterization of the paclitaxel/MPEG-PLA block copolymer conjugate. Biomaterials. 2005;26(14):2121–2128.
Abbreviations: mPEG, methoxy polyethylene glycol; PLA, polylactic acid.
Anionic ring-opening polymerization
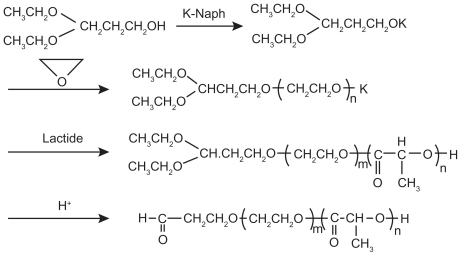
In anionic ring-opening polymerization, common catalysts include potassium alkoxide,Citation12 sodium alkoxide,Citation13 and butyl lithium. Otsuka et alCitation13 synthesized 3,3-diethoxy-potassium propanol ([C2H5O]2CHCH2OK) with the initial reactants 3,3-diethoxy-propanol ([C2H5O]2CHCH2OH) and potassium naphthalene (K-Naph) and the solvent tetrahydrofuran (THF), and then synthesized α-acetal-PEG–PLA block copolymer through anionic ring-opening polymerization with ethylene oxide and lactic acid (LA) as reactants and 3,3-diethoxy-potassium propanol as an initiator ().
Figure 2 Synthesis scheme of acetal-ended poly(ethylene glycol)–poly(lactic acid) copolymers.Citation13
Copyright © 2010, American Chemical Society. Reproduced with permission from Otsuka H, Nagasaki Y, Kataoka K. Surface characterization of functionalized polylactide through the coating with heterobifunctional poly(ethylene glycol)/polylactide block copolymers. Biomacromolecules. 2000;1(1):39–48.
Synthesis of PEG–PLA end-group derivatives
The physical and chemical properties of PEG–PLA copolymers can be improved by modifying the hydroxyl of the PEG end-group for better-loading hydrophobic, gene, and protein drugs.Citation14,Citation15 The common end-group forms of PEG–PLA derivatives include hydroformylation, propylene acylation, and amination.Citation16 For example, Salem et alCitation17 first linked biotin with PEG to synthesize biotin–PEG and then linked PLA with biotin–PEG to synthesize biotin–PEG–PLA polymer through ring-opening polymerization.
Preparation of PEG–PLA block copolymer nanoparticles
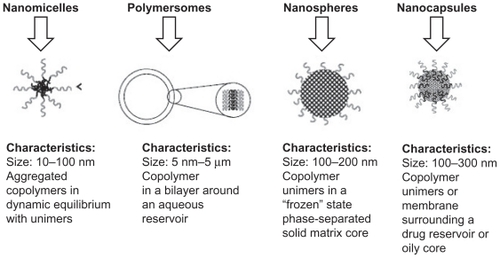
Using different compositions and preparation methods, amphiphilic block copolymers such as PEG–PLA block copolymer can be prepared into various forms of nanoparticles, including nanomicelles, polymersomes, nanospheres, and nanocapsules ().Citation18
Figure 3 Classification of nanoparticle drug delivery systems and their general characteristics.Citation18
Copyright © 2010, Elsevier Limited. Reproduced with permission from Letchford K, Burt H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: micelles, nanospheres, nanocapsules and polymersomes. Eur J Pharm Biopharm. 2007;65(3):259–269.
