?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In the present study, binary oxide (cadmium oxide [CdO])x (zinc oxide [ZnO])1–x nanoparticles (NPs) at different concentrations of precursor in calcination temperature were prepared using thermal treatment technique. Cadmium and zinc nitrates (source of cadmium and zinc) with polyvinylpyrrolidone (capping agent) have been used to prepare (CdO)x (ZnO)1–x NPs samples. The sample was characterized by X-ray diffraction (XRD), scanning electron microscopy, energy-dispersive X-ray (EDX), transmission electron microscopy (TEM), and Fourier transform infrared (FTIR) spectroscopy. XRD patterns analysis revealed that NPs were formed after calcination, which showed a cubic and hexagonal crystalline structure of (CdO)x (ZnO)1–x NPs. The phase analysis using EDX spectroscopy and FTIR spectroscopy confirmed the presence of Cd and Zn as the original compounds of prepared (CdO)x (ZnO)1–x NP samples. The average particle size of the samples increased from 14 to 33 nm as the concentration of precursor increased from x=0.20 to x=0.80, as observed by TEM results. The surface composition and valance state of the prepared product NPs were determined by X-ray photoelectron spectroscopy (XPS) analyses. Diffuse UV–visible reflectance spectra were used to determine the optical band gap through the Kubelka–Munk equation; the energy band gap was found to decrease for CdO from 2.92 to 2.82 eV and for ZnO from 3.22 to 3.11 eV with increasing x value. Additionally, photoluminescence (PL) spectra revealed that the intensity in PL increased with an increase in particle size. In addition, the antibacterial activity of binary oxide NP was carried out in vitro against Escherichia coli ATCC 25922 Gram (−ve), Salmonella choleraesuis ATCC 10708, and Bacillus subtilis UPMC 1175 Gram (+ve). This study indicated that the zone of inhibition of 21 mm has good antibacterial activity toward the Gram-positive B. subtilis UPMC 1175.
Introduction
Recently, the researches related to the field of nanomaterials have been obviously growing and thus many researchers from different fields and areas have explored the unique physicochemical nanomaterials’ features.Citation1 Therefore, several ways have allowed to create new structures, systems, nanoplatforms, or devices and can be used with many applications and fields.Citation2–Citation4 One of the powerful points of biocompatible, biodegradable, and functionalized nanomaterials applications is that, more and more research works have been attracted and addressed.Citation5,Citation6 The use of cubic cadmium oxide (CdO) and hexagonal zinc oxide (ZnO) semiconductors nanomaterials is one of the most important issues that has been considered with many research studies due to their unique properties individually or compositionally. CdO is a II–VI composite semiconductor, that is, these consist of group II and group VI elements (refer to the periodic table).Citation7 The distinctive nanomaterial structure’s features have been explored by many applications that efficiently help produce characteristic physical and chemical properties.Citation8 It contains a special structure with a unique face-centered cubic crystalline as well as a metal oxide of n-type with direct and indirect energy band gaps of 2.2–2.5 eV and 1.97 eV.Citation9 There are a number of CdO physical applications; some examples are reviewed as follows.Citation10 The existence of CdO semiconductors in a form of high viability as visible light waves inside the materials is highly useful with photovoltaic applications e.g., solar cells.Citation11 Although clear electrodes, diodes, gas sensors, and antibacterial activity, for example, are other potential physical applications, these materials have widely been used to meet desirable requirements.Citation12–Citation15 There have been several CdO nanostructures forms created at a variety of nanoscales for several purposes and applications, eg, nanoclusters,Citation16 nanowires and nanotubes,Citation17–Citation19 nanoparticles (NPs),Citation20,Citation21 nanocrystals,Citation22 nanorodes,Citation23 and many more exotic structures, eg, CdO-like nanoflowerCitation24 and CdO nanoflake arrays,Citation25 among others.Citation26,Citation27
Similarly, hexagonal ZnO is a II–VI composite semiconductor made of the metallic element zinc (II) and the non-metallic element oxygen (VI).Citation28 Thus, many ZnO semiconductor materials have shown availability of notable characteristics that has encouraged many applications to exploit these features. This structure has been used as the standard hexagonal crystal structure and classified as an n-type semiconductor with 3.27 eV–3.50 eV direct band gaps.Citation29 Hence, while the unique properties of ZnO nanomaterials derived from their distinctively crystal structure and dimensions of nanosize particle, many applications and researches will take advantage of such features.Citation30 Antibacterial activities,Citation31–Citation34 solar cells, and other optoelectronic devices have used and explored the pellucidity in the solar spectrum’s observable area.Citation35,Citation36 Antibacterial activity, catalysis, gas sensors, and diodes are further examples of suitable applications.Citation37–Citation40 Different ZnO nanomaterial forms (such as nanorods,Citation41,Citation42 nanotubes and nanowires,Citation43,Citation44 nanocrystals,Citation45,Citation46 nanoclusters,Citation47 nanofibers,Citation48,Citation49 and numerous forms nanostructures) have been produced by using various methods whereas these materials revealed that different characteristics allowed for several applications to be practically produced.Citation50,Citation51 As can be seen from literature, several techniques such as precipitation,Citation52 microwave hydrothermal,Citation53 sonochemical,Citation54,Citation55 solvothermal,Citation56 chemical,Citation57,Citation58 thermal treatment,Citation59 and sol-gelCitation60,Citation61 can be used to create ZnO nanomaterials.
On the other hand, complementary characteristics could be somehow shown by the unique composition of ZnO-CdO, eg, many band gaps and sizes are created from both oxide semiconductors, with a potential probability of showing distinctive features when comparing to other singular semiconductor components.Citation62–Citation64 One of the most important considerations related to the unique composition of CdO-ZnO nanocomposites is the use of ZnO-CdO with various purposes such as biocides and disinfectants. In addition, this composition has added much more stability property with longer life than organic-based materials. In addition, this composition has gained more attention in the scientific field for biological activity. CdO-ZnO nanocomposites prevent bacterial growth by cell membrane so that the damaged wall shows robust antibacterial activities on an expansive spectrum of bacteria.Citation65
As found in some literature, there are several methods used to produce CdO-ZnO nanostructures, such as sol-gel method,Citation66–Citation68 thermal decomposition,Citation69 high-pressure solution route,Citation70 solvothermal method,Citation71 solution method, and thermal evaporation.Citation72 But production of industrial-scale (CdO)x (ZnO)1–x nanopowder by aforementioned methods has encountered restrictions due to synthetic process complexity, such as high temperature, longer reaction time, reagents toxicity, and effluent by-product manufacture. None of these methods has produced the product in binary or powder form. In addition, no reports on thermal treatment synthesis of binary (CdO)x (ZnO)1–x NP have been found in literature in which an antibacterial activity study has been addressed. Thus, in order to overcome some of abovementioned drawbacks, this research has focused on the use of thermal treatment method to produce pure powder binary oxide NPs and examine the antibacterial activity. A simple thermal treatment route is used to produce no waste binary (CdO)x (ZnO)1–x NP products. It is then considered as an acceptable method from an environmental perspective.Citation73 The novelty of this research study can be summarized as follows: the product is aimed to be produced with steady quality, low cost, more simplicity in handling, particle size control, desirable band gap, great adaptability, and powder products. These features will be attractive for various industrial-scale uses. In addition, the present method yields no toxic or environmentally harmful by-products. Furthermore, no additional chemical agents are required. This paper is dedicated to discuss a unique method that mainly and exclusively produces a binary (CdO)x (ZnO)1–x nanopowder.
