?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose
Herbal supplements are currently available as a safer alternative to manage obesity, which has become a rising problem over the recent years. Many chemical drugs on the market are designed to prevent or manage obesity but high cost, low efficacy, and multiple side effects limit its use. Nano lipo-vesicles phytosomal thermogel of Soybean, Glycine max (L.) Merrill, was formulated and evaluated in an attempt to investigate its anti-obesity action on body weight gain, adipose tissue size, and lipid profile data.
Methods
Three different techniques were used to prepare phytosome formulations including solvent evaporation, cosolvency, and salting out. The optimized phytosome formulation was then selected using Design Expert® (version 7.0.0) depending on the highest entrapment efficiency, minimum particle size (PS), and maximum drug release within 2 hours as responses for further evaluation. The successful phytosome complex formation was investigated by means of Fourier-transform infrared spec troscopy and determination of PS and zeta potential. Phytosome vesicles’ shape was evaluated using transmission electron microscope to ensure its spherical shape. After characterization of the optimized phytosome formulation, it was incorporated into a thermogel formulation. The obtained phytosomal thermogel formulation was evaluated for its clarity, homogeneity, pH, and gel transformation temperature besides rheology behavior and permeation study. An in vivo study was done to investigate the anti-weight-gain effect of soy phytosomal ther mogel.
Results
EE was found to be >99% for all formulations, PS ranging from 51.66–650.67 while drug release was found to be (77.61–99.78) in range. FTIR and TEM results confirmed the formation of phytosome complex. In vivo study showed a marked reduction in body weight, adipose tissue weight and lipid profile.
Conclusion
Concisely, soy phytosomal thermogel was found to have a local anti-obesity effect on the abdomen of experimental male albino rats with a slight systemic effect on the lipid profile data.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Obesity is a common health problem around the world that impacts human general health and social life and may affect vital organs such as heart, kidney, and liver.Citation1 Several chemical anti-obesity drugs were withdrawn from the market because of their serious side effects, and so there is a great interest now toward herbal remedies as an alternative for treatment of obesity.
Many experimental trials were done to evaluate the anti-obesity effect of different herbal products, for example, Dioscorea nipponica was found to have anti-obesity effect upon administration as an oral emulsion,Citation2 Gambi-hwan extract was able to prevent weight gain after 10 weeks of oral administration,Citation3 and also Clerodendron glandulosum aqueous extract gave positive results in weight gain control when mixed with the diet of the experimental animals.Citation4
Recently, the topical route has received considerable attention as it is a noninvasive route and has a high bioavailability level as it delivers the drug directly to the site of action avoiding first-pass effect of liver and gastrointestinal tract-related problems.Citation5 Most of the phytoconstituents are highly water-soluble substances such as saponins and flavonoids, and thus have a poor lipid solubility that came besides their large molecular weight, which became the reason for their very poor absorption and consequently bioavailability especially in case of transdermal administration route.Citation6 However, topical administration of capsaicin, a major constituent of chillies, was found to have local anti-obesity effect,Citation7 as well as glycyrrhetinic acid, the active principle component of licorice that showed significant reduction of subcutaneous thigh fat thickness when administered topically.Citation8
Many approaches were developed to overcome the obstacle of poor absorption of the herbal products, and an important one of those approaches is phytosome complex formation. Phytosome is a complex, produced by forming a hydrogen bond between stoichiometric amounts of herbal extract functionalities and phospholipid polar heads using a suitable solvent to give nanosized vesicles with high bioavailability. Better stability of phytosomes over liposomes can be explained by the fact that liposome formation can be obtained by just mixing a water-soluble substance with phospholipids with no chemical bond formation.Citation9
Thermogel, a gel that changes in viscosity according to temperature change, is considered an important formulation that enhances the topical transdermal delivery of many drugs.Citation10
Soybean, Glycine max (L.) Merrill family Fabaceae, was known to have a significant effect on body weight control because of its content of saponin,Citation11 protein,Citation12 and phosphatidylcholine (PC).Citation13 However, the mechanism is still unexplained.Citation14
In this study, the effect of soy phytosomal thermogel in controlling high-fat diet (HFD)-induced weight gain was investigated in experimental albino rats.
