Abstract
Introduction
Silver nanoparticles (AgNPs) have been shown to promote wound healing and to exhibit antimicrobial properties against a broad range of bacteria. In our previous study, we prepared tannic acid (TA)-modified AgNPs showing a good toxicological profile and immunomodulatory properties useful for potential dermal applications.
Methods
In this study, in vitro scratch assay, antimicrobial tests, modified lymph node assay as well as a mouse splint wound model were used to access the wound healing potential of TA-modified and unmodified AgNPs.
Results
TA-modified but not unmodified AgNPs exhibited effective antibacterial activity against Pseudomonas aeruginosa, Staphylococcus aureus and Escherichia coli and stimulated migration of keratinocytes in vitro. The tests using the mouse splint wound model showed that TA-modified 33 and 46 nm AgNPs promoted better wound closure, epithelialization, angiogenesis and formation of the granulation tissue. Additionally, AgNPs elicited expression of VEGF-α, PDGF-β and TGF-β1 cytokines involved in wound healing more efficiently in comparison to control and TA-treated wounds. However, both the lymph node assay and the wound model showed that TA-modified AgNPs sized 13 nm can elicit strong inflammatory response not only during wound healing but also when applied to the damaged skin.
Conclusion
TA-modified AgNPs sized >26 nm promote wound healing better than TA-modified or unmodified AgNPs. These findings suggest that TA-modified AgNPs sized >26 nm may have a promising application in wound management.
Introduction
Skin wound healing is a complex process involving 3 stages that overlap in time and space: inflammation, new tissue formation (granulation and angiogenesis) and tissue remodeling.Citation1 These 3 phases involve well-organized interactions between various cell types such as leukocytes, fibroblasts and keratinocytes and are controlled by several factors, including cytokines, chemokines, growth factors and enzymes.Citation1,Citation2 Following the initial hemostasis, inflammation is a critical part of the normal wound healing process. Prolonged inflammation hampers entering into the proliferative phase and delays wound closure. Chronic wounds represent a major health burden and remain a challenging clinical problem. Therefore, many studies have focused on the principles and mechanisms of wound healing acceleration to develop new drugs and/or dressings that promote wound healing.Citation3,Citation4
The importance of silver as an antimicrobial agent has been known for centuries, and its use has recently increased with the development of silver nanoparticles (AgNPs).Citation5 AgNPs have a large surface area, resulting in large amounts of silver ions being released and potentially penetrating into the skin, particularly the damaged skin.Citation6 There are numerous reports indicating that AgNPs promote wound healing and show antimicrobial properties against a broad range of bacteria, including Pseudomonas aeruginosa, Escherichia coli and Staphylococcus aureus.Citation7,Citation8 Wound dressings containing AgNPs have also been suggested to enhance wound healing by decreasing the inflammatory response.Citation9,Citation10
Tannins are plant phenol derivatives of various molecular weights naturally synthesized by plants as metabolic products.Citation11–Citation13 Tannic acid (TA, penta-m-digalloyl glucose) is the simplest and principal hydrolyzable tannin that has astringent, antioxidant, antimicrobial, antiviral and anti-inflammatory properties.Citation14,Citation15 Structurally, TA has a glucose moiety as a core and the hydroxyl groups of glucose are esterified with 5 digallic acids.Citation15 Although tannin extracts have been used to improve the process of wound healing for many years, their exact mechanism of action has not been completely elucidated to date. It is believed that tannins can promote wound healing through several mechanisms: 1) scavenging of free radicals and reactive oxygen species (ROS), 2) promoting wound contraction, and 3) increasing formation of capillary vessels and proliferation of fibroblasts.Citation12–Citation14
Synthesis of AgNPs with plant extracts is known as the green synthesis, which is believed to be eco-friendly. TA is an effective reducing and stabilizing agent, which allows us to obtain AgNPs with homogeneous spherical shape and a narrow size distribution.Citation16 Moreover, synthesis of AgNPs with TA is more stable compared with other methods.Citation17
In our previous studies, we demonstrated that toxicity of TA-modified AgNPs was cell-type dependent, with monocytes but not keratinocytes, producing ROS when exposed to AgNPs.Citation18,Citation19 Furthermore, TA-modified AgNPs sized >30 nm showed a good toxicological profile in vitro in human HaCaT and VK2-E6/E7keratinocyte cell lines and possessed immunomodulatory properties useful for potential dermal applications in humans.Citation19 TA-modified but not unmodified AgNPs downregulated tumor necrosis factor-α (TNF-α)- and lipopolysaccharide (LPS)-triggered production of interleukin 8 (IL-8) in VK2-E6/E7 cells.Citation19
In this study, the wound healing potential of TA-modified AgNPs was tested. The cytotoxicity and antibacterial activity in vitro, as well as the effect on wound healing in vitro and in vivo of TA-modified AgNPs, were evaluated. It is hypothesized that TA modification can promote wound healing by influencing inflammatory reaction and exerting antimicrobial properties. The importance of nanoparticle size in the wound healing process is also shown.