The prepared method has used a thermal-based treatment process to synthesize binary (CdO)x (ZnO)1–x NPs and investigate the CdO and ZnO contents effect on the morphological, structural and optical properties of binary (CdO)x (ZnO)1–x nanopowder. This method has used a solution with nitrate metallic ions as precursors and a PVP capping as an agent whereas a necessary calcination was done to produce pure desirable products. The morphology and crystallinity of the product were studied by different techniques. This study also has studied the antibacterial activity.
Experimental section
Materials
In this experimental work, 0.20, 0.40, 0.60, 0.80, and 1.00 mmol cadmium nitrate (Cd(NO3)2 ⋅4H2O (MW =308.46 g/mol)); 0.20, 0.40, 0.60, 0.80, and 1.00 mmol zinc nitrate (Zn (NO3)2 ⋅6H2O (MW =297.47 g/mol)); PVP ((C6H9NO)n (MW =29,000 g/mol)), and deionized water as solvents were used as the starting raw materials. In this work, chemicals were purchased from Sigma-Aldrich Co., (St Louis, MO, USA); they were of standard research grade and were used without further purification.
Methodology
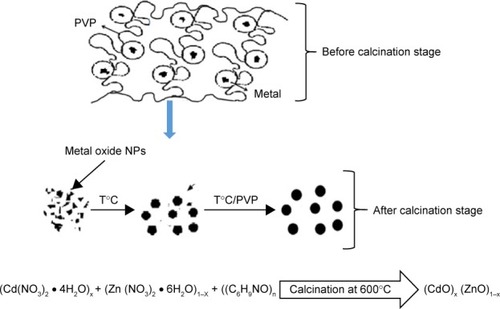
Firstly, the (CdO)x (ZnO)1–x NP product was prepared by dissolving 4 g of PVP in 100 mL of deionized water, followed by vigorous stirring for 2 h at 70°C. Then, 0.00, 0.20, 0.40, 0.60, 0.80, and 1.00 mmol of cadmium nitrate were dissolved subsequently. Next, to form a homogeneous solution, 1.00, 0.80, 0.60, 0.40, 0.20, and 0.00 mmol of zinc nitrate were added and mixed thoroughly. After that, the mixed solution was poured into a petri dish and dried in an oven for 24 h at 80°C. The resulting solid was finally crushed for 30 min in a mortar in order to construct a powder. The obtained powder was subjected to calcination temperature of 600°C in a box furnace for 90 min. The as-synthesized and calcined samples of binary oxide NPs were ready for characterization.
Characterization
To investigate products’ morphological, structural, and optical properties, powder X-ray diffraction (XRD) patterns were used; observed measurements were then recorded on an X-ray diffractometer (X’Pert Pro, PANalytical, Almelo, the Netherlands) using Cu Kα (λ=1.54187 Å) radiation at 40 kV and 30 mA. Fourier transform infrared (FTIR) spectroscopy (1650; PerkinElmer Inc., Waltham, MA, USA) measurements were made in range of 280–4,000 cm−1, and transmission electron microscopy (TEM) (2010F UHR; JEOL, Tokyo, Japan) images were obtained at an accelerating voltage of 200 kV. Energy-dispersive X-ray (EDX) spectroscopy measurement was done using an EDX spectrometer (7353; Oxford Instruments, Abingdon, UK). X-ray photoelectron spectroscopy (XPS) (ULVAC-PHI Quantera II (ULVAC-PHI, Invc)) was performed utilizing monochromatic Al-Kα (hv = 1486.6 eV); operated at 25.6 W (beam diameter of 100 µm) for the X-ray source. Wide-scan analysis was performed using a pass energy of 280 eV with 1 eV per step. Narrow scan (chemical states analysis) was performed using a pass energy of 112 eV with 0.1 eV per step. Prior to deconvolution, charge correction was performed at C 1s by setting binding energies of C-C and C-H to 284.8 eV. A UV–visible (vis) spectrophotometer (Shimadzu model UV-3600) was used in order to evaluate the optical properties of the samples at room temperature in the wavelength range between 200 and 800 nm. A PerkinElmer spectrofluorometer LS-55 equipped with a xenon lamp and room temperature conditions was used to measure photoluminescence (PL). The antibacterial activity of binary oxide NPs was investigated against bacteria on an agar plate.
Evaluation of antibacterial activity
In order to detect antibiotic proficiency of the CdO, ZnO, and CdO-ZnO NPs against bacterial infections, an antimicrobial test was performed. An investigation test was done for the samples of the in vitro antibacterial activity using a disc diffusion method. Hence, this type of investigation led to comparison of the response (sensitivity/resistance) of the microorganism with antimicrobial compounds. In addition, an activity investigation against Escherichia coli ATCC 25922 Gram, Salmonella choleraesuis ATCC 10708, and Bacillus subtilis UPMC 1175 Gram (+ve) was carried out for the prepared NPs. A paper disc (with a diameter equal to 6 mm) was absorbed in a suspension containing 100 mg of each NP in 10 mL deionized water. After that, a dry process was used on the paper disc and was left in an incubator. Finally, a number of papers were then placed onto plates to allow microbes to grow. The microbe culture was standardized to the 0.5 McFarland standard, approximately 108 cells. The plates were inverted at 30°C–37°C and also incubated for sufficient bacteria growth for a period of 2 days approximately. Subsequently, each plate was evaluated and the inhibition zone diameters were measured to the nearest millimeter after the incubation process was completed. Every test related to the evaluation process was done in triplicates and the result reported in average. Mueller Hinton agar media was used as nutrient. Streptomycin (100 mg/mL) standard and distilled water were used for each bacterium as a positive control and as a negative control, respectively. Additionally, the antibacterial test for bulk CdO, ZnO, and CdO-ZnO was compared with prepared NPs.