Materials and methods
Materials
Soybean, G. max (L.) Merrill family Fabaceae, was obtained from Haraz, Babb El-Khalk, Egypt, and kindly authenticated by Professor Dr Mohamed El-Gebaly, Taxonomy Specialist at El-Orman Botanical Garden, Giza, Egypt; methanol and acetonitrile (high-performance liquid chromatography [HPLC] grade; Cornell Lab, Cairo, Egypt); standard soy saponin B (Wako Pure Chemical Industries, Osaka, Japan); PC (Sigma-Aldrich Co., St Louis, MA, USA); methylparaben (EL-Gomhouria Co., Cairo, Egypt); polyethylene glycol (PEG 400), hydroxypropyl methylcellulose (HPMC) (E 50 LV), Pluronic F127 (Poloxamer407), propylparaben (LOBA Chemie, India); dimethyl sulfoxide (DMS), n-hexane, ethanol (ADWIC; El-Nasr Pharmaceutical Chemicals Co., Egypt); cellophane membrane (12,000–14,000 Dalton molecular weight; El-Maadi Co., Cairo, Egypt).
Preparation of soy extract
Soybean seeds were finely powdered to yield soy flour, then 100 g of soy flour was accurately weighed and soaked in 1 L of methanol at room temperature (25°C) with continuous agitation for 24 hours using a magnetic stirrer (C-MAG HS 7 digital; IKA, Staufen, Germany). The obtained extract solution was filtered and methanol was evaporated using a rotary evaporator (IKA ®RV10 basic; IKA) at 30°C and 120 rpm. After complete solvent evaporation, the yielded dry extract was stored at 4°C until further use.Citation15
Soy extract and standard soy saponin were analyzed using HPLC apparatus (Dionex Ultimate 3000, quaternary gradient HPLC [Berlin, Germany] with Smart line manager-5000 degasser and Smart line pump-1000; Knauer, Germany) at λmax =205 nm (UV-detector). This method was adapted from Zhang J’s reportCitation15 with slight modification.
Serial dilutions (0.01–0.1 mg/mL) of soy extract in both methanol and buffer solution (pH 4) were prepared. Samples were filtered using syringe filter (0.25 µm pore size; Sartorius, Gottingen, Germany), then chromatographed on Thermo Scientific ODS C18 RP column (1504.6, 5 mm) in isocratic mode, and the temperature was set at 25°C. Acetonitrile and deionized water (80:20 v/v) mixture was used as an optimized degassed mobile phase at 1.5 mL/min flow rate and with an injection volume of 50 µL. Standard calibration curve of the analyzed soy extract was established.
Experimental design
Three-level factorial design quadratic model was employed using Design Expert (version 7.0.0; Stat-Ease Inc., Minneapolis, MN, USA), to investigate the combined effect of three independent variables, PC% (1% and 3%), extract% (1% and 3%), and method of preparation (solvent evaporation, cosolvency, and salting out), on three dependent variables, entrapment efficiency percentage (EE%), particle size (PS), and percentage of drug released within 2 hours ().
Table 1 Design matrix depicting the experimental runs and values of the response variables obtained for experimental runs for soy-loaded phytosomes
Preparation of soy phytosomes
Solvent evaporation
Dried soy extract and PC (according to the ratio clarified in ) were dissolved into 10 mL of ethanol and refluxed for 2 hours under vacuum using rotary evaporator (IKA RV10 basic; IKA) at 30°C and 120 rpm. After complete evaporation of ethanol, the resulted residue was hydrated with distilled water to obtain the phytosomal suspension.Citation16
Cosolvency
Two separate flasks each containing 10 mL of methanol were used to dissolve the dried soy extract and PC (according to the ratio clarified in ); after that, the dissolved soy extract was added dropwise to the dissolved PC with stirring on a magnetic stirrer (C-MAG HS 7 digital; IKA) for 1 hour.Citation16
Salting out
Ethanol (10 mL) was used to dissolve both dried soy extract and PC (according to the ratio clarified in ) with continuous agitation on a magnetic stirrer (C-MAG HS 7 digital; IKA), then n-hexane was added dropwise to the resulted residue till complete precipitation of phytosome vesicles occurred.Citation16
Characterization of phytosomes
Entrapment efficiency
Ultracentrifugation techniqueCitation17 was used to determine the EE% of the prepared soy phytosomes. Briefly, soy phytosome formulations were centrifuged at 4°C and 15,000 rpm for 90 minutes using cooling centrifuge apparatus (Sigma Laborzentrifugen D-37520, Osterode–am-Harz, Germany). The supernatant was separated and then analyzed using the same HPLC analysis method mentioned previously.
