?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Silymarin (Sm) is a polyphenolic component extracted from Silybum marianum. It is an antioxidant, traditionally used as an immunostimulant, hepatoprotectant, and dietary supplement. Relatively recently, Sm has proved to be a valuable chemopreventive and a useful antineoplastic agent. Medical success for Sm is, however, constrained by very low aqueous solubility and associated biopharmaceutical limitations. Sm flavonolignans are also susceptible to ion-catalyzed degradation in the gut. Proven antihepatotoxic activity of Sm cannot therefore be fully exploited in acute chemical poisoning conditions like that in paracetamol overdose. Moreover, a synchronous delivery that is required for hepatic regeneration is difficult to achieve by itself. This work is meant to circumvent the inherent limitations of Sm through the use of nanotechnology. Sm nanoparticles (Smnps) were prepared by nanoprecipitation in polyvinyl alcohol stabilized Eudragit RS100® polymer (Rohm Pharma GmbH, Darmstadt, Germany). Process parameter optimization provided 67.39% entrapment efficiency and a Gaussian particle distribution of average size 120.37 nm. Sm release from the nanoparticles was considerably sustained for all formulations. Smnps were strongly protective against hepatic damage when tested in a paracetamol overdose hepatotoxicity model. Nanoparticles recorded no animal death even when administered after an established paracetamol-induced hepatic necrosis. Preventing progress of paracetamol hepatic damage was traced for an efficient glutathione regeneration to a level of 11.3 μmol/g in hepatic tissue due to Smnps.
Introduction
Paracetamol (N-acetyl-ρ-aminophenol, APAP) is an antipyretic analgesic, available over-the-counter. The drug, however, is toxic in high dosages and an overdose of 4 g/day can cause hepatic necrosis.Citation1 APAP hepatotoxicity is marked in alcoholics and in patients undergoing multiple drug treatment like that in human immunodeficiency virus HIV infections or in tuberculosis.Citation2,Citation3 A number of reports also exist on suicidal attempts by ingestion of APAP.Citation4 APAP is metabolized in the liver to form soluble sulfates and glucuronides. A small amount is converted in the microsomal cytochrome P450 enzyme system into the reactive metabolite N-acetyl-ρ-benzoquinone-imine (NAPQI).Citation5,Citation6 At low dosages, the amount of NAPQI formed gets conjugated to the hepatic reduced glutathione (GSH) store, before being eliminated. In case of APAP overdose or in conditions when the hepatic GSH store is depleted, NAPQI reacts further with cellular proteins causing oxidative stress, microsomal membrane damage, and cell death.Citation7 With the liver being the most oxidative organ, increased oxidative stress induces apoptosis.
Silymarin (Sm) is a polyphenolic component isolated from the fruits and seeds of the milk thistle plant Silybum marianum (family Asteraceae).Citation8,Citation9 Milk thistle extracts have been used for over 2000 yearsCitation10 by different civilizations as rejuvenators. Chemically, Sm is a mixture of flavonolignan isomersCitation11,Citation12 of general molecular formula C25H22O10. Silybin constitutes the principal chemical component in the purified extract.Citation13
Interest has been renewed in Sm for a variety of reasons including the discovery of its chemopreventive, anti-angiogenic, and anticancer potentials.Citation14,Citation15 The drug, however, suffers from biopharmaceutic limitations due to very poor aqueous solubility,Citation16,Citation17 inappropriate tissue distribution, and degradation in the gastric environment.Citation18 Attempts were made to solubulize Sm in order to overcome biopharmaceutic limitations but none of these have met with any pharmacological successes.Citation19,Citation20 A phospholipids complex of silybin was proposed to improve solubility and permeability.Citation21 Salts of Sm were attempted but were limited by membrane permeability. A liposomal delivery system for Sm was reportedCitation22 but suffers from high surfactant content and low entrapment efficiency.
Sm flavonolignans exert multilateral activity on hepatocytes. Sm promotes hepatocyte ribonucleic acid (RNA) polymerase I, facilitates adenosine 5′-triphosphatase (ATPase) activity, and restores GSH content.Citation23 Hepatoprotection is a synchronous activity of flavonolignans to hasten mitotic activity and thereby leads to regeneration of liver tissue.Citation24 Additionally, Sm components are strong inhibitors of leukotrienes and proinflammatory transmitters like nuclear factor kappa B (NF-κB).Citation25,Citation26 Sm has great potential for long-term hepatoprotection against chemotoxic agents like APAP and might even offset hepatic damage.Citation27–Citation29
This work was aimed to develop a slow release nanoparticle delivery device for Sm in order to circumvent solubility limitations. Nanoprecipitation technique was preferred over others for easy adaptability in scaling up. Eudragit RS100® (Rohm Pharma GmbH, Darmstadt, Germany), a polycationic acrylate copolymer, was successfully used for Sm nanoparticulation. The polymer is insoluble at physiological pH ranges but swells partially in water. Cationic Eudragit nanoparticles allow specific advantages and were previously used in oral and ophthalmic nanoparticle delivery devices.Citation30,Citation31 Polyvinyl alcohol, PVA, was used as a stabilizer. PVA can provide nanoparticle steric and mechanical stabilizationCitation32 but has not previously been evaluated with Eudragit nanoparticles.