Materials and methods
Synthesis of AgNPs
TA-modified (the size of metallic core: 13, 33 and 46 nm) and unmodified AgNPs (10–65 nm) were synthesized as described previously via the chemical reduction method in water.Citation16,Citation18–Citation20 Briefly, AgNP colloids sized 13, 33 and 46 nm were synthesized with silver nitrate and the mixture of sodium citrate and TA. In our previous study, we showed the crucial role of this mixture in the synthesis of monodisperse AgNPs compared to the synthesis carried out with sodium citrate or TA alone.Citation16 Only the combined use of citrate and TA produced an active CA–TA complex, which enabled control of the reaction conditions resulting in homogeneous size and shape of AgNPs. A colloid with a broad range of particle sizes (10–65 nm) was synthesized with only sodium citrate, and constitutes the unmodified AgNPs (the synthesis carried out without TA). This colloid was used in our biological experiments as a reference sample, since it contains particles with the sizes within the range of tested colloids. Nanoparticles were characterized for shape, size and size distribution after each synthesis by dynamic light scattering (DLS) and transmission electron microscopy (TEM), as described previously.Citation18 The colloidal stability was analyzed with ultraviolet–visible (UV-vis) spectroscopy and through zeta potential measurements.
Cell culture and treatment
Mouse L929 fibroblasts from American Type Culture Collection (ATCC CCL-1; Manassas, MD, USA) were propagated in Eagle’s Minimum Essential Medium (MEM) supplemented with 10% fetal calf serum, 10 U/mL penicillin and 100 µg/mL streptomycin (Thermo Fisher Scientific, Waltham, MA, USA). Mouse RAW 264.7 monocytes (ATCC TIB-71) were maintained in RPMI-1640 medium with 10% fetal bovine serum (FBS) and 1% antibiotics (Thermo Fisher Scientific). Human HaCaT keratinocytes were obtained from the Department of Clinical Virology, University of Gothenburg, Göteborg, Sweden and propagated in DMEM supplemented with 10% fetal calf serum, 10 U/mL penicillin and 100 µg/mL streptomycin (Thermo Fisher Scientific). The cells were seeded into 24-well plates at the density of 5×104/mL cells and cultured for 48 h before exposure to nanoparticles at the concentration range of 0.5–10 µg/mL. After another 24 h, fresh medium was applied, and the cells were used for further analyses.
Visualization of AgNPs
For microscopy detection of nanoparticles, cells were plated on Lab-tek II chamber slides (Nunc; Thermo Fisher Scientific) at a density of 1×105/mL for 18 h before exposure to nanoparticles. After 24 h of exposure to 5 µg/mL of AgNPs, the medium was discarded, and the cells were fixed with 4% paraformaldehyde (PFA) in PBS, washed twice with PBS and covered with PBS containing 2 µg/mL Hoechst 33342 (Sigma-Aldrich Co., St Louis, MO, USA). The images were captured with the DMi8 inverted microscope with MC170HD camera coupled with the laser scan confocal system TCS SP8 (Leica Microsystems, Wetzlar, Germany). Bidirectional scan was performed with the acquisition speed of 200 Hz. Images were acquired in Z axis using water objective CS2 of magnification 63× and digital zoom 1.5×. Due to the signal interference, sequential scanning was applied to all samples. The obtained images were analyzed in Leica Application Software X (LAS X) Version 2.0.
Toxicity tests
Toxicity of AgNPs was accessed using Annexin V-Apoptosis detection kit I (BD Biosciences, San Jose, CA, USA), according to the manufacturer’s protocol. The annexin V-positive, propidium iodide (PI)-negative cells were scored as apoptotic cells, while all PI-positive cells were considered to be necrotic. Changes in the mitochondrial potential were measured in cells stained with a cationic dye, 5,5′,6,6′-tetrachloro1,1′,3,3′-tetraethyl-benzimidazolylcarbocyanine iodide (JC-1) (Sigma-Aldrich), as described previously.Citation18,Citation19,Citation21 The stained cells were analyzed in FACSCalibur using Cell-Quest program (BD Biosciences). Neutral red uptake assay was performed as described previously.Citation18 The viability of the cells was expressed as a percentage of the control, untreated cells (100%).Citation18,Citation19
Antibacterial assays
S. aureus ATCC 25923, P. aeruginosa ATCC 27853 and E. coli ATCC 15597 were used to determine the antibacterial activities of AgNPs. Minimum inhibitory concentrations (MICs) were determined in Mueller–Hinton broth (MHB) medium using a serial dilution method. Test tubes containing 2-fold dilutions of AgNPs in concentrations ranging from 0.78 to 50 µg/mL were inoculated with bacterial suspension to yield the appropriate density (105 CFU/mL) and incubated for 18–20 h at 37°C. The MIC was defined as the lowest concentration of the silver additive at which no visual turbidity of the growth medium developed. All measurements of MIC values were repeated in triplicate.