Results and discussions
Mechanism of NPs
illustrates the behavior and mechanism of development of NPs during calcining. The primary purpose of PVP was to stabilize the complex metallic salts generated. Frequently, this was attained by side steric and electrostatic stabilization of the amide groups attached to the pyrrolidone rings and the methylene groups. As the mixing solution process progressed, metallic ions become suppressed once more and trapped by the amine groups via ionic-dipole generated within polymeric chains. Then, during the drying stage, the metallic cations became stationary within the polymer cavity due to loss of H2O. During the subsequent calcining stage, other gases evolved, eg, N2, NO, CO, or CO2, as organic materials decomposed. In addition, during the calcining stage, PVP influenced the generation of metal oxide NP nuclei. So, without the presence of PVP, the Ostwald ripening process would progress, increasing NP size with increased surface energy levels. However, the presence of PVP led to deactivation of the steric hindrance affecting the conglomeration of NPs.Citation74,Citation75 Therefore, amendment with PVP causes a decrease in NP grain size by restricting and countering breakdown of metal ions on NP surfaces.Citation76–Citation78
XRD analysis
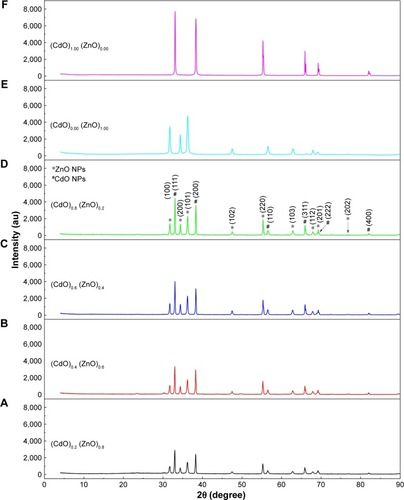
The XRD pattern revealing (CdO)x (ZnO)1–x nanoparticles formation following calcining at 600°C for 3 h is depicted in . Diffraction peaks of ZnO and CdO NPs are marked with star and sharp, respectively, as shown in . These peaks of CdO at 2θ=33.06°, 38.340°, 55.33°, and 69.29° having relations to (111), (200), (220), and (222) planes, respectively, are clearly observed. Additionally, ZnO at 2θ=31.77°, 34.46°, 36.25°, 56.58°, 62.89°, 65.92°, and 67.93° relate to (100), (002), (101), (110), (103), (200), and (112) planes, respectively. As for the hexagonal and cubic phases of ZnO (lattice constants are a=0.325 nm; c=0.521 nm) and CdO (a=0.469 nm) NPs, these reflections are somehow assigned to the standard powder pattern. The (h k l) values are compatible with the standard card of ZnO (JCPDS file no: 36-1451) and with the standard card of CdO (JCPDS file no: 05-0640). The observed diffraction pattern is compatible with the other reported ZnCdO nanomaterial.Citation69,Citation74,Citation79,Citation80 The synthesized binary of (CdO)X (ZnO)1−X NPs exhibited a mixture of cubic phase of CdO NPs and hexagonal ZnO NPs, and no other impurity peak was detected in XRD pattern of the samples. The crystal size (D) of the NPs produced can be determined following Scherrer’s formula:
where λ is the wavelength of X-rays employed (1.5406Å), β is the full width at half maximum, and θ is the angle of diffraction. It can therefore be seen that the crystallite size has increased from 14 to 32 nm as a consequence of x values increasing to 1.00 mmol cadmium nitrate. The results demonstrate increasing x value results in sharper and narrower diffraction peaks, with increased intensity as depicted in . Thus, the increment in the nuclei particle size with respect to crystalline volume ratio increment has enhanced crystallinity.
Figure 2 XRD patterns of (A) (CdO)0.20 (ZnO)0.80, (B) (CdO)0.40 (ZnO)0.60, (C) (CdO)0.60 (ZnO)0.40, (D) (CdO)0.80 (ZnO)0.200, (E) (CdO)0.00 (ZnO)1.00, and (F) (CdO)1.00 (ZnO)0.00 nanoparticles calcined at 600°C.
Note: *ZnO NPs; #CdO NPs.
Abbreviations: XRD, X-ray diffraction; CdO, cadmium oxide; ZnO, zinc oxide.
SEM analysis
Scanning electron microscopy (SEM) in the range 0.00–1.00 mmol was used in order to examine the surface morphology of binary oxide NPs. Micrographs of (CdO)x (ZnO)1–x NPs, at six concentrations, are presented in . The CdO and ZnO compounds appear as porous and grain shaped, respectively. Similar results were obtained within the research works reviewed by Karami et alCitation81 and Raja et al.Citation82 At high x value, as shown in . CdO has a dark color and is nearly porous with regularities, whereas the ZnO looks white and semispherical in shape. In , CdO seems as a plate form with porous shape due to tendency increment in its grains to merge, disintegrate, and overlap with x value decrement; in the ZnO image, the decrement in x is easily noticeable to produce a ZnO dispersal increment throughout the matrix due to the disintegration impact.
EDX spectrum analysis
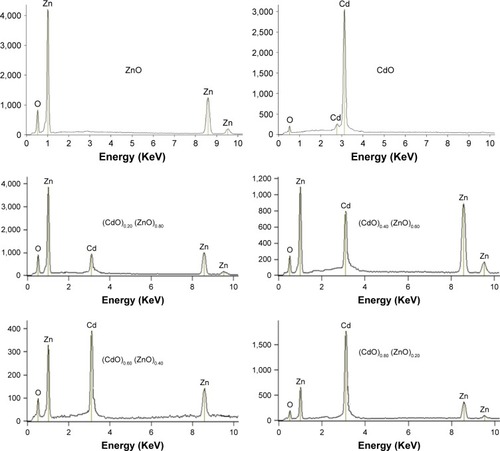
The elemental compositions of (CdO)x (ZnO)1–x NPs at varying precursor concentrations following thermal treatment technique were assessed by EDX spectroscopy. The EDX spectrum derived from (CdO)x (ZnO)1–x NPs following calcination at 600°C is depicted in . These spectra reveal that the Zn:O and Cd:O elements are obviously present as the respective peaks. The atomic percentages of Zn:O and Cd:O are detailed in . This shows that the final product comprises pure binary oxide NPs. The atomic percentage of Zn:O and Cd:O appears equivalent in the final product.