Factorial design experiments were attempted to optimize the nanoparticle size and entrapment efficiency. Both protective and restorative animal experiments were used to assess the efficacy of Sm nanoparticles (Smnps) as an impediment to APAP-induced necrosis. Mouse models were preferred over rat, as NAPQI-mediated hepatic damage is more pronounced.Citation33,Citation34
Materials and facilities
Borosil® (Mumbai, India) glassware was used for preparation and analysis experiments. A precision balance 0.00001 g Mettler® Toledo AL54 (Mettler, Columbus, OH), an ultracentrifuge Himac CS120GHXL (Hitachi Koki, Tokyo, Japan), and Accupipet Tarsons (Tarsons, Kolkata, India) were used in preparative processes. Zetasizer® Nano ZS (Malvern Instruments, Malvern, UK), UV-vis spectrophotometer UV-2550 (Shimadzu, Kyoto, Japan), Atomic Force Microscope Nanoscope 3A (Veeco, Plainview, NY), and FT/IR-670 plus (Jasco, Tokyo, Japan) were used for analytical and particle characterization. Homogenizer TH 02 (Omni International, Kennesaw, GA) and a microscope (B1 series, Motic, Xiamen, China) were used for biochemical analysis and animal experiments. Solvents and water used were of high-performance liquid chromatography (HPLC) grade and were procured from E Merck or Spectrochem (Mumbai, India). Dialysis tubing D9652 (MW cut off 12,400 kD), Sm, PVA (89,000–98,000 kD), 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB) were purchased from Sigma-Aldrich (St Louis, MO). Diagnostic kits for biochemical studies were obtained from Merck Specialties Private Ltd (Mumbai, India). Eudragit RS100® was a gift from Rohm Pharma GmbH. Paracetamol was a gift sample from Dey’s Medical Stores (Mfg) Ltd (Kolkata, India). Windows Excel (v 2003; Redmond, WA) and Sigmaplot (v 6.0; Jandel Scientific) were used for most data analysis purposes.
Methods
Preparation of Smnps
Smnps were prepared following a nanoprecipitation technique. Different preparations were designed varying in stabilizer PVA and the Eudragit RS100® polymer mass used ().
Table 1 Particle size, zeta potential, and silymarin entrapment in nanoparticles
Particle size and polydispersity index (PDI)
The particle size of the Smnps was determined by photon correlation spectroscopy (PCS) in Zetasizer® Nano ZS against a 4 mW helium–neon (He–Ne) laser beam, 633 nm, and a back scattering angle of 173°. Particle size and PDI of preparations were determined in triplicate.
Zeta potential
Zeta potentials were measured using the Zetasizer® Nano ZS using disposable zeta cells. Aliquots from each preparation type were injected in electrophoretic zeta cells and zeta potentials were analyzed using the Smoluchowski equation.
Atomic force microscopy (AFM)
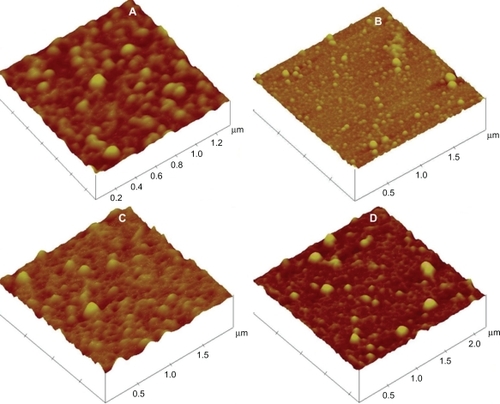
The morphological examination of the nanoparticles was carried out using a AFM setup. Smnp suspensions (100 μL) from different preparations were deposited onto fused mica substrates and the particles were visualized in tapping mode using RTESP tips (Veeco, Santa Barbara, CA) at 267–328 kHz resonance frequency at a scan speed of 1.2 Hz.