Preparation of PEG–PLA block copolymer nanomicelles
The method of preparation of PEG–PLA block copolymer nanomicelles mainly depends on the hydrophilicity of copolymers. The hydrophilic copolymer can form micelles by self-assembly in water. The direct dissolution method is most commonly used, ie, after being dissolved directly in water (they are able to be heated), the copolymers may form the transparent micellar solution immediately above its critical micelle concentration.Citation19 Another is the film rehydration method, ie, copolymers and drugs are dissolved in volatile solvent to form a membrane after vaporizing solvent, and then the micelles can be formed by adding buffer solution or water as well as stirring and dissolving copolymer membranes.Citation20 If the copolymer is insoluble in water, organic solvents may be used. The copolymer is first dissolved in the organic solvent (or water-mixed solvent), and then the organic solvent is removed by dialysis or evaporation.Citation21
Preparation of PEG–PLA block copolymer polymersomes
Some preparation methods of liposomes, such as the injection method, film rehydration method, and ultrasonic dispersion method, can also be used to prepare copolymer polymersomes. The solvent injection method was adopted by Jain and KumarCitation22 to prepare amphotericin B-loaded polymersomes. In the film rehydration method, copolymers are dissolved in volatile organic solvents, the membranes form after the rotary evaporation of organic solvents, and then the copolymer polymersomes form after adding buffer solution and stirring constantly with sonication and extrusion.Citation23
Preparation of PEG–PLA block copolymer nanospheres
The preparation methods of copolymer nanospheres include the emulsification solvent evaporation method and emulsification solvent diffusion method. The process is generally divided into two steps. The first step is emulsification, ie, the copolymer and drugs are dissolved in organic solvent and then the emulsion is formed by adding into the water phase and stirring. The second step is to remove the organic solvent in the emulsion by evaporation or dialysis.Citation24 Venkatraman et alCitation25 used the emulsification solvent evaporation method to prepare the PLA–PEG–PLA nanospheres. First, the copolymer was dissolved in organic solvent (acetone, THF, dimethylformamide, or dimethylacetamide) and mixed with deionized water by stirring. Then, acetone or THF was removed by evaporation, dimethylformamide or dimethylacetamide was removed by dialysis, and finally the nanospheres were obtained after freeze dehydration.
Preparation of PEG–PLA block copolymer nanocapsules
The interfacial polymerization method is commonly used to prepare PEG–PLA nanocapsules, ie, drugs and block copolymers are dissolved in water-miscible organic solvent, and then nanocapsules can be prepared by slowly dripping the mixed solvent into aqueous solution by stirring with or without surfactants.Citation26
The release of PEG–PLA block copolymer nanoparticles
The release mechanism of PEG–PLA block copolymer nanoparticles
The release mechanism of PEG–PLA nanoparticles is similar to that of general nanoparticles, and the common mechanisms include three types: 1) adsorption and desorption of drugs on nanoparticles surface; 2) diffusion release; and 3) degradation of nanomatrix or degradation/diffusion collaborative process. Citation27 The drug release mainly depends on the following: 1) desorption of drugs absorbed on the surface or interface; 2) diffusion by nanoparticle matrix; 3) diffusion through the copolymer wall (nanocapsules); 4) dissolution of nanomatrix; and 5) dissolution or diffusion of bond compounds. Therefore, the process of drug release is controlled by drug diffusion and matrix degradation.Citation28
The release mechanism for most nanoparticles can be divided into two phases: the burst release phase and controlled release phase. In the burst release phase, drugs diffuse quickly in the solvent medium due to drugs adsorbing or weak bonding onto the surface of nanoparticles with a large surface area. Because drugs are not uniformly distributed/dissolved in the matrix (hydrophobic chain), in the controlled release phase the drug release by diffusion or erosion (degradation) depends on the characteristics of the drug delivery system. The drug release mainly depends on diffusion when the rate of drug diffusion is greater than that of matrix degradation; otherwise, it mainly depends on matrix degradation when the rate of drug diffusion is less.Citation29 Li et alCitation21 used the volatile dialysis method with organic solvent to prepare all-trans retinoic acid-loaded nanomicelles and then analyzed the release mechanism of nanomicelles. It was found that in ensuring the integrity of nanoparticles, by using the mixture of phosphate-buffered saline (PBS) (pH 7.4) and ethanol (with the proportion of 9:1) as the release medium, the burst release occurred in the first 15 h, and then the controlled release occurred. Because nanoparticles were complete and not degraded in the above condition, the drug release mechanism was considered as diffusion after dissolution. Over 80% of drugs had been released after 5 days. Mean-while, in another method, the drug-loaded nanomicelles were incubated in PBS, and then the molecular weight was analyzed by nuclear magnetic resonance. It was found that the nanomicelles were slowly degraded. The degradation time of 27.6% of mPEG5–PLA5 and 30.1% of mPEG2–PLA16 was more than 30 days. Therefore, the release mechanism of all-trans retinoic acid-loaded mPEG–PLA nanomicelles was considered as mainly relying on drug diffusion rather than matrix degradation.