Wound assay in vitro
HaCaT cells were plated in 24-well dishes and cultured to 100% confluence. Scratches were produced using 200 µL pipette tips, as described by Liang et al.Citation22 After washing, the cells were treated with medium containing AgNPs at 5 µg/mL. After 24 h treatment, the cells were fixed with 4% PFA buffered with phosphate-buffered saline (Avantor, Gliwice, Poland) and stained with crystal violet. Micro-photographs were taken using a Zeiss Axiovert A1 (Zeiss, Oberkochen, Germany) inverted microscope using 5× objective and analyzed with ImageJ software.
Ethical statement
This study was performed in strict accordance with the recommendations of the Polish Act of 21 January 2005 on animal experiments (OJ no 33, item 289) and Directive 2010/63/EU of the European Parliament and the Council of 22 September 2010 on the protection of animals used for scientific purposes. The protocol was approved by the 4th Local Committee on the Ethics of Animal Experiments in Warsaw, Poland (permit number: 58/2013 and 76/2015). The use of HaCaT cell line was approved by the Scientific Board of the Military Institute of Hygiene and Epidemiology as part of the PhD thesis of Piotr Orłowski (December 2014).
Local lymph node assay (LLNA)
Female C57BL6 mice (Mossakowski Medical Research Centre, Warsaw, Poland), 7 weeks of age, were assigned to groups (3 animals per group) using a stratified random grouping method based on individual body weights. Ears were stripped 10 times with an ordinary adhesive tape (Polopor, Viscoplast; Polfa Warszawa, Poland). For each stripping, a fresh piece of tape was lightly pressed onto the ear and pulled off. After tape stripping, vehicle (acetone: olive oil, 3:1) – negative control, 25% w/v citral in vehicle (Sigma-Aldrich) – positive control, and 25% w/v of AgNPs in vehicle were applied to the dorsum of each ear (25 µL/ear) once daily for 3 consecutive days (days 1–3). On day 5, the draining auricular lymph nodes from each mouse ear were excised and processed separately in PBS for each animal. Cells were seeded in 96-well round bottom plates at 104 cells per well in RPMI medium without phenol red supplemented with 10% FBS, 10 U/mL penicillin, 100 µg/mL streptomycin (Thermo Fisher Scientific), 2 µg/mL concanavalin A (Sigma-Aldrich), 10% resazurin (Thermo Fisher Scientific) and cultured for 72 h. Fluorescence was measured at 540 nm (emission) and at 600 nm (reference) using a FLUOstar Omega counter (BMG Labtech, Ortenberg, Germany).
Wound model in vivo
Female C57BL6 mice (Mossakowski Medical Research Centre, Warsaw, Poland), 7 weeks of age, were assigned to groups (5 animals per group), as described earlier. Mice were anesthetized by intraperitoneal injection of 85 mg/kg ketamine (Biowet, Pulawy, Poland) and 11 mg/kg xylazine (Xylopan; Polypharm SA, Warsaw, Poland). Hair removal and sterilization were performed routinely. Two full-thickness circular excisions that included the panniculus carnosus on either side of the mouse’s midline at the level of the shoulders were created using a sterile 4 mm biopsy punch (Kruuse, Langeskov, Denmark). Next, a 5 mm silicone splint (Zegir, Warsaw, Poland) was applied around the excision with a cyanoacrylate adhesive (Super Glue) and anchored with interrupted 6-0 nylon sutures to ensure positioning. AgNPs or TA was applied at 5 µg/mL in saline in the volume of 100 µL to 1 wound, and a vehicle control to the other. The wound was covered with a transparent occlusive dressing using incise drape (3M Health Care, Neuss, Germany). A total of 5 mg/kg of meloxicam (Loxicom; ScanVet, Gniezno, Poland) was administered once daily via subcutaneous injection for post-operative pain relief during the first 3 days. At days 3, 6 and 14, mice were euthanized, and then the wounds were excised and used for further tests.