Fourier transform infrared (FTIR) spectroscopy
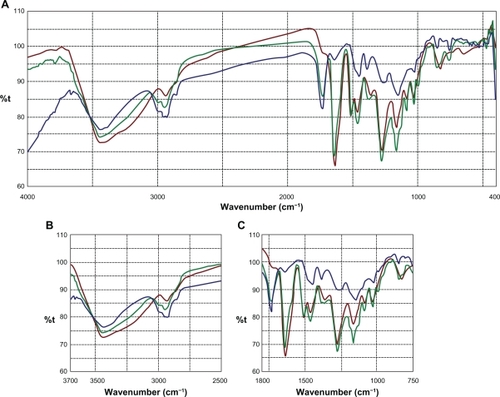
FTIR studies were carried out to observe for Sm and polymer interactions if any. Samples of Sm, Eudragit RS100®, and an eutectic mixture were diluted separately with IR grade KBr in the ratio of 1:100 and were pelletized in a hydraulic press. The pellets were scanned over a wavenumber range of 4000–400 cm−1 and the data stacked in KnowItAll® (Bio-Rad, Hercules, CA) software to compare and search for chemical interactions.
Nanoparticle entrapment efficiency
Sm mass encapsulation in nanoparticle was determined from the amount of Sm originally taken and the amount remaining in the supernatant after harvesting. A validated spectrophotometric analysis method was used throughout. Briefly, 100 μL of supernatant from each preparation was diluted in pH 7.4 phosphate buffer: methanol, 48:62, v/v and Sm λmax of 326 nm was used for analysis.Citation36 The limit of determination (LOD) for Sm was observed as 1.167 μg/mL and the limit of quantitation (LOQ) was 3.896 μg/mL. Sm entrapment efficiency for each preparation was determined using the formula:
In vitro release studies
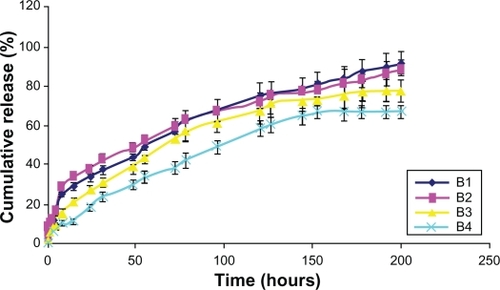
Smnp equivalent to 5 mg of payload was suspended in 5 mL of 100 mM, pH 7.4 phosphate buffer and transferred quantitatively in dialysis bags. Lightly tied bags were placed in glass vials containing 100 mL of phosphate buffer maintained at 37°C over a shaking water bath set at 50 rpm. At every predetermined time interval, 5 mL of the buffer medium was withdrawn from the external media and 20 mL of fresh buffer was added to maintain sink conditions. The amount of Sm released at each time point was estimated spectrophotometrically as described earlier with necessary corrections for the dilution factors. The in vitro release studies for all preparations were completed in triplicate and the cumulative percentage release over time was plotted. In order to understand the release mechanism, the Korsmeyer–Peppas model was applied and release exponent (n) and the constant incorporating structural and geometric characteristics of the system (K) values were calculated using SigmaPlot.
Smnp in APAP-induced hepatotoxicity
Animal care
Swiss albino mice (25–30 g) procured from Central Research Institute (Kolkata, India) were used in all experiments. Animals were housed in polypropylene cages in standard laboratory conditions of relative humidity 50% ± 10%, temperature 22°C ± 2°C, and 12/12 hour light–dark cycle for 10 days prior to experiments. Access of water was ad libitum and standard pellet food (Hindustan Unilever, Mumbai, India) supply was provided twice a day. All animal experiments were conducted as per the approval and guidelines of the institutional animal ethical committee under the Government of India registration number 506/01/a/CPCSEA. Mice were fasted for 12 hours before the start of experiments in order to deplete hepatic GSH store. APAP and test drugs were administered intraperitoneally (i.p.) either dissolved or dispersed in saline at 37°C. Food was restored 1 hour after administration; water, however, was allowed ad libitum.
APAP hepatotoxicity
Animals were individually weighed and randomly divided into 12 animals per group. Group A, received normal saline and served as the control. APAP hepatotoxicity was induced in Group B animals by single i.p. injection of 300 mg/kgCitation37 and served as positive control. Group C mice were treated with empty nanoparticles on alternate days for 7 days, prior to administration of APAP. Effects of Sm and Smnp over APAP-induced hepatotoxicity were analyzed. An alternate day treatment protocol was followed in Group D animals receiving 125 mg/kg of Sm for 7 daysCitation38 prior to a single injection of 300 mg/kg APAP. Similarly, Group E animals were treated with Smnp with an equivalent of 125 mg/kg Sm payload for 7 days prior to an injection of APAP. Group F animals received a single injection of 125 mg/kg Sm 1 hour after APAP-induced hepatotoxicity while group G animals received a single injection of Smnp equivalent to 125 mg/kg of Sm payload 1 hour after APAP administration.