However, drug release can be induced by physical or chemical methods. Light,Citation30 temperature,Citation31 pH,Citation32 power,Citation33 magnetic field,Citation34 and ultrasoundCitation19 have been used to control drug release from copolymer nanoparticles.
Influencing factors on drug release of PEG–PLA block copolymer nanoparticles
PEG–PLA block copolymer is an amphiphilic polymer with good stability in vivo. With good biocompatibility, the PEG hydrophilic layer can increase the solubility of insoluble drugs, effectively prevent the protein absorbed on the nanoparticle surface, make nanoparticles unrecognizable by the reticuloendothelial system as foreign bodies, and thereby show a characteristic of long circulation. Many factors may influence drug release of PEG–PLA block copolymer nanoparticles. The main factors are as follows:
Molecular weight, chain length of PEG or PLA, and PEG/PLA ratio in the polymer: The chain length of PEG and PLA can be controlled by changing the molecular weight of PEG and the concentrations of PEG and PLA. The longer the PLA chain length, the larger would be the nanoparticle size and the drug loading of hydrophobic drugs. As the PEG content and weight-average molecular weight (Mw) of PLA–PEG–PLA copolymers increased, the amount of drug release increased and the total Mw of copolymers of nanoparticles decreased. Drug release from nanoparticles could potentially be controlled by changing the content of PEG, Mw of PEG, and total Mw of copolymer.Citation35 It was found by Yang et alCitation36 that the longer the PLA chain length, the larger the diameter of micelles and drug-loaded micelles would be. An in vitro release test showed that the longer the PLA chain length, the greater the interaction between PLA chain and hydrophobic drug would be and the slower the drug release rate of micelles would be. In case of a low Mw of PEG, deformation occurs easily due to the small molecular chain and low flexibility. The greater the molecular weight of PEG, the longer the PEG molecular chain length will be and the more stable the structure will be. The increase of PLA block Mw in the copolymer will reduce significantly the stability of nanoparticles and even lead to condensation in the solvent.Citation21
Preparation methods and conditions of PEG–PLA block copolymer nanoparticles: Different preparation technologies of nanoparticles will influence the crystal shape of polymer, drug distribution, and stability in the carrier materials; influence the surface morphology, particle size, and internal compactness of nanoparticles; and thus influence the rate and degree of drug release.Citation37
Particle size of PEG–PLA block copolymer nanoparticles: The particle size of PEG–PLA block copolymer nanoparticles can be changed by adjusting PEG chain length and PEG/PLA ratio or using a different preparation method. PEG–PLA block copolymer nanoparticles with different sizes will lead to different degradation or diffusion rates of nanomatrix, resulting in differences in drug release.Citation38
Drug loading of PEG–PLA block copolymer nanoparticles: Drug loading of PEG–PLA block copolymer nanoparticles is also an important influencing factor for drug release. The relationship between drug loading and micelle stability was studied by Huh et al,Citation39 and it was found that due to the incorporation of hydrophobic drugs, the micellar hydrophilic–hydrophobic balance was destroyed, and the stability of micelles would be increased with drug loading decreased. When the paclitaxel loading of PEG–PLA micelles was 17.6%, the paclitaxel would be dissolved completely in sodium salicylate solution after 5 days, whereas in the case of loading capacity of 27.6%, the paclitaxel would be dissolved completely after 3 days.