Table 1 EDX spectra showing the atomic percentages of Zn, Cd, and oxygen species in four positions
TEM analysis
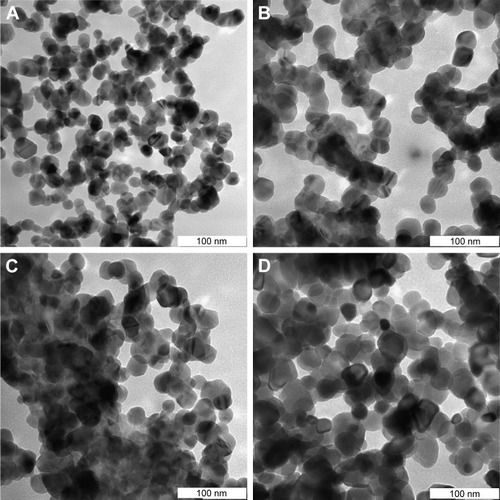
Characterization of the NP microstructure was determined by TEM assessment. Typically, generated samples exhibit homogeneous morphology, as illustrated in depicting TEM images of (CdO)x (ZnO)1–x NPs calcined at 600°C. TEM analysis corroborates the uniform spherical morphology of the NPs generated. As discussed in the preceding section, increasing x value is associated with a significant increase in particle size. This particle size increase is due to the conglomeration of many adjacent particles as a consequence of surface melting at higher x values and calcination temperatures.Citation83–Citation86 All results showed that the binary (CdO)x (ZnO)1–x NPs were sphericalCitation87 and homogeneous and not subject to crystal entanglement as demonstrated by TEM scans of the synthesized binary (CdO)x (ZnO)1–x NPs. This standard procedure has demonstrated its efficiency in generating a variety of binary oxide NPs, where a demonstrable PVP presence is shown to influence NP size as part of an agglomeration–suppression mechanism. compares XRD and TEM results, demonstrating correlation between particle diameters of 16 and 34 nm and calcining at 600°C. PVP is demonstrated to function as a particle stabilizer, facilitating nucleation and development of the NP and promoting consistency. It is therefore employed to restrict NP size and prevent NP agglomeration.Citation75–Citation78,Citation88
Table 2 XRD and TEM results for binary (CdO)x (ZnO)1–x nanoparticles at various x
FTIR analysis
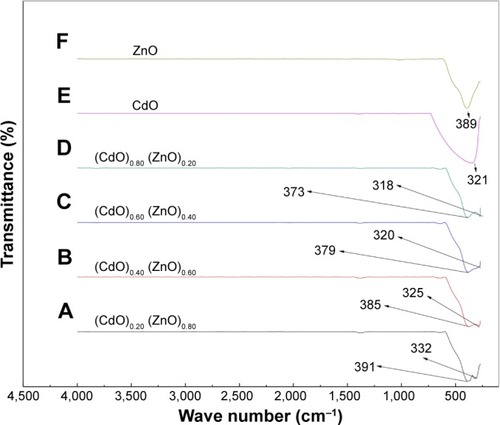
depicts the FTIR spectrum obtained at 280–4,000 cm−1 for the binary oxide NPs produced by the thermal treatment method outlined above. The double absorption peaks are thought to be due to the generation of exceptionally pure binary oxide NPs, also indicated by a shift in the wave number for the (CdO)x (ZnO)1–x NPs spectra associated with increasing x values. This x effect is corroborated by the crystallinity enhancement of the (CdO)x (ZnO)1–x NPs generated. Therefore, demonstrates the x-related increment results in characteristic sharper peaks for the binary oxide, indicating that these increases in x value are associated with a more pronounced crystalline nature of the generated binary oxide.
Figure 6 FTIR spectra of (CdO)x (ZnO)1–x nanoparticles in the range 280–4,500 at (A) (CdO)0.20 (ZnO)0.80, (B) (CdO)0.40 (ZnO)0.60, (C) (CdO)0.60 (ZnO)0.40, (D) (CdO)0.80 (ZnO)0.200, (E) (CdO)1.00 (ZnO)0.00, and (F) (CdO)0.00 (ZnO)1.00 nanoparticles calcined at 600°C.
Abbreviations: FTIR, Fourier transform infrared; CdO, cadmium oxide; ZnO, zinc oxide.
XPS analysis
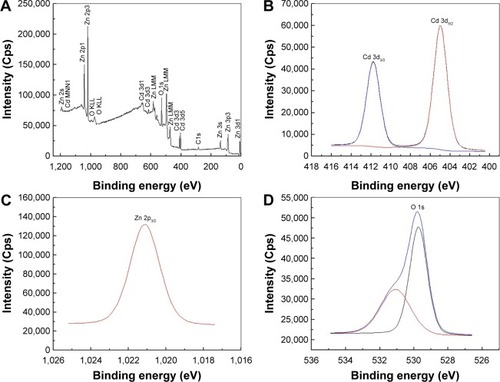
XPS analysis was employed to determine phase compositions and chemical state of Cd, Zn, and O elements. depicts the XPS survey spectra of binary oxide NPs, corroborating the presence of Zn, Cd, O, and C elements. depict the high-resolution XPS spectra for Cd 3d and Zn 2p. The XPS spectrum for Cd relates to respective binding energies of 404.9 eV and 411.7 eV for Cd 3d5/2 and Cd 3d3/2 peaks as depicted in ; similar results have been reported previously.Citation89,Citation90 shows that the Zn 2p3/2 state at a binding energy of 1,021.1 eV corroborates the 2+ oxidation state of zinc.Citation91,Citation92 depicts the deconvoluted O 1s spectrum highlighting the presence of two types of oxygen with binding energies at 529.7 eV and 531.1 eV; these correlate with ZnO and CdO, respectively.Citation93 These results indicate that each element within the binary NP comprises pure oxidation states with no impurities present.
Band gap analysis
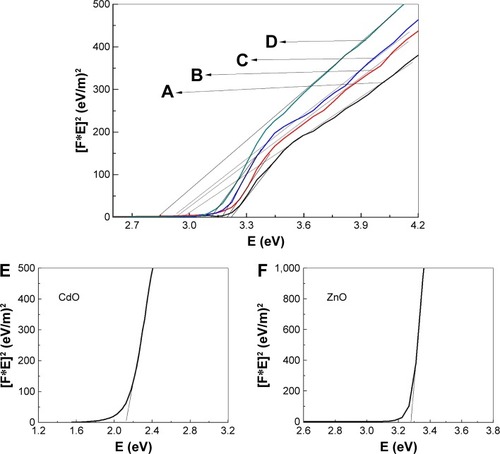
The Kubelka–Munk function was utilized by plotting the square of the Kubelka–Munk function (F(R∞)hv)Citation2 versus energy and extrapolating the linear part of the curve to F (R)Citation2=0. This was employed to determine NP energy band gaps from diffuse reflectance spectra for samples calcined at temperature 600°C, as depicted in , which indicates that the band gap energy for binary oxide NPs has been produced. The energy band gaps values are observed to decrease with increasing x value. This increased x value is considered to be due to a quantum size effect. Furthermore, the reduction occurring over the band gap may be caused by the transitions between the partially suitable valance and conduction bands of the d-shell electrons of Zn2+ ions.Citation94 Therefore, exclusion of the particle size effect on the band gap presented a challenge. As a consequence of the particle size reduction, an alteration in the band structure and material properties is achievable. Conversely, NP size is increased with the band gap reduction. Therefore, at higher energy regimes, the conduction bands of s-electrons and p-electrons are separated from each other, meaning that an overlap occurrence is feasible at conditions involving smaller sized particles. At a Fermi level distance, which is extremely remote from the particle center, the nuclear potential for the conduction of electrons is very low and therefore any transition with permitted quantum numbers will possess an absorption energy equal to the conduction band energy.Citation95 For comparison, the band gap values were decreased with increasing x values, as depicted in . It is considered that the increase in x value may result in an increase of defected states leading to an absorption coefficient increment. Photon absorption generates electron-hole pairs. In turn, the field generated by such pairs may be able to alter the electronic structure and optical nanomaterial characteristics.