EE% was calculated using the following equation:
PS and polydispersity index (PDI)
The PS and PDI were determined at 25°C by photon correlation spectroscopy using zeta seizer (Malvern ZetaSizer 3000; Malvern Instruments, Malvern, UK) at a fixed angle of 90°.Citation18
In vitro release
Percentage of soy extract released within 2 hours from the prepared phytosome vesicles was determined using modified USP Dissolution Tester apparatus II method (Paddle type-Erweka DT-720; Erweka GmbH, Heusenstamm, Germany). Briefly, cellophane membrane with a diffusion area of 4.9 cm2 was used as a diffusion membrane, cut out, stretched over two open edges of a glass tube, and tied well. Soy phytosome preparations were added into the cellophane-covered tubes, which were then fixed on the dissolution tester apparatus replacing its paddles. The speed of the dissolution apparatus was set at 100 rpm and the temperature was maintained at 32°C±0.5°C, while the release medium was 500 mL of phosphate buffer (pH 4). Samples were withdrawn from the release medium at predetermined time intervals (5, 10, 15, 30, 60, and 120 minutes) and immediately replaced by an equal volume of fresh phosphate buffer (pH 4), and then they were analyzed using the same HPLC method mentioned earlier.Citation19
Zeta potential
The charge of the diluted phytosome vesicles of the optimized formulation was determined by light scattering technique using zeta sizer (Malvern ZetaSizer 3000; Malvern Instruments).Citation18
Fourier-transform infrared (FTIR) spectroscopy study
FTIR spectroscopy study was done to the optimized phytosome formulation and then compared with that of soy extract and PC using an FTIR spectrophotometer (Bruker-alpha; Specac, Ettingen, Germany). Briefly, 0.5 mL of the sample was placed just below the fixed probe of the FTIR spectrophotometer and scanned over the 3,500–1,000 cm−1 wavenumber region.Citation20
Morphology
Successful phytosome vesicles formation was confirmed by high-resolution transmission electron microscopy (HRTEM) (Technai G2 Spirit Twin; Okolab, Naples, Italy) after dilution of the optimized phytosome formulation with 5 mL distilled water, and a drop of it being withdrawn and placed over a carbon-coated copper grid at an accelerating voltage of 120 kV, which was captured by Veleta camera.Citation21
Preparation of phytosomal thermogel
Thermogel was prepared according to the cold method.Citation22 Briefly, on a magnetic stirrer (C-MAG HS 7 digital; IKA), HPMC 3% w/v was slowly added to the phosphate buffer (0.1 M, pH 4) at 4°C with gentle mixing. Methylparaben (0.02% w/v) and propylparaben (0.02% w/v) as preservatives, PEG400 (4% w/v) as a humectant, and DMS and ethanol as penetration enhancers (2% w/v) were then added to the polymer solution as well as Pluronic F127 (18% w/v) that acted as a gel-transition polymer. Optimized soy phytosome formulation was then added dropwise to the prepared thermogel with stirring on a magnetic stirrer (C-MAG HS 7 digital; IKA) at 4°C to obtain soy phytosomal thermogel that was allowed to dissolve overnight at 4°C.Citation23
Characterization of phytosomal thermogel
Clarity, homogeneity, and pH
Phytosomal thermogel was evaluated for its clarity and homogeneity by visual inspection while pH was determined using pH-meter (Jenway 3510 pH meter; Staffordshire, UK).
Gel transformation temperature
Gel transformation temperature was determined using modified “Tilting tube method”.Citation24 Briefly, 2 mL of the prepared soy phytosomal thermogel was transferred to a test tube and then tilted into a thermostatic water bath (Memmert, Schwabach, Germany) at 90° angle with gradual raising of the water bath temperature. The temperature at which the formulation showed no flow upon tilting was recorded.
Rheology study
Soy phytosomal thermogel rheology behavior was investigated over a wide range of speed (10–100 rpm) and at different temperatures (−4°C, 25°C, and 32°C) using Brookfield viscometer (RV-TD; Brookfield Engineering Laboratories, Stoughton, MA, USA) with a suitable spindle (CP52 model HB), and there was a 30-second time gap between every two successive measurements.Citation25
Permeation study
Permeation profile of soy from the optimized phytosomal thermogel preparation was studied with and without addition of penetration enhancers (ethanol and DMS) using the USP Dissolution Tester method previously mentioned and with the same experimental conditions but using shaved abdominal area (4.9 cm2) of male albino rat skin instead of the cellophane membrane.Citation26 Cumulative percentage of soy extract permeated was calculated using Fick’s law:
The enhancement ratio (Er) that resulted from adding the penetration enhancers was calculated from the following equation:
In vivo study
Twenty male albino rats (225±5 g) aged 6 weeks were obtained from the animal house of Nahda University Beni-Suef, Egypt, and divided into four main groups (n=5) as follows: Group 1, treated with plain thermogel; Group 2, treated with crude soy extract thermogel; Group 3, treated with 2.5% soy phytosomal thermogel; Group 4, untreated control group. All groups were housed in separate cages and were allowed to access 50% HFD (standard forage mixed with 3% milk powder) and water ad libitum. The room temperature was maintained at 23°C±2°C and 12 hours light-and-dark cycle.