Morphological analyses of wound re-epithelialization, inflammatory infiltration and angiogenesis
Skin specimens were fixed in 4% PFA buffered with PBS (pH 7.4) and embedded in paraffin. Five micrometer sections were prepared on a microtome. Hematoxylin/eosin and Masson’s trichrome stainings were performed according to the manufacturer’s instructions (Sigma-Aldrich). The images were captured with the Zeiss Axio Imager.M2 (Zeiss). The wound closure was calculated as percentage of wound area at the time point in comparison to the initial wound area. The epithelial sheet area (re-epithelialization) was measured in the cross sections stained with hematoxylin/eosin. Cell numbers and blood vessels were counted in 5 randomly selected microscope fields per section in the wound cavity at 400× magnification (0.234 mm2).
Immunohistochemistry
The tissues were fixed and processed as described earlier. Antigens were unmasked in 0.1 M citrate buffer (pH =6.0) for 10 min. Macrophages were detected using F4/80 antibody (clone A3-1) (Santa Cruz Biotechnology Inc., Dallas, TX, USA), while neutrophils were detected with anti-Gr-1 antibody (RB6-8C5) (BD Biosciences) in PBS with 1% bovine serum albumin, followed by 30 min incubation with biotinylated anti-rat IgG antibody (1:200) (BD Biosciences) and streptavidin peroxidase (BD Biosciences) (1:500) in the same buffer. Sections were developed with 3,3′-diaminobenzidine (DAB) and counterstained with Harris’s hematoxylin solution (Sigma-Aldrich). Image capture, analysis and processing were performed using the Zeiss Axio Imager.M2 microscope and ZEN 2011 software (Zeiss). For all stainings, isotype control antibodies (rat monoclonal IgG) were used (BD Biosciences).
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA was isolated from the wound tissues preserved in RNAlater (Sigma-Aldrich) using Universal RNA Purification Kit (Eurx, Gdansk, Poland). cDNA was reverse-transcribed from 1 µg of total RNA using High Capacity RNA-to-cDNA Kit (Thermo Fisher Scientific) according to the manufacturer’s protocol. Transcripts of IL-1β, TNF-α, platelet-derived growth factor-α (PDGF-α), vascular endothelial growth factor-α (VEGF-α), transforming growth factor β1 (TGF-β1) and GADPH were quantified using Taqman(R) Gene Expression Assays (Thermo Fisher Scientific). All PCR reactions were carried out with TaqMan Gene Expression Master Mix using 7500 Real Time PCR System (Thermo Fisher Scientific) according to the manufacturer’s protocol. The 2Δ−DCt method was used for calculating the relative ratio. mRNA levels were counted from 3 PCR reactions for each sample.
Statistical methods
Data are shown as mean ± standard error of the mean (SEM) from at least 3 independent experiments. Data were analyzed using a 2-tailed paired Student’s t-test (normal distribution), or non-parametric Kruskal–Wallis and Wilcoxon tests were applied using SigmaPlot software. In every analysis, p-value of ≤0.05 was considered significant.
Results
Characterization of AgNPs
To find a correlation between the NPs size and their biological properties, all AgNPs were precisely characterized using UV-vis spectroscopy, electron microscopy, DLS and zeta potential measurements. UV-vis spectroscopy is a simple, fast and sensitive method for characterization of metallic NPs. The maxima of the adsorption bands of the tested AgNP colloids were detected in the region characteristic for AgNPs at 405, 408, 414 and 430 nm for , respectively. It can be easily seen that the adsorption band maximum shifts to longer wavelengths with the increase in the particle size.
Figure 1 DLS histograms and STEM images with size distribution histograms of AgNPs (A) TAm-13, (B) TAm-33, (C) TAm-46 nm AgNPs and (D) UN 10–65 nm AgNPs.
Abbreviations: AgNPs, silver nanoparticles; DLS, dynamic light scattering; STEM, scanning transmission electron microscopy; TAm, tannic acid-modified; UN, unmodified.
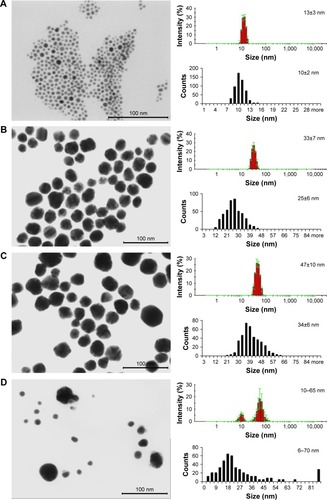
The morphology, shape and the mean size of the metallic core of AgNPs were tested using the electron microscopy. shows representative Scanning Transmission Electron Microscopy (STEM) images of AgNPs. STEM images confirm the formation of monocrystalline AgNPs with a regular spherical shape. The mean size of AgNPs metallic core was 10±2, 25±6, 34±6 and 6–70 nm for , respectively. Furthermore, all AgNP colloids were tested by the DLS technique for the hydrodynamic size of particles and colloidal stability. The hydrodynamic sizes of particles were as follows: 13±3 nm (), 33±7 nm (), 46±10 nm () and 10–65 nm (). All AgNP colloids were stable and any additional signals from larger particles or agglomerates were not observed. However, it is well-known that for DLS measurements, there is a risk that small objects present in the sample can be screened by bigger ones and will not be seen during the measurement.Citation20 However, DLS results along with the STEM images allowed us to conclude that the tested colloids were monodisperse.