Table 3 Energy band gap of product at different concentrations of x
PL analysis
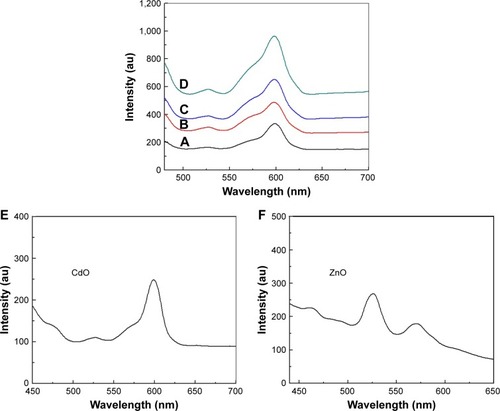
The PL spectra of binary oxide NPs synthesized in PVP and different concentration of precursors were recorded at room temperature under excitation of 425 nm. illustrates that PL spectra associated with the binary oxide NPs prepared in PVP exhibits a broad emission scattering over a range of ~500–575 nm as a consequence of both the compounded effect and the energy states in the valence and conduction bands. These broad peaks comprise two less distinctive sub-bands at ~525 and 565 nm.Citation96 The first of these is thought to be due to the recombination of electron-hole pairs in oxygen and metal vacancies. The second peak (green-yellow emissions centered at 570 nm) is distinctly apparent in PL spectra of binary oxide NPs transitioning between valence and conduction bands. The peak in the wavelength range 600 nm is correlated with the deep energy levels’ CdO emission, caused by the intrinsic anomalies of CdO NPs.Citation96,Citation97 The PL intensities exhibit an intensification with increasing x value and attain their maximum at x=1.00 mmol, which also correlates with the maximum crystallinity attained. Comparing various precursor concentrations where increases in x values produced increases in intensity, it was observed that the peak with greatest intensity versus the weak spectral bands generated for x values below 0.80 demonstrated that x values are significant in promoting a product morphology with minimal structural and surface anomalies.
Antibacterial activity
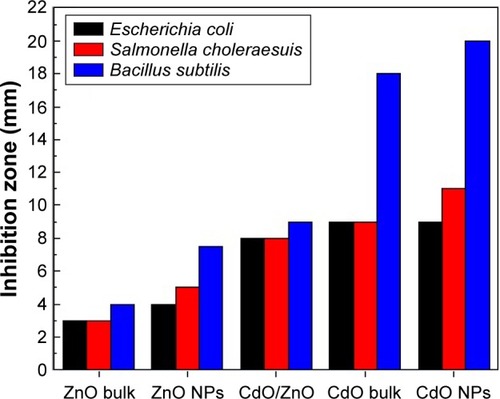
illustrates the antibacterial activity of the prepared materials ZnO bulk, ZnO NPs, binary oxide NPs, CdO bulk, and CdO NPs against E. coli ATCC 25922 Gram, S. choleraesuis ATCC 10708, and B. subtilis UPMC 1175 Gram (+ve). In this figure, inhibition zone diameters values used with agar plate are measured in millimeters. Results provided in and are of average values. With every treated sample, three times of repeated tests were implemented and related results are provided in . Zinc oxide suspensions (4, 5) showed less antibacterial activity, while the CdO and binary NPs (1, 2, and 5) suspensions for all tested bacteria showed high antibacterial activity. These antibacterial activities for CdO and binary NPs are due to several proposed methods and mechanisms. In one of these methods, a reverse effect with the antibacterial activity increment in terms of CdO NPs size has been found. Another mechanism is that the Cd2+ ion release has a meaningful impact on the antibacterial activity.Citation98 Thus, CdO and binary NPs showed significant antibacterial activity. That has effectively showed a fatal effect versus investigated bacteria as per the E. coli E266 N B. subtilis B29 N S. choleraesuis ATCC 10708 sequence. The CdO-ZnO NPs antibacterial efficiency has continued to relief peroxides into the used medium despite the lifeless bacteria surface has been totally shielded by binary CdO/ZnO NPs. That has shown high bactericidal ability.Citation99 CdO–ZnO NPs have attracted research studies to be addressed in the field of cell inhibition more than regular antibiotics have. Therefore, NP characterization for biomedical applications is desirable in the so near future.
Table 4 Average inhibition zone for ZnO bulk, ZnO NPs, ZnO/CdO NPs, CdO bulk, and CdO NPs against Escherichia coli ATCC 25922 Gram, Salmonella choleraesuis ATCC 10708, and Bacillus subtilis UPMC 1175 Gram (+ve)
Figure 10 Inhibition zone test for (A) Escherichia coli ATCC 25922 Gram (−ve), (B) Salmonella choleraesuis ATCC 10708 Gram (−ve), and (C) Bacillus subtilis UPMC 1175 Gram (+ve).
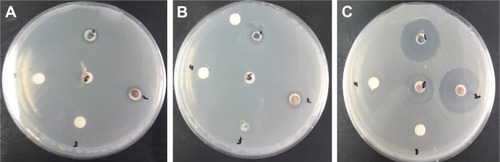
Figure 11 Average inhibition zone graph for ZnO bulk, ZnO NPs, ZnO/CdO NPs, CdO bulk, and CdO NPs against Escherichia coli ATCC 25922 Gram, Salmonella choleraesuis ATCC 10708, and Bacillus subtilis UPMC 1175 Gram (+ve).
Abbreviations: ZnO, zinc oxide; NP, nanoparticle; CdO, cadmium oxide.
shows the comparison in the inhibition zone (mm) for the tested bacteria between the previously reported studies and the product obtained from this method. It is clear that this product showed an enhanced inhibition zone, which reflects a noticeably antibacterial activity. This product has the advantages of being cost-effective, ease of preparation, and higher stability.