Experimental protocol was done according to the guidelines of Beni-Suef Animal House approved by Pharmacology and Toxicology Department, Faculty of Pharmacy, Beni-Suef University, on 20 June 2009, which was based on the guidelines suggested by the recommendations of the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No 85-23, revised 1985).
Rats were treated locally after hair removal of the treated abdominal skin area (4.9 cm2) with a razor. All preparations, according to each group, were administered topically to the rat’s abdomen once daily for 1 month.Citation8 Food intake amount of all rats was detected daily.
All rats were reweighed after 1 month of the treatment and the amount of weight gain and its percentage were calculated from the following equations:
Blood samples were collected from the retro-orbital plexus vein and centrifuged at 3,000 rpm for 20 minutes. Supernatant serum was transferred to Eppendorf tubes and analyzed for lipid profile data and lipase enzyme inhibition.Citation27
Rats were killed by cervical dislocation at the end of the experiment, and adipose tissue of the hairless treated abdominal area (4.9 cm2) of all rats was dissected, weighed, and fixed in 10% formalin/saline solution,Citation28,Citation29 then sectioned and embedded in paraffin, and after that it was stained with hematoxylin and eosin for histological evaluation (magnification ×100).Citation7,Citation30
Statistical analysis
The significance of differences was established by two-way analysis of variance using Graph Pad Prism version 6.0 for Windows, Graph Pad Software (San Diego, CA, USA). The results were expressed as mean ± SD and P-value <0.0001 was considered statistically significant.
Results
Standard calibration curve of soy extract
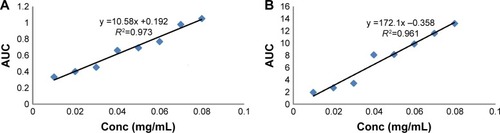
Saponin extract exhibited identical bands to those of the reference soy saponin in HPLC chromatogram. According to Beer–Lambert law, showed the standard calibration curves of soy extract in methanol and buffer solution (pH 4), and both curves showed a linear relationship between the soy extract concentration and area under the curve over a concentration range of 0.01–0.08 mg/mL. The regression equations were found to be (y =10.58x +0.192) with (R2=0.9731) and (y =0.172x −0.358) with (R2=0.961) for methanol and buffer solution (pH 4), respectively.
Optimization data analysis and validation of optimization model
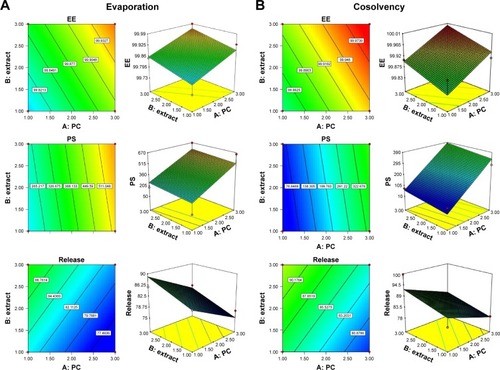
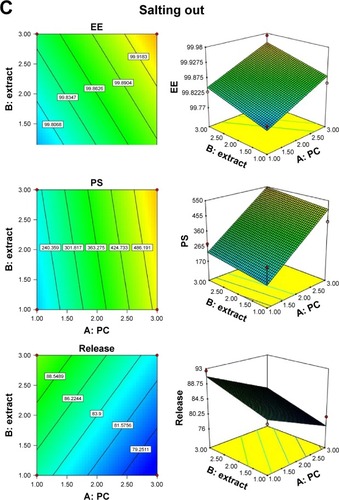
The coefficient of determination (R2), adjusted R2, predicted R2, and percentage coefficient of variation values were used to evaluate the fitness of the quadratic model to the experimental data (). The response surface analysis was carried out on three-dimensional (3D) response surface plots and 2D contour plots in order to understand the interaction if present (). The fitted models could be viewed as regression equations (Equation6(6) , Equation7
(7) , and Equation8
(8) ) generated by the software (Design Expert version 7.0.0; Stat-Ease Inc.), and the possible experimental solutions obtained from the numerical optimization with desirability values are illustrated in .
Table 2 Summary of results of regression analysis of responses
Table 3 Results of numeric optimization for soy-loaded phytosomes
Evaluation of phytosome preparations
Entrapment efficiency
Perfect loading of soy on the phytosome nanoparticles and minimal loss of it throughout the preparation steps were easily reflected in the high EE% as clarified in .