In addition to the above major influencing factors, drug release will be influenced by copolymer concentration,Citation40 pH,Citation41 zeta potential,Citation42 and solvents.Citation43
Surface modification of PEG–PLA block copolymer nanoparticles
By modifying the surface of PEG–PLA block copolymer nanoparticles, various special nanoparticles may be prepared in order to increase the therapeutic effect of drugs, such as long-circulating nanoparticles, immunonanoparticles, thermosensitive nanoparticles, and pH-sensitive nanoparticles. Common materials for surface modification include three types: 1) polysaccharides such as cyclodextrin and chitosan; 2) surfactants such as polysorbate; and 3) PEG poloxamer. Due to their core shell structure, the surface of PEG–PLA block copolymer nanoparticles is often modified by folic acid, peptide, lectin, and albumin. Compared with PEG–PLA block copolymer nanoparticles with passive targeting, these modified nanoparticles can actively target special locations for enhancing drug efficacy and decreasing drug toxicity. For example, PEG–PLA nanoparticles with surface-bound lectins (biorecognitive ligands) were established by Gao et al.Citation44 Due to abundant N-acetyl-d-glucosamine and sialic acid in the nasal cavity, wheat germ agglutinin was selected as a model lectin to bind them. The resulting nanoparticles increased significantly the uptake of drugs in the brain associated with nanoparticles through intranasal delivery, which might provide a novel effective noninvasive technique for drug delivery to the brain, particularly for biotech drugs such as peptides, proteins, and DNA. Yu et alCitation45 synthesized the aldehyde–PEG–PLA block copolymer by ring-opening polymerization and conjugated a peptide (K237 ligand) to the aldehyde group of PEG chain by using the N-terminal PEGylation technique. The K237-conjugated paclitaxel nanoparticles could be significantly internalized by human umbilical vein endothelial cells through the K237-KDR (K237-vascular endothelial growth factor receptor) interaction. This facilitated uptake of paclitaxel led to the enhanced antiangiogenic activity, migration, and tube formation compared with cells treated with paclitaxel nanoparticles and commercial taxol. PEG–PLA block copolymer nanoparticles modified with folic acid or its salts as target materials can accumulate in cancer locations, increase drug concentration in cancer locations, and prolong the action time. Tsai et alCitation46 prepared poly(HEMA-co-histidine)-g-PLA and folate-PEG–PLA nanomicelles. Folate for cancer-specific target was bound at the end of the polymer chain. It was found that cellular uptake of folate micelles was higher than that of non-folate micelles due to the folate-binding effect on the cell membrane. An in vivo study of folate micelles exhibited cancer targeting and effective inhibition of tumor growth.
Application of PEG–PLA block copolymer nanoparticles in pharmaceutical preparation
The PEG–PLA copolymer has the advantages of both PEG and PLA. As a drug carrier, PEG–PLA copolymer nanoparticles have some advantages, eg, 1) reducing the first-pass effect and increasing bioavailability;Citation47 2) increasing drug loading and encapsulation efficiency;Citation48 3) reducing particle size and burst release while improving targeting;Citation49 4) avoiding recognition and removal by the reticuloendothelial system, thereby prolonging the circulation time of drugs in the blood and improving stability;Citation50 and 5) good safety.Citation9 In many studies, PEG–PLA block copolymer nanoparticles were used as carriers for vaccine, protein, and gene drugs, particularly in a sustained/controlled release drug delivery system and targeted-drug delivery system that could enhance drug efficacy and reduce drug resistance.Citation51
Sustained and controlled release drug delivery system
PEG–PLA copolymer nanoparticles are mainly diffusion and degradation controlled release systems. Hydrophobic drugs mainly accumulate in the hydrophobic matrix. In diffusion controlled systems, drugs are dissolved or dispersed in PLA polymers, and the release rate is controlled by drug diffusion through a PLA matrix. The drugs adjacent to the membrane surface can be released smoothly, whereas drugs inside the membrane are required to be diffused to the membrane surface first and then are released successfully. In the degradation controlled system, drugs are dispersed in PLA, and the drug release rate is determined by degradation rate due to influences from PLA chain length, drug loading of nanoparticles, release medium, and other factors.
PEG–PLA and PLA nanoparticle mixture loaded with betamethasone disodium phosphate was prepared by Ishihara et al.Citation52 Taking inflammation mice as a model, the anti-inflammatory activity was found to be partly weakened by nanoparticles. With in vitro detection on nanoparticle degradation, PEG chains on the surface of nanoparticles disappeared in a few days and then the drug was slowly released. These nanoparticles accumulated at inflammation and injury locations preferentially, and a large amount of drugs was gradually degraded from these locations with the duration of more than 14 days.