After 12 hours of APAP injection, animals were anesthetized with anesthetic ether and blood was collected and allowed to coagulate at room temperature. Serum was separated and stored at −80°C for biochemical evaluations. Mice were then euthanized with ether overdose and the liver removed. Portions of the liver from both lobes were quickly dissected, blotted, weighed and stored in −80°C. A portion of the liver was preserved in formalin for histological sections. Tissue homogenates (10% w/v) were prepared in ice cold phosphate buffer (50 mM, pH 7.0) containing 0.1 mM ethylenediaminetetraacetic acid (EDTA). Homogenates were further centrifuged at 5000 rpm for 15 minutes at 4°C and the supernatant was stored at −80°C until further use.
Biochemical parameters and histopathology
Biochemical evaluations for hepatic injury were recorded by assessment of different enzyme markers in serum using specific reagent kits. Analytical procedures were followed as directed in the kits. Reduced GSH level was measured from liver homogenate following Ellman’s method.Citation39 Standard slide preparation techniques were followed for histology and visualization after staining with hematoxylin and eosin.
Statistical analysis
All experiments were completed in triplicate unless mentioned otherwise and the results were presented as mean ± standard deviation. Graphs were plotted with the mean values including the error bars. Statistical differences of mean values were analyzed using Student’s t-test. Differences were considered significant when P < 0.01.
Results
Limited solubility and specific polymer association were the major challenges in Sm nanoparticulation. Among many techniques available for drug nanoparticulation; nanoprecipitation takes an interesting advantage of solubility gradient by controlled deposition of both polymer and the drug payload.Citation32 The technique also appeared simple and reproducible for later application. The current study has utilized the similar solubility characteristics of polymer Eudragit RS100® and Sm.Citation31,Citation17,Citation18 Eudragit RS100® has widely been used for ocular nanoparticle drug delivery with good pH stability, biocompatibility, and localization properties.Citation40 PVA was used as an efficient stabilizer as it can entrap an array of Eudragit nanoparticles.
There were only a limited number of variables in the nanoprecipitation technique. A 22 factorial design study was run to study the effect of Eudragit and PVA mass as independent variables on nanoparticle size and Sm entrapment efficiency. High–low combination batches were tested and particle size, zeta potential, and PDI were recorded ().
Particles in the vicinity of 100 nm have a higher possibility for specific tissue localization.Citation41 Preparations B2, B3, and B4 were in the appropriate size range for localization in the liver, whereas preparation B2 carried a higher Sm load. The nanoparticles carried a small positive charge due to a combined effect of the cationic polymer Eudragit RS100® and the associated stabilizer PVA. A small surface charge was instrumental to keep the nanoparticles stable in solution.
Factorial design
A 22 (two level two factors) factorial design study was used to understand the effects of two independent variables, the amount of Eudragit RS100® mass (X1) and the proportional amount of PVA (X2) used over the nanoparticle size range and Sm mass entrapment efficiency.Citation42 Results are expressed in Equationequation (1)(1) :
Table 2 Summary of regression analysis and ANOVA for measured responses
Table 3 Effects of process variables on silymarin entrapment and particle size
AFM studies
Smnps were evenly distributed in AFM with almost no aggregation. Particles from all preparation types appeared spherical and smooth in surface. AFM study samples from all four preparations are depicted in .
FTIR studies
Sm flavonolignans were evidenced using FTIR analysis by the typical presence of benzopyran ring vibrations at 1084 cm−1 with concomitant presence for out of plane ─C─H deformations at 821 cm−1 (). The reactive flavono-lignan ketoneCitation43 responded at 1636 cm−1 and the aromatic ring stretching vibrations were observed at 1509 cm−1. Eudragit RS100® polymer linear branch ketone responded at 1733 cm−1 which associated with linear ─C─H stretching vibrations at 2849 cm−1. When an eutectic mixture of Sm and Eudragit polymer was analyzed using FTIR, the Sm ketone was unaffected at 1636 cm−1 alongside the polymer ketone stretching at 1733 cm−1, indicating no chemical interaction between Sm and the polymer. In addition, the structurally sensitive Sm benzopyran at 1084 cm−1 and specific Eudragit response at 2849 cm−1 were observable in the Sm-Eudragit blend, which suggests no chemical interactions.
In vitro release
In vitro release was gradual over time. In the case of Smnp B1 and B2, an initial faster Sm release phase was observed over a period of 8 hours. This was possibly due to nanoparticle surface-adsorbed Sm molecules. Release thereafter was steady and 90% of the load entrapped was traced during the study period of 200 hours. Preparation B1 and B2 release were persistent whereas B4 release was not very stable (). Polymer mass here appeared an important parameter for Sm entrapment and release profiling and no polymer burst effect was observed during the in vitro release studies. Korsmeyer–Peppas kinetic model was applied for Smnp release up to 60% of the mass load release. The exponent component n for B2 and B3 formulation was near 0.4 (),
Table 4 Korsmeyer–Peppas release kinetics for nanoparticles
APAP hepatotoxicity and liver biochemistry
Effects of Sm and Smnp on APAP mouse hepatotoxicity are presented in .