It is well-known that the charge affects physical stability of nanoparticles in a colloidal solution. The colloidal stability of AgNPs was analyzed through zeta potential measurements which are an indirect measure of the surface charge and allow evaluation of the expected storage stability of a colloidal dispersion (high positive or negative zeta potential values indicate the high stability of colloids). The zeta potentials of the tested samples were as follows: −31±7, −58±2, −55±2 and 64±1 mV for colloids 1, 2, 3 and 4, respectively. These values along with the DLS results confirm the high stability of all investigated colloids.
Antimicrobial activity of modified and unmodified AgNPs
P. aeruginosa and S. aureus are the most common bacteria responsible for wound infection.Citation5,Citation6 The inhibitory effects of TA-modified AgNPs were size-dependent with 13 nm AgNPs showing the lowest MIC values (1.56 µg/mL for S. aureus, 3.13 µg/mL for P. aeruginosa and 0.78 µg/mL for E. coli) (). TA-modified 33 and 46 nm AgNPs showed the same MIC values (3.13 µg/mL for S. aureus, 12.5 µg/mL for P. aeruginosa and 6.25 µg/mL for E. coli) (). Unmodified 10–65 nm AgNPs showed the lowest inhibitory activity against S. aureus, P. aeruginosa and E. coli (>50 µg/mL) ().
Table 1 Antibacterial activities of AgNPs (MIC in µg/mL) against 3 pathogenic bacterial strains
Internalization of AgNPs
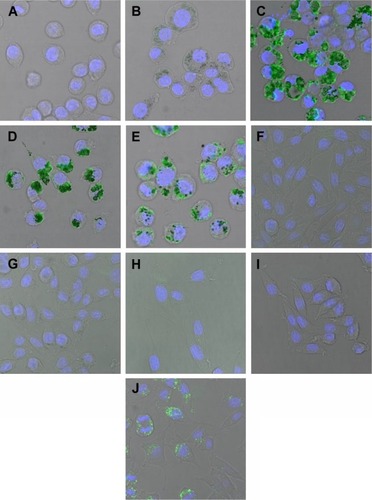
shows representative images of RAW 264.7 monocytes () and L929 fibroblasts () with internalized nanoparticles (green fluorescence). Fibroblasts internalized significantly less nanoparticles than monocytes for all types of AgNPs. In both cell types, bigger sizes (33 and 46 nm TA-modified AgNPs) () were accumulated more than 13 nm TA-modified AgNPs (). However, L929 fibroblasts accumulated unmodified 10–65 nm AgNPs more efficiently than monocytes ().
Figure 2 Fibroblasts internalize AgNPs less effectively than monocytes.
Notes: Confocal microscopy images showing TA and unmodified AgNPs inside the cells (green). Nuclei were stained with Hoechst 33342. Images were captured using 63× objective lens and 1.5× digital zoom. (A) RAW control; (B) RAW TAm-13 nm; (C) RAW TAm-33 nm; (D) RAW TAm-46 nm; (E) RAW UN 10–65 nm; (F) L929 control; (G) L929 TAm-13 nm; (H) L929 TAm-33 nm; (I) L929 TAm-46 nm; (J) L929 UN 10–65 nm.
Abbreviations: AgNPs, silver nanoparticles; TAm, tannic acid-modified; UN, unmodified.
Toxicity of TA-modified AgNPs
As shown in , the cytotoxic effect of AgNPs upon the mitochondrial potential in L929 cells was dose-dependent. Addition of TA, 13, 33, and 46 nm TA-modified AgNPs at 5 µg/mL to L929 cell cultures resulted in a significant increase in the percentage of cells with a decreased mitochondrial potential (19.81%±0.53%, 29.65%±4.87% and 27.82%±3.57%, respectively) (p≤0.05) (). TA-modified 13, 33 and 46 nm AgNPs at 10 µg/mL resulted in the further increase in the percentage of cells with a decreased mitochondrial potential (p≤0.05) (). No significant influence of unmodified 10–65 nm AgNPs upon the mitochondrial potential in L929 was found (). Effective concentration 50 (EC50) values for unmodified 10–65 nm AgNPs remained higher in comparison to EC50 values for TA-modified 13, 33 and 46 nm AgNPs as well as TA, and the values were as follows: 39.56±11.82 vs 23.96±3.46, 13.09±2.51, 12.46±2.75 and 11.13±2.3 µg/mL, respectively.