Table 5 Zone of inhibition presented by previously reported against Salmonella choleraesuis, Escherichia coli ATCC 25922 Gram (−ve), and Bacillus subtilis
Conclusion
This paper has shown that binary (CdO)x (ZnO)1–x NPs were successfully prepared through thermal treatment method. Nanocrystalline binary (CdO)x (ZnO)1–x were produced and characterized by face-centered cubic and hexagonal structures, respectively, at all x values, as investigated by the XRD samples analysis. The NP size was shown to be increased with an increase in x value; sizes varied from 14 nm at x=0.20 to 32 nm at x=0.80. SEM image shows a porous and grain shape, and the presence of Zn, Cd, and O atoms has been proved by EDAX analysis corresponding to those in the initial mixture. The FTIR spectrum represents the characteristic vibrational modes of Zn-O and Cd-O. The optical band gap is observed to decrease with increasing x value as demonstrated by UV–vis absorption spectrum. The luminescence spectrum shows that the intensity of PL increased with an increase in particle size. These materials have revealed that they could be used to absorb particular wavelengths of solar energy and solar cell applications.
The biological activities of the fabricated binary oxide NPs showed meaningful antibacterial activity against four pathogenic organisms. This study has indicated that the inhibition zone of 18 mm has good antibacterial activity toward the Gram-positive B. subtilis UPMC 1175. Experiments have demonstrated that our method outperforms the other competitive methods and can be used with a variety of biomedical and nanotechnology applications.
Acknowledgments
The authors would like to thank the Faculty of Science, Universiti Putra Malaysia for providing a suitable environment to conduct this research.
Disclosure
The authors report no conflicts of interest in this work.
References
- FinardiUNanosciences and nanotechnologies: evolution trajectories and disruptive featuresNanotechnology: Concepts, Methodologies, Tools, and ApplicationsIGI Global2014120
- SekhonBSNanotechnology in agri-food production: an overviewNanotechnol Sci Appl20147315324966671
- TianJXuJZhuFLuTSuCOuyangGApplication of nanomaterials in sample preparationJ Chromatogr A2013130021623611621
- TangHQXuMRongQJinRWLiuQJLiYLThe effect of ZnO nanoparticles on liver function in ratsInt J Nanomed20161142754285
- ChanWWChhowallaMGlotzerSNanoscience and nanotechnology impacting diverse fields of science, engineering, and medicineACS Nano20161012106151061728024354
- GeYLiSWangSMooreRNanomedicine: Principles and PerspectivesSpringer-Verlag New York IncUSA2014
- SenPHegdeMSRaoCNRSurface oxidation of cadmium, indium, tin and antimony by photoelectron and Auger spectroscopyAppl Surf Sci19821016374
- BlumJLXiongJQHoffmanCZelikoffJTCadmium associated with inhaled cadmium oxide nanoparticles impacts fetal and neonatal development and growthToxicol Sci2012126247848622240978
- ZhaiTFangXLiaoMA comprehensive review of one-dimensional metal-oxide nanostructure photodetectorsSensors (Basel)2009986504652922454597
- GuptaRKGhoshKPatelRMishraSRKaholPKStructural, optical and electrical properties of In doped CdO thin films for optoelectronic applicationsMater Lett2008621933733375
- ManeRSPathanHMLokhandeCDHanSHAn effective use of nanocrystalline CdO thin films in dye-sensitized solar cellsSol Energy2006802185190
- ShuklaMKumariSShuklaSShuklaRKPotent antibacterial activity of nano CdO synthesized via microemulsion schemeJ Mater Environ Sci201234678685
- KumarSOjhaAKSynthesis, characterizations and antimicrobial activities of well dispersed ultra-long CdO nanowiresAIP Adv201335052109
- YakuphanogluFCaglarMCaglarYIlicanSElectrical characterization of nanocluster n-CdO/p-Si heterojunction diodeJ Alloys Compd20105061188193
- ChandiramouliRJeyaprakashBGReview of CdO thin filmsSolid State Sci201316102110
- SrinivasaraghavanRChandiramouliRJeyaprakashBGSeshadriSQuantum chemical studies on CdO nanoclusters stabilitySpectrochim Acta A Mol Biomol Spectrosc201310224224923220663
- FanDHCatalyst-free growth and crystal structures of CdO nanowires and nanotubesJ Cryst Growth2009311823002304
- PengXSWangXFWangYWWangCZMengGWZhangLDNovel method synthesis of CdO nanowiresJ Phys D: Appl Phys20023520L101
- DhawaleDSMoreAMLattheSSRajpureKYLokhandeCDRoom temperature synthesis and characterization of CdO nanowires by chemical bath deposition (CBD) methodAppl Surf Sci20082541132693273
- DongWZhuCOptical properties of surface-modified CdO nanoparticlesOpt Mater2003223227233
- Al-HadaNMSaionEBShaariAHA facile thermal-treatment route to synthesize the semiconductor CdO nanoparticles and effect of calcinationMater Sci Semicond Process201426460466
- GhoshalTKarSDeSKMorphology controlled solvothermal synthesis of Cd(OH)2 and CdO micro/nanocrystals on Cd foilAppl Surf Sci20092551880918097
- HenríquezRGrezPMuñozEDalchieleEAMarottiREGómezHTemplate-free non-aqueous electrochemical growth of CdO nanorodsThin Solid Films201152014146
- FuXLiuJHanTZhangXMengFLiuJA three-dimensional hierarchical CdO nanostructure: preparation and its improved gas-diffusing performance in gas sensorSens Actuators B: Chem2013184260267
- ZhangYQLiZLingTKulinichSADuXWSuperior gas-sensing performance of amorphous CdO nanoflake arrays prepared at room temperatureJ Mater Chem A201642287008706
- RajputJKPathakTKKumarVPurohitLPInfluence of sol concentration on CdO nanostructure with gas sensing applicationAppl Surf Sci2017409816
- SreekanthTVMPanduranganMDillipGRKimDHLeeYRToxicity and efficacy of CdO nanostructures on the MDCK and Caki-2 cellsJ Photochem Photobiol B: Biol2016164174181
- ChenYLHuZAChangYQZinc oxide/reduced graphene oxide composites and electrochemical capacitance enhanced by homogeneous incorporation of reduced graphene oxide sheets in zinc oxide matrixThe J Phys Chem C2011115525632571
- Kołodziejczak-RadzimskaAJesionowskiTZinc oxide – from synthesis to application: a reviewMaterials2014742833288128788596