Table 5 Effects of silymarin and silymarin nanoparticles against APAP-induced hepatotoxicity
The levels of reduced GSH in the APAP-treated group were markedly low at 6.9 ± 1.21 μmol/g of tissue. This was indicative of NAPQI-mediated damage. In the case of Group D and E, the reduced GSH level was restored to a higher level possibly due to a Sm-mediated regeneration response.
A differential response was recorded when the group F and group G animals were administered with Sm or Smnp 1 hour after APAP overdose. Sm failed to elicit significant hepatoprotection. The APAP-elevated levels of marker enzymes and the hepatic GSH levels were not significantly altered. In the case of Smnp, however, hepatic GSH store was restored with concomitant decrease in serum marker enzymes. Perhaps Smnps hepatoprotection was possible due to the rapid localization of nanoparticles in hepatic tissues during the 12-hour exposure period.
Histopathology
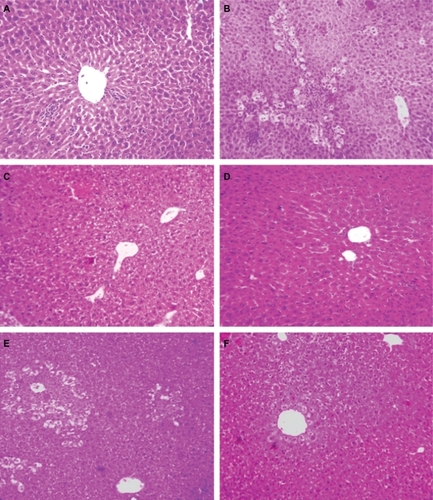
Elevated levels of marker enzymes in serum were an indication of disrupted structural integrity of the hepatocellular membrane which caused leakage of the liver enzymes into the blood due to APAP toxicity. APAP-treated group (Group B) animals demonstrated loss of normal hepatic structure, with necrotic damage which was characterized by the disruption of the lattice nature of the hepatocyte, damaged cell membranes, reduced diameter of nuclei, disintegrated central vein, dilated sinusoids and moderate infiltration of monocytes, and neutrophils in the cytoplasm (). The Sm-treated group (Group D) showed improved histological changes in comparison to Group B animals. This was implied by the presence of mild necrotic lesions (). Smnp-treated groups (), however, showed significant regeneration, visible by the presence of increased numbers of hepatocytes and lack of any prominent centrilobular necrosis or sinusoidal congestion. The inflammatory cell infiltration caused by APAP was also significantly decreased following Smnp treatment.
Discussion
Antihepatotoxic activity of Sm is due to a synchronous effect of principal flavonolignans.Citation45 Our initial attempts in Sm nanoparticulation with poly(lactic-co-glycolic acid) (PLGA),Citation46 casein, and alginateCitation47 were unsuccessful due to very low Sm mass loading. Eudragit RS100® is a safe and biocompatible polycation and has previously been used in ophthalmic nanoparticle delivery devices.Citation31,Citation48 PVA resulted in a lowering of interfacial tension and nanoparticle steric and mechanical stabilization.Citation32 The effect of PVA concentration in particle sizing was shown in factorial design experiments. Higher initial concentration of PVA resulted in smaller particles. Similar PVA-induced stabilization effects have been reported by others in the case of PLGA nanoparticles.Citation49,Citation50 Sm payloading on the other hand was not dependent on any one parameter and the effect of the interaction term was significant. AFM details indicated smooth and spherical particles in all cases of PVA stabilized Eudragit nanoparticles. Chemical interaction studies in FTIR indicated no significant peak shifts for Sm in the presence of the entrapment polymer Eudragit RS100®. Structurally reactive Sm ketone and benzopyran groups responded strongly at 1636 cm−1 and 1084 cm−1, both as free compounds and in the Eudragit polymer mixture environment.
Smnps exhibited a sustained release profile and type B1 and B2 accounted for a 90% payload release during the 200 hours of the in vitro study period. The biphasic Sm release was possibly due to nanoparticle surface-adsorbed molecules, low aqueous solubility of Sm, and the insoluble nature of the polymer. Smnp B2 type carried a higher pay-load and was selected for detailed APAP-induced mouse hepatotoxicity studies.