Particle size and PDI
Mean of the PS (n=3) of all phytosome formulations was found to be in the range of 51.66–667.24 nm. PDI of the optimized formulation was found to be 0.11, which indicates a homogeneous vesicle size distribution as small values of PDI (<0.1) indicate a homogeneous population, while PDI values (>0.4) are more heterogenic.Citation31
Drug release
Release profile of all soy phytosome formulations showed a great enhancement in the released percentage within 2 hours in comparison with the crude soy extract (77.16% within 2 hours) ().Citation32
Zeta potential
Phytosome stability was confirmed when zeta potential value was >±30 Mv, so optimized phytosome formulation (−51±7.06 mV) can be considered highly stable.Citation33
FTIR
FTIR spectra () confirmed the formation of phytosome vesicles by comparing the spectra of the prepared phytosome with that of PC and soy extract as the difference between them in fingerprint region (below 1,500 cm−1) indicates the new phytosome complex formation. The band at 3,276 cm−1 was attributed to the stretching vibration of the phenolic-OH group in natural soy. Moreover, sharp absorption bands were observed at 1,645 cm−1 (C=O vibration) and 1,004 cm−1 (aromatic C−O stretching vibration). In PC, the strong peaks at 1,736 and 1,229 cm−1 were due to C=O absorption and P=O absorption, respectively.
Figure 3 Fourier-transform infrared spectra of the optimized soy phytosome formulation (A) in comparison with that of phosphatidylcholine (B) and soy extract (C).
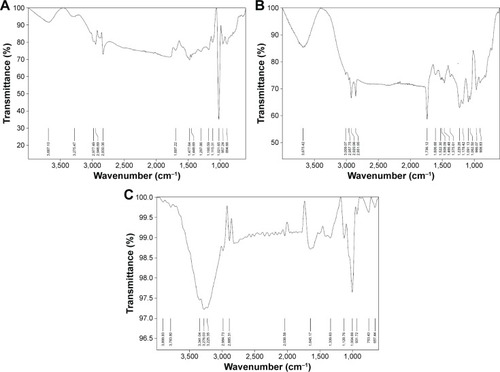
The strong peaks at 2,920 and 2,851 cm−1 and the weak peak at 1,375 cm−1 in PC could be due to stretching and deformation of methyl groups. The peak at 1,466 cm−1 observed could be due to bending vibration of CH2, while 3,009 cm−1 was for C=C-H and 2,957 cm−1 was for C−C-H in PC. A shift from 3,675 cm−1 of PC to 3,687 cm−1 compared with natural soy, 3,276 cm−1, was exhibited by phytosome complex besides C−C-H that appears at 2,977 cm−1 for phytosome, 2,957cm−1 for PC, and 2,984 cm−1 for soy. These changes indicated that soy and PC formed the phytosome complex by hydrogen bond formation between the OH group of soy phenol group and the P=O group of the PC.Citation34,Citation19
Morphology
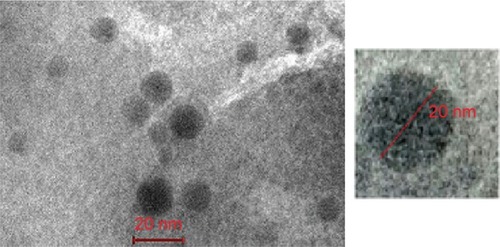
Transmission electron micrograph showed spherical, self-closed, rough surface vesicles with no indication of particle aggregation ().Citation21
Evaluation of phytosomal thermogel
Clarity, homogeneity, and pH
Soy phytosomal thermogel was found to be clear, translucent, and homogeneous with a pH=4, so safe skin application was assured with no expectation of any skin irritation or discomfort upon application.Citation35
Gel transformation temperature
Gel transformation temperature of soy phytosomal thermogel was found to be 31.5°C, which is suitable for skin delivery.Citation35
Rheology study
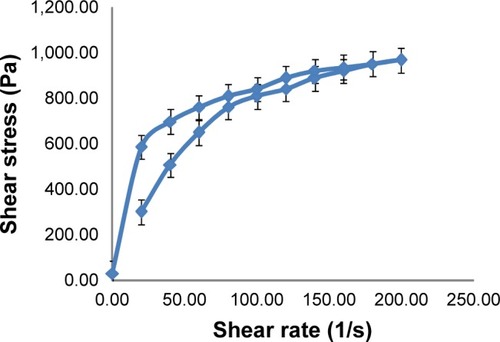
Soy phytosomal thermogel showed a thixotropic behavior, which is an important property for thermogel preparations, which means a decrease in viscosity and shear stress with increasing shear rate and vice versa ().Citation25
Permeation study
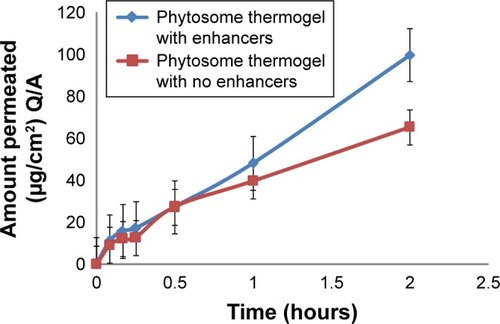
Permeation flux of soy extract from the phytosomal thermogel was found to be 9.7309 and 5.2062 µg/cm2⋅h with and without the addition of penetration enhancers, respectively, with r2=0.99 for both and Er =1.8690, which indicates the positive effect of penetration enhancers on the permeation profile of soy ().Citation26