PEG–PLA nanoparticles do not change the spatial configuration of proteins, antigens, and other bioactive substances to maintain their biological activities. Meanwhile, PEG–PLA copolymer nanoparticles loaded with proteins may avoid the degradation of proteins by proteases in the blood, reduce the recognition of immune cells, and enhance stability, so that protein inactivation is not able to occur easily. Thus, they are particularly suitable as a biotech drug carrier, especially for oral controlled release delivery of proteins and enzymes.Citation53,Citation54 The protein drug and gene therapeutic agents encapsulated in PEG–PLA block copolymer nanoparticles for sustained/controlled release may improve therapeutic effects of drugs and the quality of life of patients, and consequently the nanoparticles have become popular carriers for proteins and gene drugs. For example, Rafat et alCitation15 evaluated PEG–PLA microparticles for encapsulation and delivery of a transactivator of transcription-enhanced green fluorescent protein fusion (Tat-EGFP) to retinal cells. The results suggested that PEG–PLA microparticles can deliver proteins in cell culture, allowing protein internalization in as little as 1 h. In vivo, protein was shown to localize within the photoreceptor layer of the retina and persist for at least 9 weeks with no observed toxicity.
Targeted drug delivery system
PEG–PLA nanoparticles with a narrow distribution of particle size have a smaller particle size than PLA nanoparticles. Thus, they may accumulate easily in inflammation locations and then slowly release the drugs. In particular, targeting molecules can be introduced in the PEG terminal to enhance active targeting. Therefore, the combination of passive and active targeting can act effectively on lesion locations. The carboxy-PEG–PLA block copolymer was synthesized by Ueki et alCitation55 and was used to prepare camptothecin nanoparticles. The results showed that nanoparticles can effectively improve the delivery efficiency of camptothecin to the tumor location. Wang et alCitation56 successfully prepared combretastatin A4(CA4)-loaded nanomicelles. Arg–Gly–Asp (RGD) peptides were coupled to the surface of nanomicelles. It was concluded that RGD-targeted micelles significantly enhanced the cell uptake of encapsulated drug in angiogenic tumor endothelial cells, which also resulted in increased antiproliferative activity of antivascular agent. Additionally, a lectin-PEG–PLA nanoparticle drug delivery system was established by Gao et al.Citation44 The retention of the biorecognitive activity of lectin after the covalent coupling procedure was confirmed by hemagglutination test. Taking coumarin as a fluorescent marker, the results showed that the nanoparticles modified by lectin could be quickly delivered into the brain by nasal administration, and the brain’s uptake of coumarin carried by lectin-functionalized nanoparticles was about two-fold in different brain tissues compared with that of coumarin incorporated in the unmodified ones. In the meantime, it has a higher application security. Due to the hydrophilic chain of PEG, it can stay longer in the nasal cavity and facilitate nanoparticles through cell transit. Moreover, after linking modifiers of special biological activity in the PEG–PLA chain end, such as peptide, amino acid, and protein,Citation57–Citation59 drugs in PEG–PLA nanoparticles can enter the brain through the blood–brain barrier.
Conclusions and prospects
In conclusion, with their biodegradability, good biocompatibility, and amphiphilic characteristics, PEG–PLA block copolymers can be prepared into various nanoparticles. By adjusting the content and Mw of PLA and PEG and the PEG/PLA ratio, the block copolymers can increase the drug loading and encapsulation efficiency of hydrophobic drugs, reduce particle sizes, avoid recognition by the reticuloendothelial system, and prolong blood circulation time. However, the long circulating property of PEG–PLA block copolymer nanoparticles is required to be improved further. Additionally, due to small particle sizes and large surface areas of nanoparticles with surface adsorption, the first-pass effect still exists, and blood clearance can also be accelerated. Otherwise, the synthesis of PEG–PLA block copolymer by the lactide ring-opening polymerization method is expensive and not suitable for large-scale production, whereas the polymer prepared by a direct method has a lower Mw with a wider distribution. The current studies on PEG–PLA nanoparticles are still performed in the laboratory, and there is a long way to go on how to make technologies more stable and mature and ultimately promote large-scale production for clinical application. PEG–PLA nanoparticles can be expected to provide more tools and possibilities for the clinical treatment of diseases with broad application prospects in pharmaceutical preparations.