- WangZLZinc oxide nanostructures: growth, properties and applicationsJ Phys: Condens Matter20041625R829R858
- AliSSMorsyREl-ZawawyNAFareedMFBedaiwyMYSynthesized zinc peroxide nanoparticles (ZnO2-NPs): a novel antimicrobial, anti-elastase, anti-keratinase, and anti-inflammatory approach toward polymicrobial burn woundsInt J Nanomed20171260596073
- BraynerRFerrari-IliouRBrivoisNDjediatSBenedettiMFFiévetFToxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal mediumNano Lett20066486687016608300
- JonesNRayBRanjitKTMannaACAntibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganismsFEMS Microbiol Lett20082791717618081843
- SalahNHabibSSKhanZHHigh-energy ball milling technique for ZnO nanoparticles as antibacterial materialInt J Nanomed20116863869
- HauSKYipHLBaekNSZouJO’MalleyKJenAKYAir-stable inverted flexible polymer solar cells using zinc oxide nanoparticles as an electron selective layerAppl Phys Lett20089225225
- FacchettiAMarksTTransparent Electronics: from Synthesis to ApplicationsChichester, U.KJohn Wiley & Sons2010
- EllmerKKleinARechBTransparent Conductive Zinc Oxide: Basics and Applications in Thin Film Solar Cells104Springer-VerlagBerlin Heidelberg Germany2007
- BecheriADürrMNostroPLBaglioniPSynthesis and characterization of zinc oxide nanoparticles: application to textiles as UV-absorbersJ Nanopart Res2008104679689
- HuangMHWuYFeickHTranNWeberEYangPCatalytic growth of zinc oxide nanowires by vapor transportAdv Mater2001132113116
- ZhangYNayakTRHongHCaiWBiomedical applications of zinc oxide nanomaterialsCurr Mol Med201313101633164524206130
- HsuJWTianZRSimmonsNCMatzkeCMVoigtJALiuJDirected spatial organization of zinc oxide nanorodsNano Lett200551838615792417
- FooKLHashimUMuhammadKVoonCHSol–gel synthesized zinc oxide nanorods and their structural and optical investigation for optoelectronic applicationNanoscale Res Lett20149142925221458
- KatwalGPauloseMRusakovaIAMartinezJEVargheseOKRapid growth of zinc oxide nanotube–nanowire hybrid architectures and their use in breast cancer-related volatile organics detectionNano Lett20161653014302127045345
- KimHSigmundWZinc oxide nanowires on carbon nanotubesAppl Phys Lett2002811120852087
- KachynskiAVKuzminANNykMRoyIPrasadPNZinc oxide nanocrystals for non-resonant nonlinear optical microscopy in biology and medicineJ Phys Chem C Nanomater Interfaces200811229107211072419633706
- BaiSQuanLTangPControllable synthesis and gas-sensing properties of zinc oxide nanocrystals with exposed different percentage of facetsIEEE Sens J2016164866872
- LaiLZhaoCSuMIn vivo target bio-imaging of Alzheimer’s disease by fluorescent zinc oxide nanoclustersBiomater Sci2016471085109127229662
- ViswanathamurthiPBhattaraiNKimHYLeeDRThe photoluminescence properties of zinc oxide nanofibres prepared by electrospinningNanotechnology2003153320
- KavoshMMoallemianHMolaeiHMehranniyaHSalamiMDehdashtiMEStudy of optical and structural properties of zinc oxide nanofibers by using Zn(ac) 2/PVA precursorsSynth React Inorg Met-Org Nano-Metal Chem2016462225229
- KumarRAl-DossaryOKumarGUmarAZinc oxide nanostructures for NO2 gas–sensor applications: a reviewNano-Micro Lett20157297120
- CaudaVPuglieseDGarinoNMulti-functional energy conversion and storage electrodes using flower-like zinc oxide nanostructuresEnergy201465639646
- KumarSSVenkateswarluPRaoVRRaoGNSynthesis, characterization and optical properties of zinc oxide nanoparticlesInt Nano Lett20133130
- QuirinoMROliveiraMJCKeysonDLucenaGLOliveiraJBGamaLSynthesis of zinc oxide by microwave hydrothermal method for application to transesterification of soybean oil (biodiesel)Mater Chem Phys20171852430
- XiongHMShchukinDGMöhwaldHXuYXiaYYSonochemical synthesis of highly luminescent zinc oxide nanoparticles doped with magnesium(II)Angew Chem Int Ed Engl200948152727273119267379
- RayathulhanRSodipoBKAzizAANucleation and growth of zinc oxide nanorods directly on metal wire by sonochemical methodUltrason Sonochem201735Pt A27027527756524
- AtchudanREdisonTNJIPerumalSKarthikeyanDLeeYRFacile synthesis of zinc oxide nanoparticles decorated graphene oxide composite via simple solvothermal route and their photocatalytic activity on methylene blue degradationJ Photochem Photobiol B201616250051027459420
- GnanasangeethaDSaralaThambavaniDOne pot synthesis of zinc oxide nanoparticles via chemical and green methodRes J Mater Sci201323206055
- DasDDattaAKKumbhakarDVGhoshBPramanikAGuptaSConditional optimisation of wet chemical synthesis for pioneered ZnO nanostructuresNano-Structures Nano-Objects201792630
- Al-HadaNMSaionEBShaariAHA facile thermal-treatment route to synthesize ZnO nanosheets and effect of calcination temperaturePLoS One201498e10313425093752
- FarragAABalboulMRNano ZnO thin films synthesis by sol–gel spin coating method as a transparent layer for solar cell applicationsJ Sol-Gel Sci Technol2017821269279
- El GhoulJBarthouCEl MirLSynthesis by sol–gel process, structural and optical properties of nanoparticles of zinc oxide doped vanadiumSuperlattices Microstruct2012516942951
- ZhengZGanLLiHA fully transparent and flexible ultraviolet–visible photodetector based on controlled electrospun ZnO-CdO hetero-junction nanofiber arraysAdv Funct Mater2015253758855894
- PathakTKRajputJKKumarVPurohitLPSwartHCKroonRETransparent conducting ZnO-CdO mixed oxide thin films grown by the sol-gel methodJ Colloid Interface Sci201748737838727810506
- ChoiYSLeeCGChoSMTransparent conducting ZnxCd1− xO thin films prepared by the sol-gel processThin Solid Films19962891–2153158
- KarthikKDhanuskodiSGobinathCSivaramakrishnanSMicrowave-assisted synthesis of CdO–ZnO nanocomposite and its antibacterial activity against human pathogensSpectrochim Acta A: Mol Biomol Spectrosc201513971225546491
- SahuNDuchaniyaRKSynthesis of ZnO-CdO nanocompositesJ Mater Sci Surf Eng2013111114
- SaravananRGraciaFKhanMMZnO/CdO nanocomposites for textile effluent degradation and electrochemical detectionJ Mol Liq2015209374380