Figure 3 Cytotoxicity assays in L929 cell line (A) and in vitro scratch assay in HaCaT cell line (B) exposed to TAm and unmodified AgNPs.
Notes: (A) L929 cell line was exposed to TAm AgNPs sized 13, 33, 46 nm and UN 10–65 nm AgNPs at 0.5–10 µg/mL for 24 h and subjected to measurement of mitochondrial potential with JC-1. The results are expressed as the percentage of cells with decreased mitochondrial potential. (B) Quantitative analysis of scratch assay in HaCaT cell line exposed to TAm AgNPs sized 13, 33, 46 nm and UN 10–65 nm AgNPs at 5 µg/mL for 24 h. Migration of cells is expressed as percentage increase in relation to control, unexposed culture with scratch. Each bar represents the mean from 5 experiments (N=5) ± SEM, *Significant differences with p≤0.01.
Abbreviations: AgNPs, silver nanoparticles; JC-1, 1H-benzimidazolium, 5,6-dichloro-2-[3-(5,6-dichloro-1,3-diethyl-1,3-dihydro-2H-benzimidazol-2-ylidene)-1-propenyl]-1,3-diethyl-, iodide; TAm, tannic acid-modified; SEM, standard error of the mean; UN, unmodified.
![Figure 3 Cytotoxicity assays in L929 cell line (A) and in vitro scratch assay in HaCaT cell line (B) exposed to TAm and unmodified AgNPs.Notes: (A) L929 cell line was exposed to TAm AgNPs sized 13, 33, 46 nm and UN 10–65 nm AgNPs at 0.5–10 µg/mL for 24 h and subjected to measurement of mitochondrial potential with JC-1. The results are expressed as the percentage of cells with decreased mitochondrial potential. (B) Quantitative analysis of scratch assay in HaCaT cell line exposed to TAm AgNPs sized 13, 33, 46 nm and UN 10–65 nm AgNPs at 5 µg/mL for 24 h. Migration of cells is expressed as percentage increase in relation to control, unexposed culture with scratch. Each bar represents the mean from 5 experiments (N=5) ± SEM, *Significant differences with p≤0.01.Abbreviations: AgNPs, silver nanoparticles; JC-1, 1H-benzimidazolium, 5,6-dichloro-2-[3-(5,6-dichloro-1,3-diethyl-1,3-dihydro-2H-benzimidazol-2-ylidene)-1-propenyl]-1,3-diethyl-, iodide; TAm, tannic acid-modified; SEM, standard error of the mean; UN, unmodified.](/cms/asset/ac5b1ac6-9518-46d9-929f-3c8b138ac666/dijn_a_154797_f0003_c.jpg)
Since a decrease in mitochondrial potential usually precedes induction of apoptosis, we used annexin V staining to confirm AgNPs toxicity. The results of the annexin V/PI assay showed that only TA-modified 13 nm AgNPs induced apoptosis in L929 cells at 5 and 10 µg/mL (p≤0.05) (), while only TA-modified 13 and 33 nm AgNPs at 10 µg/mL induced necrosis (p≤0.05) ().
Table 2 Percentage of apoptotic and necrotic mouse L929 fibroblasts at 24 h after exposure to TAm 13, 33, 46 nm AgNPs and UN 10–65 nm AgNPs at 1, 5 and 10 µg/mL
In vitro scratch assay
Next, we chose to investigate the effect of TA-modified and unmodified AgNPs on the migration of keratinocytes in cell culture. The scratch assay using human HaCaT cell line is a well-characterized method to measure keratinocyte migration in vitro.Citation19 The scratch area of cells treated with all AgNPs at 5 µg/mL appeared to have a greater number of keratinocytes present within the scratch region when compared to control cells (). However, only TA-modified AgNPs showed a significant increase in cell migration (p≤0.05) ().