In vivo study
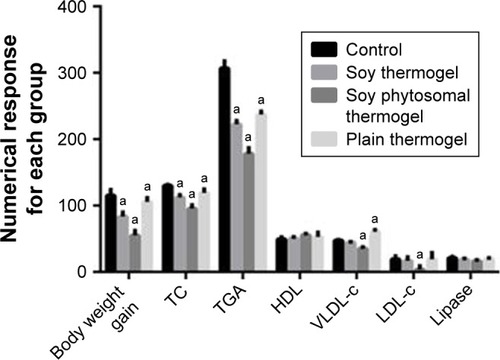
Soy phytosomal thermogel-treated rats group showed a marked decrease in body weight, adipose tissue weight, and food consumption than the other treated rat groups and the control group ().Citation2
Table 4 Effect of soy extract phytosomal thermogel on the body weight, adipose tissue weight, and food consumption of rats after 1 month of treatment
Moreover, from the lipid profile data illustrated in and , it was observed that there was a significant decrease in total cholesterol, triglycerides, low-density lipoprotein cholesterol, and very low-density lipoprotein cholesterol, which can be attributed to a slight systemic action of soy phytosomal thermogel in comparison with the control-treated group.Citation2
Table 5 Effect of soy extract phytosomal thermogel on the blood parameters in rats after 1 month of treatment
Figure 7 Two-way analysis of variance of body weight gain and lipid profile analysis for each animal group.
Note: aHas a significant difference at P<0.0001, when compared with model control (Group IV) values.
Abbreviations: HDL, high-density lipoprotein; LDL-c, low-density lipoprotein cholesterol; TC, total cholesterol; TGA, triglycerides; VLDL-c, very low-density lipoprotein cholesterol.
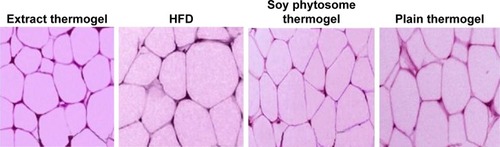
The histological analysis of epididymal adipose tissue () showed a decrease in the size of adipose cells in comparison with the control and crude soy thermogel groups and revealed the enhancement effect of phytosomal thermogel formulation on soy anti-obesity effect.Citation2
Discussion
Soy was approved by many researchers to have an anti-obesity effect because of its saponin,Citation11 protein,Citation12 and PCCitation13 content, so in this study, this anti-obesity action was investigated topically.
Soy was extracted by continuous agitation method at 24°C,Citation15 then 12 soy phytosome formulations were prepared with three different techniques and characterized to optimize one formulation with the highest EE%, minimum PS, and maximum release percentage within 2 hours as independent factors using Design Expert version 7.0.0 (Stat-Ease Inc.).
Regression equations for each response were produced by the model design (EquationEqs 6(6) –Equation8
(8) ), and a positive factor in polynomial equations means that increasing the factor causes increase in the response while negative sign represents that increasing the factor decreases the response. These equations showed that both PC% and soy extract% have a positive effect on both EE% and PS, and this may be because soy extract also contains PC and it enhances its effect, and by increasing solid lipids percentage, the aggregation facilitated and leads to increasing the PS.Citation36
High EE% of phytosome formulations (>99%) was expected because of the hydrogen bond formation between soy extract and PC.Citation7 PS obtained is good for enhancement of EE%,Citation37 release profile, and skin permeation.Citation23 Soy extract% was found to have a positive effect on the release response within 2 hours. On the other hand, PC factor was found to have a negative effect on it, which may be due to that increasing PC increases the bond strength between the extract and the PC in phytosome, which may delay the release of soy. The 3D response surface and 2D contour plots were obtained by varying magnitudes of PC and soy extract%, keeping the method of preparation constant, and results showed a significant effect of these factors on EE%, PS, and drug release% ().