Acknowledgment
This work was supported by grants from the Foundation of Zhejiang Science and Technology Department (2009C33005), National Natural Science Foundation of China (81001647), China Postdoctoral Science Foundation (20100471757), and National Natural Science Foundation of China (20906016).
Disclosure
The authors report no conflicts of interest. The authors are solely responsible for the content and writing of the article.
References
- LeeWCLiYCChuIMAmphiphilic poly(D,L-lactic acid)/poly(ethylene glycol)/poly(D,L-lactic acid) nanogels for controlled release of hydrophobic drugsMacromol Biosci200661084685417039577
- KulkarniRKPaniKCNeumanCLeonardFPolylactic acid for surgical implantsArch Surg19669358398435921307
- KimKYuMZongXControl of degradation rate and hydrophilicity in electrospun non-woven poly(D,L-lactide) nanofiber scaffolds for biomedical applicationsBiomaterials200324274977498514559011
- GrefRLückMQuellecPStealth’ corona-core nanoparticles surface modified by polyethylene glycol (PEG): influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorptionColloids Surf B Biointerfaces20001834301313
- BradleyAJMuradKLReganKLScottMDBiophysical consequences of linker chemistry and polymer size on stealth erythrocytes: size does matterBiochim Biophys Acta20021561214715811997115
- ZhuALuPWuHImmobilization of poly([var epsilon]-caprolactone)-poly(ethylene oxide)-poly([var epsilon]-caprolactone) triblock copolymer on poly(lactide-co-glycolide) surface and dual biofunctional effectsAppl Surf Sci2007253632473253
- HuDSGLiuHJEffect of soft segment on degradation kinetics in polyethylene glycol/poly (L-lactide) block copolymersPolym Bull1993306669676
- YounesHCohnDPhase separation in poly(ethylene glycol)/poly(lactic acid) blendsEur Polym J1988248765773
- IgnatiusAAClaesLEIn vitro biocompatibility of bioresorbable polymers: poly(L,DL-lactide) and poly(L-lactide-co-glycolide)Biomaterials19961788318398730968
- ZhangXLiYChenXSynthesis and characterization of the paclitaxel/MPEG-PLA block copolymer conjugateBiomaterials200526142121212815576187
- KricheldorfHRHachmann-ThiessenHSchwarzGTelechelic and star-shaped poly(L-lactide)s by means of bismuth(III) acetate as initiatorBiomacromolecules20045249249615003011
- StefaniMCoudaneJVertMIn vitro ageing and degradation of PEG-PLA diblock copolymer-based nanoparticlesPolym Degrad Stab2006911125542559
- OtsukaHNagasakiYKataokaKSurface characterization of functionalized polylactide through the coating with heterobifunctional poly(ethylene glycol)/polylactide block copolymersBiomacromolecules200011394811709841
- PulkkinenMPikkarainenJWirthTThree-step tumor targeting of paclitaxel using biotinylated PLA-PEG nanoparticles and avidin-biotin technology: formulation development and in vitro anticancer activityEur J Pharm Biopharm2008701667418555675
- RafatMClérouxCAFongWGPEG-PLA microparticles for encapsulation and delivery of Tat-EGFP to retinal cellsBiomaterials201031123414342120149443
- LiXRYuanXYPoly(ethylene glycol)-poly(lactic acid) copolymers for drug carriersProg Chem2007196973981
- SalemAKCannizzaroSMDaviesMCSynthesis and characterisation of a degradable poly(lactic acid)-poly(ethylene glycol) copolymer with biotinylated end groupsBiomacromolecules20012257558011749223
- LetchfordKBurtHA review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: micelles, nanospheres, nanocapsules and polymersomesEur J Pharm Biopharm200765325926917196803