APAP hepatotoxicity is primarily due to the pharmacokinetic generation of the reactive metabolite NAPQI.Citation5 NAPQI binds to cellular macromolecules causing the collapse of cell membranes and subsequent cell death. A dose of 300 mg/kg APAP resulted in profound hepatotoxicity and correlated well with the rise in serum aspartate transaminase (AST), 4943.3 ± 220.5 IU/L, and alanine transaminase (ALT), 5161.7 ± 339.8 IU/L levels. Test compounds were i.p. administered and the serum enzyme levels were monitored as markers for hepatic conditions. Hepatic GSH store was monitored (), as the increased level of NAPQI is linked directly to depletion of hepatic GSH store.Citation6 APAP inflicted a rapid depletion of GSH store within 1 hour of treatment.Citation51 Higher doses of APAP generate NAPQI, an oxidative product of cytochrome p450, in hepatocytes. GSH can neutralize the highly electrophilic NAPQI but in conditions of depleted GSH stores, NAPQI leads to a cascade of adverse events leading to the generation of reactive oxygen species, membrane protein damage, and adenosine triphosphate (ATP) depletion leading to hepatic necrosis.Citation52 Other contributing factors for APAP necrosis are intracellular Ca2+ imbalance and activation of inflammatory mediators such as tumor necrosis factor alpha (TNF-α).Citation6,Citation53
Sm possesses strong free radical scavenging activity, inhibits lipid peroxidation, and promotes regeneration of damaged hepatocytes. This effect is due to the increased synthesis rate of ribosomal RNA (rRNA) for the activation of RNA polymerase. In addition, Sm inhibits the 5-lipoxygenase pathway and prevents liver fibrosis. Sm also possesses membrane stabilizing propertiesCitation54 and these factors together contribute to its hepatoprotective activity.
Group D and group E animals pretreated with Sm or Smnp did not show a marked increase in serum marker enzymes. Hepatic GSH level did not decline and 100% survival was recorded. Smnp provided an incremental improvement over Sm in serum marker enzyme levels. This was likely due to Sm-induced membrane stabilization effects. These observations corroborated well with the histological examinations of treated and control groups (). However, when Sm was administered 1 hour after established APAP-induced hepatic necrosisCitation55 there was no significant lowering of serum marker enzymes. Necrosis and damage were also observed in the liver. Animal mortality recorded was significant and comparable to that of group B. Group G animals, however, when treated with Smnp 1 hour after APAP challenge survived and serum transaminase factors reverted to a lower concentration. A significant amount of hepatic GSH was also recorded.
Sm is membranotropic in nature, and Sm flavonolignans are known to exert a differential hepatic membrane stabilization response.Citation54 Silibin, a major component of Sm, is known to induce dual perturbation on membrane bilayers.Citation56 Silibin effects a concentration-dependent transition of bilayers to micellar structure. Isolated membrane studies have provided ample evidence that Sm prevents membrane disruption with increasing concentration. Silibin protective effects were, however, far less marked and were independent of the concentration gradient. Nanoparticle synchronous delivery therefore is important and exerts hepatoprotection activity in case of APAP overdose. Moreover, it can be reasoned that Smnp-induced rapid replenishment of hepatic nonprotein-SH concentration was due to particle effects. Sm flavonolignans are known to increase nuclear rRNA synthesis to a significant level within 8 hours of injection.Citation23 Biopharmaceutic enhancement of Sm through nanoparticulation can therefore provide a significant solution for conditions of acute APAP poisoning.
Conclusion
A new nanoparticle delivery device for Sm in Eudragit RS100® was successfully designed and protective properties against APAP-induced hepatotoxicity were established. Increased Smnp-induced rapid regeneration of hepatic GSH levels was demonstrated, along with downregulation of serum enzyme parameters, and marked increase in survival even when administered after APAP-induced hepatic damage. This appeared possible due to nanoparticle-assisted improved solubilization of Sm.
Acknowledgements
The authors are thankful to Anirban Chakraborty for his assistance in AFM studies. Financial assistance to Suvadra Das and Partha Roy from the Council of Scientific and Industrial Research (CSIR), India, and Indian Council Of Medical Research (ICMR), respectively, is gratefully acknowledged. Partial support from the Department of Biotechnology, Government of India, is also acknowledged.
Disclosure
The authors report no conflicts of interest in this work.