Dermal inflammatory reaction in mice
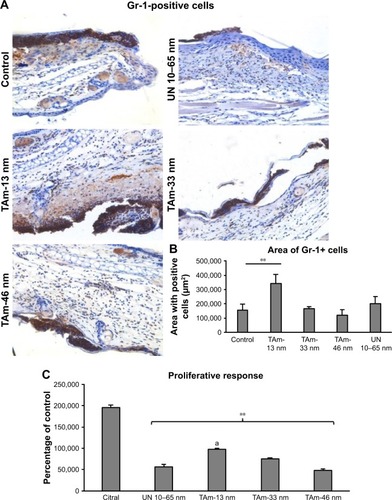
To explore the potential of TA-modified AgNPs for dermal inflammatory reaction, we performed staining with the anti-granulocyte receptor-1 (Gr-1) monoclonal antibody (mAb), in ears with TA-modified and unmodified AgNPs applied onto the dorsal part of scarified mouse ears. This antibody binds to Ly6G, which is present on neutrophils, and to Ly6C, which is expressed on neutrophils, and subpopulations of monocytes (Ly6G+ and Ly6C+). Both cell populations actively participate in early inflammation.Citation23 In AgNP-treated and untreated ears, we observed strong inflammatory reaction of the thickened epidermis and infiltrating inflammatory cells within dermis (). However, ears treated with TA-modified 13 nm AgNPs showed the strongest inflammatory reaction with necrosis within the epidermis and strong involvement of dermis (). The ears treated with TA-modified 33 and 46 nm AgNPs and unmodified 10–60 nm AgNPs showed only a small inflammatory reaction within the epidermis and no dermal thickening (). The area occupied by Gr-1+ cells was the biggest in the group of TA-modified 13 nm AgNP-treated ears in comparison to other groups (p=0.001) (). To assess activation of the local immune system, we stimulated lymphocytes isolated from the auricular lymph nodes with concanavalin A and measured cell proliferation after 72 h (). The lymphocytes isolated from mice treated with the irritating citral solution showed the strongest proliferative response (). Treatment with modified or unmodified AgNPs induced significantly lower proliferative response in comparison to citral (p≤0.001) (). Of all TA-modified AgNPs, the smallest 13 nm AgNPs showed the highest induction of proliferation (p≤0.05) ().
Figure 4 Inflammatory ear reaction upon AgNPs exposure. Mice were subjected to ear stripping followed by 3 applications of TAm AgNPs sized 13, 33, 46 nm and UN 10–65 nm AgNPs (25 µg per each ear, every 24 h).
Notes: Five days after the first exposure mice were euthanized to collect ears and auricle lymph nodes. (A) Gr-1-positive cells (brown) in the ears of ear stripped, AgNP-exposed mice. The nuclei in the slides were counterstained with Harris hematoxylin (violet). Magnification 400×. (B) Area of the ear tissue occupied by Gr-1-positive cells in the stained sections expressed in micrometers squared. Each bar represents the mean from 3 animals (N=3) ± SEM. **Significant differences with p≤0.001. (C) Proliferative response of lymphocytes isolated from auricle lymph nodes of citral and AgNP-exposed mice at 72 h after stimulation with concanavalin A. N=3, **significant differences with p≤0.001, while “a” means p=0.05 for 13 nm AgNPs in comparison to other AgNPs.
Abbreviations: AgNPs, silver nanoparticles; SEM, standard error of the mean; TAm, tannic acid-modified; UN, unmodified.
TA-modified 33 and 46 nm AgNPs improve wound healing
Wound closure was observed in all treatment groups within 14 days. All tested animals formed scabs at the wound site, which lasted for several days and left residual lesions on the skin tissue after they fell off. Wound closure was clearly observed at day 6 in all experimental groups, except for TA-modified 13 nm AgNPs, as shown in and . The TA-modified 13 nm AgNPs group also showed a delayed wound closure at day 14 ( and ). The TA-modified 33 and 46 nm AgNP groups showed an improvement in the wound closure at days 6 and 14 as well as better epithelialization at all tested days in comparison to the control group (p≤0.05) ( and ). In contrast, TA significantly increased epithelial sheet thickness only at day 3 (p=0.05) ( and ).
Figure 5 TA AgNPs improve healing in a mouse wound model.
Notes: Silver nanoparticles (TAm TAgNPs sized 13, 33, 46 nm and UN 10–65 nm AgNPs) or tannic acid were applied at 5 µg/mL in saline, and the wounds were subjected to further tests at 3, 6 and 14 days from injury. Each bar represents the mean from 5 animals ± SEM (N=5). (A) Percentage of the original wound size. *p≤0.05 versus untreated control. (B) Re-epithelialization, expressed as the epithelial sheet thickness in micrometers measured in hematoxylin–eosin stained tissue sections at respective days after injury. *p≤0.05 versus untreated control.
Abbreviations: AgNPs, silver nanoparticles; SEM, standard error of the mean; TAm, tannic acid-modified; UN, unmodified.
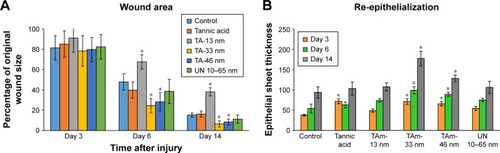
Figure 6 General morphology of wound after treatment with TA and unmodified AgNPs.
Notes: Representative microphotographs of the tri-chrome Masson-stained wound sections at days 3, 6 and 14 postinjury. Silver nanoparticles (TAm AgNPs sized 13, 33 and 46 nm and UN 10–65 nm AgNPs) or tannic acid were applied at 5 µg/mL in saline. Magnification 400×.