The optimized phytosome formulation was found to be the one prepared using cosolvency method with a PC =1% and extract =3% (), which was then evaluated for zeta potential using TEM and FTIR. Zeta potential was found to be −51 mV, and shifting in zeta potential value toward negative value can be attributed to the incorporation of soy into the phytosome and it indicates a high repulsion force between vesicles that prevents its aggregation and consequently gives a high stability for the phytosome suspension with high skin penetration ability.Citation18
Soy phytosomal thermogel formulation was prepared and evaluated, and it was found to be clear, homogeneous, with pH value =5.5 and gel transformation temperature =31.5°C, which matches the required characteristics for skin delivery. Rheology study showed thixotropic behavior, which is a very important property for semisolid topical preparations as it gives indication of the physical and chemical changes occurred.Citation25
In vivo study of soy phytosomal thermogel showed a marked decrease in the body weight gain, adipose tissue weight, food consumption, and the size of adipose cells for treated groups. Body weight gain amounts are 85.030±6.43 g in soy thermogel and 55.260±6.94 g in soy phytosomal thermogel. This trend can be caused by the effect of different soy extract components that were approved to have anti-obesity effect including triglyceride soy saponin, which was investigated by Kim et al to have anti-obesity effect in vitro,Citation11 and soy protein oral consumption showed anti-obesity effect as reported by Velasquez and BhathenaCitation12 besides soy PC that was approved by Lee et al to have valuable effect on obesity.Citation13
Although rats were treated topically, lowering effect on the serum lipid profile was observed, which showed slight systemic anti-obesity effect of soy phytosomal thermogel which can be explained by the fact that nanosized particles of phytosome vesicles have a high skin permeation ability that may let it reach the blood circulation showing the cholesterol-lowering property of orally administered soy proteins.Citation38
In conclusion, soy phytosomal thermogel was found to have a local anti-obesity effect on rats’ abdomen with slight systemic effect on the lipid profile data.
Conclusion
In this study, a novel topical drug delivery system of soy extract has been developed in an attempt to investigate the anti-obesity action of soy topically. Phytosomal thermogel of soy extract was formulated and evaluated, and the optimized phytosome formulation was found to have an EE% of 99.89, PS equal to 64.44 nm, and a release percentage up to 92.50 within 2 hours. FTIR study confirmed the formation of a soy–PC complex that was also found to have a high permeation rate profile. The optimized phytosome formulation was then incorporated into the thermogel formulation and characterized for skin delivery suitability.
In vivo study on rats proved that soy extract has the ability to locally decrease the size of adipose cells in the abdominal area of rats with a slight systemic lowering effect on the serum lipid profile. Therefore, topical phytosomal thermogel of soy extract can be considered a hopeful formulation for the treatment of obesity.
Acknowledgments
Authors are thankful to Nahda University Beni-Suef central lab for providing the facilities to bring out this work and for Professor Dr Mohammed El-Gebaly for his great effort in plant authentication.
Disclosure
The authors report no conflicts of interest in this work.
References
- AdeneyeAAAdeyemiOOAgbajeEOAnti-obesity and antihyperlipidaemic effect of Hunteria umbellata seed extract in experimental hyperlipidemiaJ Ethnopharmacol2010130230731420471465
- KwonCSSohnHYKimSHAnti-obesity effect of Dioscorea nipponica Makino with lipase-inhibitory activity in rodentsBiosci Biotechnol Biochem20036771451145612913286
- LeeBJRyuJHKimJWParkJHParkJWThe anti-obesity effects of Gambi-hwan extract on obese rats induced by high-fat diet through the expression of UCP-1 and PPAR-δKorean J Orient Med2007284136147
- JadejaRNThounaojamMCRamaniUVDevkarRVRamachandranAAnti-obesity potential of Clerodendron glandulosum. Coleb leaf aqueous extractJ Ethnopharmacol2011135233834321397678
- ElnaggarYSEl-RefaieWMEl-MassikMAAbdallahOYLecithin-based nanostructured gels for skin delivery: an update on state of art and recent applicationsJ Control Release2014180102424531009
- PritiLHeenSAn overview of phytosomes as an advanced herbal drug delivery systemRes J Topical Cosmet Sci20134265
- LeeGRShinMKYoonDJTopical application of capsaicin reduces visceral adipose fat by affecting adipokine levels in high-fat-diet-induced obese miceObesity (Silver Spring)201321111512223505175
- ArmaniniDNacamulliDFrancini-PesentiFBattaginGRagazziEFioreCGlycyrrhetinic acid, the active principle of licorice, can reduce the thickness of subcutaneous thigh fat through topical applicationSteroids200570853854215894038