References
- KnightTRFarissMWFarhoodAJaeschkeHRole of lipid peroxidation as a mechanism of liver injury after acetaminophen overdose in miceToxicol Sci200376122923612944590
- ZimmermanHJMaddreyWCAcetaminophen (paracetamol) hepatotoxicity with regular intake of alcohol: analysis of instances of therapeutic misadventureHepatology19952237677737657281
- NolanCMSandblomREThummelKSlatteryJTNelsonSDHepatotoxicity associated with acetaminophen usage in patients receiving multiple drug therapy for tuberculosisChest199410524084117508362
- BreenKJBuryRWDesmondPVForgeBHMarshfordMLWhelanGParacetamol self-poisoning: diagnosis, management, and outcomeMed J Aust19821277797070335
- DahlinDCMiwaGTLuAYNelsonSDN-acetyl-p-benzoquinone imine: a cytochrome P-450-mediated oxidation product of acetaminophenProc Natl Acad Sci U S A1984815132713316424115
- VermeulenNPEBessemsJGMVan de StraatRMolecular aspect of paracetamol-induced hepatotoxicity and its mechanism based preventionDrug Metab Rev19922433674071628537
- KaushalRDaveKRKatyareSSParacetamol hepatotoxicity and microsomal functionEnviron Toxicol Pharmacol199971677421781911
- WagnerHDieselPSeitzMChemistry and analysis of silymarin from Silybum marianum GaertnArzneimittelforschung1974244466471 German.4408125
- HahnGLehmannHDKurtenNUebelHVogelGOn the pharmacology and toxicology of silymarin, an antihepatotoxic active principle from Silybum marianum (L.) GaertnArzneimittelforschung1968186698704 [German].5755807
- CorchetePSilybum marianum (L) Gaertn: the source of silymarinRamawatKGMerillonJMBioactive Molecules and Medicinal PlantsBerlin, GermanySpringer-Verlag2008124140
- LeeJINarayanMBarrettJSAnalysis and comparison of active constituents in commercial standardized silymarin extracts by liquid chromatography–electrospray ionization mass spectrometryJ Chromatogr B Analyt Technol Biomed life Sci2007845195103
- ShibanoMLinASItokawaHLeeKHSeparation and characterization of active flavonolignans of Silybum marianum by liquid chromatography connected with hybrid ion-trap and time-of-flight mass spectrometry (LC-MS/IT-TOF)J Nat Prod20077091424142817764149
- KvasnickaFBíbaBSevcíkRVoldrichMKrátkáJAnalysis of the active components of silymarinJ Chromatogr A20039901 Pt 223924512685603
- RamasamyKAgarwalRMultitargeted therapy of cancer by silymarinCancer Lett2008269235236218472213
- KaurMAgarwalRSilymarin and epithelial cancer chemoprevention: how close we are to bedside?Toxicol Appl Pharmacol2007224335035917184801
- GabettaBBombardelliEPifferiG inventor; Inverni Della Beffa S.p.A., assignee. Complexes of flavonolignans with phospholipids, preparation thereof and associated pharmaceutical compositions. United States patent US 4764508.1988816
- MadausRHHalbachGTrostW inventor; Dr. Madaus and Co, assignee. Salt of the silymarin group with aminopolyhydroxy alcohols. United States patent US 3994925.19761130
- SchulzHUSchurerMKrumbiegelGWachterWWeyhenmeyerRSeidelGThe solubility and bioequivalence of silymarin preparationsArzneimittelforschung19954516164 [German].7893272
- WooJSKimTSParkJHChiSCFormulation and biopharmaceutical evaluation of silymarin using SMEDDSArch Pharm Res2007301828917328246
- ZhangHLBaiTCYanGBHuYJSolubility of silybin in aqueous poly(vinylpyrrolidone) solutionFluid Phase Equilibr20052382186192
- ComoglioATomasiAMalandrinoSPoliGAlbanoEScavenging effect of silipide, a new silybin-phospholipid complex, on ethanol-derived free radicalsBiochem Pharmacol1995508131313167488251
- EI-SamaligyAfifiNNMahmoudEAEvaluation of hybrid liposomes-encapsulated silymarin regarding physical stability and in vivo performanceInt J Pharm20063191 Pt 212112916837151
- SonnenbichlerJMattersbergerJRosenHStimulation of RNA synthesis in rat liver and isolated hepatocytes by silybin, an antihepatotoxic agent from Silybum marianum L. GaertnHoppe Seylers Z Physiol Chem1976357811711180 [German].976944
- ShakerEMahmoudHMnaaSSilymarin, the antioxidant component and Silybum marianum extracts prevent liver damageFood Chem Toxicol201048380380620034535
- SaliouCRihnBCillardJOkamotoTPackerLSelective inhibition of NF-kappa-B activation by the flavonoid hepatoprotector silymarin in HepG2: evidence for different activating pathwaysFEBS Lett19984401 Pt 28129862414
- DehmlowCErhardJde GrootHInhibition of Kupffer cell functions as an explanation for the hepatoprotective properties of silybininHepatology19962347497548666328
- CamposRGarridoAGuerraRValenzuelaASilybin dihemisuccinate protects against glutathione depletion and lipid peroxidation induced by acetaminophen on rat liverPlanta Med19895554174192813577