- ZhangHXiaHWangJLiYHigh intensity focused ultrasound-responsive release behavior of PLA-b-PEG copolymer micellesJ Control Release20091391313919523500
- ZhanCGuBXieCLiJLiuYLuWCyclic RGD conjugated poly(ethylene glycol)-co-poly(lactic acid) micelle enhances paclitaxel anti-glioblastoma effectJ Control Release2010143113614220056123
- LiYQiXRMaitaniYNagaiTPEG-PLA diblock copolymer micelle-like nanoparticles as all-trans-retinoic acid carrier: in vitro and in vivo characterizationsNanotechnology200920505510619417337
- JainJPKumarNDevelopment of amphotericin B loaded polymersomes based on (PEG)3-PLA co-polymers: factors affecting size and in vitro evaluationEur J Pharm Sci201040545646520580669
- LiSByrneBWelshJPalmerAFSelf-assembled poly(butadiene)-b-poly(ethylene oxide) polymersomes as paclitaxel carriersBiotechnol Prog200723127828517269699
- HammadyTRabanelJMDhanikulaRSLeclairGHildgenPFunctionalized nanospheres loaded with anti-angiogenic drugs: cellular uptake and angiosuppressive efficacyEur J Pharm Biopharm200972241842719462478
- VenkatramanSSJiePMinFFreddyBYCLeong-HuatGMicelle-like nanoparticles of PLA-PEG-PLA triblock copolymer as chemotherapeutic carrierInt J Pharm2005298121923215946811
- PereiraMAMosqueiraVCVilelaJMAndradeMSRamaldesGACardosoVNPLA-PEG nanocapsules radiolabeled with 99m Technetium-HMPAO: release properties and physicochemical characterization by atomic force microscopy and photon correlation spectroscopyEur J Pharm Sci2008331425117983736
- XuBNano Medicine1st edBeijing2004
- SoppimathKSAminabhaviTMKulkarniARRudzinskiWEBiodegradable polymeric nanoparticles as drug delivery devicesJ Control Release2001701212011166403
- NiwaTTakeuchiHHinoTKunouNKawashimaYPreparations of biodegradable nanospheres of water-soluble and insoluble drugs with D,L-lactide/glycolide copolymer by a novel spontaneous emulsification solvent diffusion method, and the drug release behaviorJ Control Release199325128998
- LeeHIWuWOhJKLight-induced reversible formation of polymeric micellesAngew Chem20071191425052509
- NakayamaMOkanoTMiyazakiTKohoriFSakaiKYokoyamaMMolecular design of biodegradable polymeric micelles for temperature-responsive drug releaseJ Control Release20061151465616920217
- BaeYJangWDNishiyamaNFukushimaSKataokaKMultifunctional polymeric micelles with folate-mediated cancer cell targeting and pH-triggered drug releasing properties for active intracellular drug deliveryMol BioSyst20051324225016880988
- MurdanSElectro-responsive drug delivery from hydrogelsJ Control Release2003921211714499181
- HuSHLiuTYLiuDMChenSYControlled pulsatile drug release from a ferrogel by a high-frequency magnetic fieldMacromolecules2007401967866788
- MatsumotoJNakadaYSakuraiKNakamuraTTakahashiYPreparation of nanoparticles consisted of poly(L-lactide)-poly(ethylene glycol)-poly(L-lactide) and their evaluation in vitroInt J Pharm199918519310110425369
- YangZLLiXRYangKWLiuYAmphotericin B-loaded poly(ethylene glycol)-poly(lactide) micelles: preparation, freeze-drying, and in vitro releaseJ Biomed Mater Res A200885253954617729259
- BlancoEBeyEADongYBeta-Lapachone-containing PEG-PLA polymer micelles as novel nanotherapeutics against NQO1-overexpres sing tumor cellsJ Control Release2007122336537417574288
- VilaASánchezAÉvoraCSorianoIMcCallionOAlonsoMJPLA-PEG particles as nasal protein carriers: the influence of the particle sizeInt J Pharm200529212435215725549
- HuhKMLeeSCChoYWLeeJJeongJHParkKHydrotropic polymer micelle system for delivery of paclitaxelJ Control Release2005101135968