- ZiabariAAGhodsiFEOptoelectronic studies of sol–gel derived nanostructured CdO–ZnO composite filmsJ Alloys Compd20115093587488755
- SaravananRShankarHPrakashTNarayananVStephenAZnO/CdO composite nanorods for photocatalytic degradation of methylene blue under visible lightMater Chem Phys20111251277280
- GhoshMRaychaudhuriAKStructure and optical properties of Cd-substituted ZnO (Zn1−xCdxO) nanostructures synthesized by the high-pressure solution routeNanotechnology20071811115618
- SundarSMMahadevanCKRamanathanPOn the preparation of ZnO–CdO nanocompositesMater Manuf Processes2007223400403
- SenthilKTakYSeolMYongKSynthesis and characterization of ZnO nanowire–CdO composite nanostructuresNanoscale Res Lett20094111329133420628464
- KamariHMAl-HadaNMSaionECalcined solution-based PVP influence on ZnO semiconductor nanoparticle propertiesCrystals2017722
- Al-HadaNMSaionEKamariHMStructural, morphological and optical behaviour of PVP capped binary (ZnO) 0.4 (CdO) 0.6 nanoparticles synthesised by a facile thermal routeMater Sci Semicond Process2016535665
- IzuNUchidaTMatsubaraIItohTShinWNishiboriMFormation mechanism of monodispersed spherical core–shell ceria/polymer hybrid nanoparticlesMater Res Bull201146811681176
- KoczkurKMMourdikoudisSPolavarapuLSkrabalakSEPolyvinylpyrrolidone (PVP) in nanoparticle synthesisDalton Trans20154441178831790526434727
- ThanhNTMacleanNMahiddineSMechanisms of nucleation and growth of nanoparticles in solutionChem Rev2014114157610763025003956
- VisaveliyaNKöhlerJMControl of shape and size of polymer nanoparticles aggregates in a single-step microcontinuous flow process: a case of flower and spherical shapesLangmuir20143041121801218925251615
- SaravananRMansoob KhanMGuptaVKZnO/Ag/CdO nanocomposite for visible light-induced photocatalytic degradation of industrial textile effluentsJ Colloid Interface Sci201545212613325935283
- LiXFCaoYSuiYRStructure and optical properties of ternary ZnCdO nanopowder synthesized by sol–gel methodSuperlattices Microstruct201469187193
- KaramiHAminifarATavallaliHNamdarZAPVA-based sol–gel synthesis and characterization of CdO–ZnO nanocompositeJ Cluster Sci201021119
- RajaSBhuvaneswariPVRamesh BabuRGokulakrishnanVRamamurthiKStudies on the structural, morphological, electrical and optical properties of (CdO) x (ZnO)1−x thin films deposited by spray pyrolysis methodJ Mater Sci: Mater Electron201627881118117
- Al-HadaNMSaionEBShaariAHKamarudeenMAFlaifelMHGeneSASynthesis, structural and morphological properties of cadmium oxide nanoparticles prepared by thermal treatment methodAdv Mater Res20151107291294
- BaqerAAMatoriKAAl-HadaNMShaariAHSaionEChyiJLYEffect of polyvinylpyrrolidone on cerium oxide nanoparticle characteristics prepared by a facile heat treatment techniqueResults Phys20177611619
- LeePJSaionEAl-HadaNMSoltaniNA simple up-scalable thermal treatment method for synthesis of ZnO nanoparticlesMetals20155423832392
- SalemASaionEAl-HadaNMSynthesis and characterization of CdSe nanoparticles via thermal treatment techniqueResults in Physics2017715561562
- MosqueraEdel PozoIMorelMStructure and red shift of optical band gap in CdO–ZnO nanocomposite synthesized by the sol gel methodJ Solid State Chem2013206265271
- Al-HadaNMSaionETalibZAShaariAHThe impact of polyvinylpyrrolidone on properties of cadmium oxide semiconductor nanoparticles manufactured by heat treatment techniquePolymers201684113
- YousefABarakatNAAl-DeyabSSNirmalaRPantBKimHYEncapsulation of CdO/ZnO NPs in PU electrospun nanofibers as novel strategy for effective immobilization of the photocatalystsColloids Surf A: Physicochem Eng Aspects2012401816
- KingPDVealTDSchleifeAValence-band electronic structure of CdO, ZnO, and MgO from x-ray photoemission spectroscopy and quasi-particle-corrected density-functional theory calculationsPhys Rev B20097920205205
- AnandanSOhashiNMiyauchiMZnO-based visible-light photocatalyst: band-gap engineering and multi-electron reduction by co-catalystAppl Catal B20101003502509
- KumarPSSelvakumarMBhagabatiPBharathiBKaruthapandianSBalakumarSCdO/ZnO nanohybrids: facile synthesis and morphologically enhanced photocatalytic performanceRSC Adv20144623297732986
- SaravananRKhanMMGuptaVKZnO/Ag/CdO nanocomposite for visible light-induced photocatalytic degradation of industrial textile effluentsJ Colloid Interface Sci201545212613325935283
- Tabet-DerrazHBenramdaneNNacerDBouzidiAMedlesMInvestigations on Zn x Cd 1− x O thin films obtained by spray pyrolysisSol Energy Mater Sol Cells2002733249259
- CaglarYCaglarMIlicanSAtesAMorphological, optical and electrical properties of CdZnO films prepared by sol–gel methodJ Phys D: Appl Phys2009426065421
- ZhangJZhaoSQZhangKZhouJQCaiYFA study of photoluminescence properties and performance improvement of Cd-doped ZnO quantum dots prepared by the sol–gel methodNanoscale Res Lett20127140522809232
- VijayalakshmiSVenkatarajSJayavelRCharacterization of cadmium doped zinc oxide (Cd: ZnO) thin films prepared by spray pyrolysis methodJ Phys D: Appl Phys20084124245403
- KhanZRehmanAHussainSZNisarMAZulfiqarSShakooriARCadmium resistance and uptake by bacterium, Salmonella enterica 43C, isolated from industrial effluentAMB Express2016615427491862
- KohenRNyskaAOxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantificationToxicol Pathol200230662065012512863
- XavierARRavichandranATRavichandranKManthaSRavinderDSm doping effect on structural, morphological, luminescence and antibacterial activity of CdO nanoparticlesJ Mater Sci: Mater Electron201627111118211187
- SalehiBMehrabianSAhmadiMInvestigation of antibacterial effect of Cadmium Oxide nanoparticles on Staphylococcus aureus bacteriaJ Nanobiotechnol201412126
- KhanMFHameedullahMAnsariAHFlower-shaped ZnO nanoparticles synthesized by a novel approach at near-room temperatures with antibacterial and antifungal propertiesInt J Nanomed20149853864