- MurielPGarciapinaTPerez-AlvarezVMourelleMSilymarin protects against paracetamol-induced lipid peroxidation and liver damageJ Appl Toxicol19921264394421360480
- NenciniCGiorgiGMicheliLProtective effect of silymarin on oxidative stress in rat brainPhytomedicine2007142 Pt 312913516638633
- UbrichNSchmidtCBodmeierRHoffmanMMaincentPOral evaluation in rabbits of cyclosporin-loaded Eudragit RS or RL nanoparticlesInt J Pharm2005288116917515607269
- PignatelloRBucoloCFerraraPMalteseAPuleoAPuglisiGEudragit RS100 nanosuspensions for the ophthalmic controlled delivery of ibuprofenEur J Pharm Sci2002161 Pt 2536112113891
- Galindo-RodriguezSAllémannEFessiHDoelkerEPhysicochemical parameters associated with nanoparticle formation in the salting-out, emulsification-diffusion, and nanoprecipitation methodsPharm Res20042181428143915359578
- MillerRPFischerLJUrinary excretion of acetaminophen by the ratJ Pharm Sci19746369699704855343
- PrescottLFFactors influencing paracetamol metabolismPrescottLFParacetamol (Acetaminophen): a Critical Bibliographic ReviewLondon, UKTaylor and Francis1996708715
- GovenderTStolnikSGarnettMCIllumLDavisSSPLGA nanoparticles prepared by nanoprecipitation: drug loading and release studies of a water soluble drugJ Control Release19995721711859971898
- MaheshwariHAgarwalRPatilCKatareOPPreparation and pharmacological evaluation of silibinin liposomesArzneimittelforschung200353642042712872613
- HinsonJAPikeSLPumfordNRMayeuxPRNitrotyrosine-protein adducts in hepatic centrilobular areas following toxic doses of acetaminophen in miceChem Res Toxicol19981166046079625727
- SinghaPKRoySDeySProtective activity of andrographolide and arabinogalactan proteins from Andrographis paniculata Nees. against ethanol-induced toxicity in miceJ Ethnopharmacol20071111132117127022
- EllmanGLTissue sulfhydryl groupsArch Biochem Biophys1959821707713650640
- PignatelloRBucoloCPuglisiGOcular tolerability of Eudragit RS100 and RL100 nanosuspensions as carriers for ophthalmic controlled drug deliveryJ Pharm Sci200291122636264112434408
- LanjuanLHuayingWZhanYOPolymer- and lipid-based nanoparticle therapeutics for the treatment of liver diseasesNano Today201054296312
- ArmstrongNAJamesKCPharmaceutical Experimental Design And InterpretationLondon, UKTaylor and Francis1996131134
- BellamyLJThe Infrared Spectra of Complex Molecules3rd ed1London, UKChapman and Hall1975107128
- LinCCMettersATHydrogels in controlled release formulations: network design and mathematical modelingAdv Drug Deliv Rev20065812 Pt 131379140817081649
- LuCLuYChenJZhangWWuWSynchronized and sustained release of multiple components in silymarin from erodible glyceryl monostearate matrix systemEur J Pharm Biopharm200766221021917196805
- RoyPDasSBeraTMondolSMukherjeeAAndrographolide nanoparticles in leishmaniasis: characterization and in vitro evaluationsInt J Nanomedicine201051113112121270962
- BhowmikBBSaBMukherjeeAPreparation and in vitro characterization of slow release testosterone nanocapsules in alginatesActa Pharm20065641742919839134
- PignatelloRBucoloCSpedalieriGMalteseAPuglisiGFlurbiprofen-loaded acrylate polymer nanosuspensions for ophthalmic applicationBiomaterials200223153247325512102196
- VandervoortJLudwigABiocompatible stabilizers in the preparation of PLGA nanoparticles: a factorial design studyInt J Pharm20022381 Pt 2779211996812
- MurakamiHKawashimaYNiwaTHinoTTakeuchiHKobayashiMInfluence of the degrees of hydrolyzation and polymerization of poly(vinylalcohol) on the preparation and properties of poly(DL -lactide-co-glycolide) nanoparticleInt J Pharm19971494349
- MinerDJKissingerPTEvidence for the involvement of N-acetyl-p-quinoneimine in acetaminophen metabolismBiochem Pharmacol1979282232853290526335
- MitchellJRJollowDJPotterWZGilletteJRBrodieBBAcetaminophen-induced hepatic necrosis. IV. Protective role of glutathioneJ Pharmacol Exp Ther197318712112174746329
- Martin-MurphyBVHoltMPJuCThe role of damage associated molecular pattern molecules in acetaminophen-induced liver injury in miceToxicol Lett2010192338739419931603
- BasiglioCLSánchez PozziEJMottinoADRomaMGDifferential effects of silymarin and its active component silibinin on plasma membrane stability and hepatocellular lysisChem Biol Interact20091792 Pt 329730319135039
- RobertsDWBucciTJBensonRWImmunohistochemical localization and quantification of the 3-(cystein-S-yl)-acetaminophen protein adduct in acetaminophen hepatotoxicityAm J Pathol199113823593711992763
- WesolowskaOLania-PietrzakBKuzdzalMInfluence of silybin on biophysical properties of phospholipid bilayersActa Pharmacol Sin200728229630617241534