- AminTBhatSVA review of phytosome technology as a novel approach to improve the bioavailability of nutraceuticalsInt J Adv Res Tech201213115
- MoonHJKo duYParkMHJooMKJeongBTemperature-responsive compounds as in situ gelling biomedical materialsChem Soc Rev201241144860488322688789
- KimHJHwangJTKimMJThe inhibitory effect of saponin derived from Cheonggukjang on adipocyte differentiation in vitroFood Sci Biotechnol201423412731278
- VelasquezMTBhathenaSJRole of dietary soy protein in obesityInt J Med Sci200742728217396158
- LeeHSNamYChungYHBeneficial effects of phosphatidylcholine on high-fat-diet-induced obesity, hyperlipidemia and fatty liver in miceLife Sci2014118171425445436
- AndersonJWJohnstoneBMCook-NewellMEMeta-analysis of the effects of soy protein intake on serum lipidsNew Engl J Med199533352762827596371
- ZhangWPopovichDGChemical and biological characterization of oleanane triterpenoids from soyMolecules20091482959297519701138
- SinghRParpaniSNarkeRChavanRPhytosome: recent advance research for novel drug delivery systemAsian J Pharmaceut Res Health Care201421529
- AhmedTAPreparation of transfersomes encapsulating sildenafil aimed at transdermal drug delivery: Plackett–Burman design and characterizationJ Liposome Res201525111025148294
- Chen-yuGChun-fenYQi-luLDevelopment of a quercetin-loaded nanostructured lipid carrier formulation for topical deliveryInt J Pharm20124301–229229822486962
- FreagMSElnaggarYSAbdallahOYLyophilized phytosomal nanocarriers as platforms for enhanced diosmin delivery: optimization and ex vivo permeationInt J Nanomedicine201382385239723861584
- DasMKKalitaBDesign, and evaluation of phyto-phospholipid complexes (Phytosomes) of rutin for transdermal applicationJ App Pharm Sci20144105157
- AllamANKomeilIAAbdallahOYCurcumin phytosomal softgel formulation: development, optimization and physicochemical characterizationActa Pharm201565328529726431106
- ParmarVJLumbhaniANFormulation and development of thermo-reversible mucoadhesive intranasal in situ hydrogel by using a combination of polymersBull Pharm Res201223167174
- GargBJGargNKBegSSinghBKatareOPNanosized ethosomes-based hydrogel formulations of methoxsalen for enhanced topical delivery against vitiligo: formulation optimization, in vitro evaluation and preclinical assessmentJ Drug Target2016243233246
- LuCLiuMFuHNovel thermosensitive in situ gel based on poloxamer for uterus deliveryEur J Pharm Sci201577242825981887
- PingaliPSSrinivasPReddyBMMiconazole loaded novel phytosomal topical gelsWorld J Pharm Sci201541023052320
- El-BadryMFathyMEnhancement of the dissolution and permeation rates of meloxicam by the formation of its freeze-dried solid dispersions in polyvinylpyrrolidone K-30Drug Dev Ind Pharm200632214115016537195
- TanSGaoBTaoYGuoJSuZQAntiobesity effects of capsaicin–chitosan microsphere (CCMS) in obese rats induced by high-fat dietJ Agric Food Chem20146281866187424479662
- TanQLiuWGuoCZhaiGPreparation and evaluation of quercetin-loaded lecithin-chitosan nanoparticles for topical deliveryInt J Nanomedicine201161621163021904452
- KazmiIAfzalMRahmanSIqbalMImamFAnwarFAntiobesity potential of ursolic acid stearoyl glucoside by inhibiting pancreatic lipaseEur J Pharmacol20137091–3283623500199
- JooHKimCTKimIHKimYAnti-obesity effects of hot water extract and high hydrostatic pressure extract of garlic in rats fed a high-fat dietFood Chem Toxicol20135510010523306787
- CentisVVermettePPhysico-chemical properties and cytotoxicity assessment of PEG-modified liposomes containing human hemoglobinColloids Surf B Biointerfaces200865223924618538549
- VarshosazJPardakhtyAHajhashemiVINajafabadiARDevelopment and physical characterization of sorbitan monoester niosomes for insulin oral deliveryDrug Deliv200310425126214612341
- WangXGuanQChenWHuXLiLNovel nanoliposomal delivery system for polydatin: preparation, characterization, and in vivo evaluationDrug Des Devel Ther2015918051813
- ZhangJTangQXuXLiNDevelopment and evaluation of a novel phytosome-loaded chitosan microsphere system for curcumin deliveryInt J Pharm2013448116817423524117
- XuXShenYWangWPreparation and in vitro characterization of thermosensitive and mucoadhesive hydrogels for nasal delivery of phenylephrine hydrochlorideEur J Pharm Biopharm2014883998100425257714
- PhatakAAChaudhariPDDevelopment and evaluation of nanostructured lipid carrier (NLC) based topical delivery of an anti-inflammatory drugJ Pharm Res201378677685
- El-MenshaweSFHusseinAKFormulation and evaluation of meloxicam niosomes as vesicular carriers for enhanced skin deliveryPharmaceutical Dev Technol2013184779786
- WilsonTANicolosiRJKotylaTFleckingerBSoy protein without isoflavones reduces aortic total and cholesterol ester concentrations greater than soy protein with isoflavones compared with casein in hypercholesterolemic hamstersNutr Res2007278498504