- ZhangYWuXHanYMoFDuanYLiSNovel thymopentin release systems prepared from bioresorbable PLA-PEG-PLA hydrogelsInt J Pharm201038612152219874880
- AhmedFPakunluRISrinivasGShrinkage of a rapidly growing tumor by drug-loaded polymersomes: pH-triggered release through copolymer degradationMol Pharm20063334035016749866
- SantSPoulinSHildgenPEffect of polymer architecture on surface properties, plasma protein adsorption, and cellular interactions of pegylated nanoparticlesJ Biomed Mater Res A200887488589518228249
- SasatsuMOnishiHMachidaYIn vitro and in vivo characterization of nanoparticles made of MeO-PEG amine/PLA block copolymer and PLAInt J Pharm2006317216717416621360
- GaoXTaoWLuWLectin-conjugated PEG-PLA nanoparticles: preparation and brain delivery after intranasal administrationBiomaterials200627183482349016510178
- YuDHLuQXieJFangCChenHZPeptide-conjugated biodegradable nanoparticles as a carrier to target paclitaxel to tumor neovasculatureBiomaterials20103182278229220053444
- TsaiHCChangWHLoCLGraft and diblock copolymer multifunctional micelles for cancer chemotherapy and imagingBiomaterials20103182293230120042234
- JainAKGoyalAKMishraNVaidyaBMangalSVyasSPPEG-PLA-PEG block copolymeric nanoparticles for oral immunization against hepatitis BInt J Pharm20103871225326219883743
- WeiQWeiWTianRWangLYSuZGMaGHPreparation of uniform-sized PELA microspheres with high encapsulation efficiency of antigen by premix membrane emulsificationJ Colloid Interface Sci2008323226727318501376
- Lim SooPChoJGrantJHoEPiquette-MillerMAllenCDrug release mechanism of paclitaxel from a chitosan-lipid implant system: effect of swelling, degradation and morphologyEur J Pharm Biopharm200869114915718164931
- MaedaHWuJSawaTMatsumuraYHoriKTumor vascular permeability and the EPR effect in macromolecular therapeutics: a reviewJ Control Release2000651227128410699264
- MolinaJUrbinaJGrefRBrenerZRodriguesJMJuniorCure of experimental Chagas’ disease by the bis-triazole DO870 incorporated into ‘stealth’ polyethyleneglycol-polylactide nanospheresJ Antimicrob Chemother200147110110411152439
- IshiharaTTakahashiMHigakiMMizushimaYMizushimaTPreparation and characterization of a nanoparticulate formulation composed of PEG-PLA and PLA as anti-inflammatory agentsInt J Pharm20103851217017519819319
- SimoneEADziublaTDArguiriELoading PEG-catalase into filamentous and spherical polymer nanocarriersPharm Res200926125026018956141
- TakedaMMaedaTIshiharaTSynthesis of prostaglandin E1 phosphate derivatives and their encapsulation in biodegradable nanoparticlesPharm Res20092671792180019415470
- UekiKOnishiHSasatsuMMachidaYPreparation of carboxy-PEG-PLA nanoparticles loaded with camptothecin and their body distribution in solid tumor-bearing miceDrug Dev Res2009707512519
- WangYYangTWangXWangJZhangXZhangQTargeted polymeric micelle system for delivery of combretastatin A4 to tumor vasculature in vitroPharm Res20102791861186820559700
- RenWHChangJYanCHDevelopment of transferrin functionalized poly(ethylene glycol)/poly(lactic acid) amphiphilic block copolymeric micelles as a potential delivery system targeting brain gliomaJ Mater Sci Mater Med20102192673268120535631
- HuKLiJShenYLactoferrin-conjugated PEG-PLA nanoparticles with improved brain delivery: in vitro and in vivo evaluationsJ Control Release20091341556119038299
- ParikhTBommanaMMSquillanteE3rdEfficacy of surface charge in targeting pegylated nanoparticles of sulpiride to the brainEur J Pharm Biopharm201074344245019941957