Abbreviations: AgNPs, silver nanoparticles; TAm, tannic acid-modified; UN, unmodified.
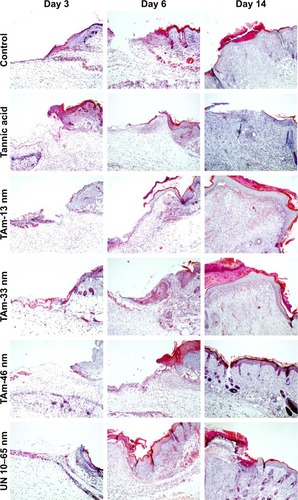
shows the wound healing histology for each treatment group at days 3, 6 and 14 after wounding, stained with Masson’s trichrome procedure. This staining shows stained collagen fibers in pale blue, the cytoplasm in pale purple, the nuclei in blue and the red blood cells in cherry red. At the early stage of the healing processes (days 3–6), wounds in all groups displayed evident inflammatory cell infiltration, granulation tissue formation and epidermal proliferation. However, wounds treated with TA-modified 13 nm AgNPs showed stronger and prolonged inflammatory reaction in comparison to other groups (). The inflammatory cells in the TA-modified 33 and 46 nm AgNP groups disappeared more quickly, and the formation of hair follicles was observed (). Interestingly, all AgNP groups showed an increased number of new vessels in the overall appearance of the wound ( and ).
Figure 7 Formation of new vessels and inflammatory infiltration in wounds subjected to treatment with TAm and unmodified AgNPs.
Notes: Wounds were collected at days 3, 6 and 14 from injury and analyzed with the microscopic techniques. (A) Number of new vessels per field in the skin sections subjected to tri-chrome Masson’s staining (magnification 400×). (B) Number of neutrophils per field in the skin sections subjected to hematoxylin–eosin staining (magnification 400×). (C) Number of F4/80+ cells (macrophages) per field in the skin sections subjected to immunohistochemical staining with anti-F4/80 antibody (magnification 400×). Each bar represents the mean from 5 animals ± SEM (N=5). *p≤0.05 versus untreated control.
Abbreviations: AgNPs, silver nanoparticles; SEM, standard error of the mean; TAm, tannic acid-modified; UN, unmodified.
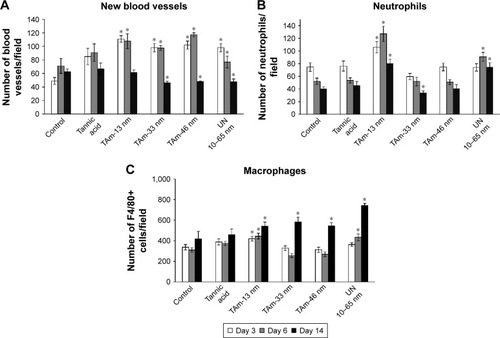
Formation of new vessels was evaluated by counting the number of new vessels per field (). Wounds treated with all tested AgNPs and TA at day 3 after wounding showed significantly increased number of vessels in comparison to control (p≤0.05) (). Later during healing, all AgNP-treated wounds showed significantly increased number of blood vessels at day 6 (p≤0.05) (). However, the number of new vessels at day 14 in wounds subjected to TA-modified 33, 46 nm AgNPs and unmodified 10–65 nm AgNPs were significantly decreased in comparison to control wounds (p≤0.05) ().
To evaluate inflammation in wounds, the number of neutrophils and macrophages per field was calculated (). In the TA-modified 13 nm AgNP group, the neutrophil and macrophage content in the wound tissue was significantly increased during the whole period of healing (p≤0.05) (). In the unmodified 10–65 nm group, both neutrophil and macrophage numbers were significantly increased at days 6 and 14 after wounding (p≤0.05) (). The wounds treated with TA-modified 33 and 46 nm AgNPs showed an increased number of macrophages at day 14 (p≤0.05) (), while for 33 nm AgNPs, the number of neutrophils was significantly decreased at day 14 (p=0.035) ().
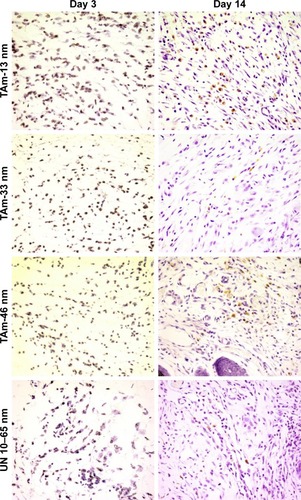
The presence of AgNPs within the wound was found in all tested groups. AgNPs were found not only in the wound bed at day 3 but also within the inflammatory reaction and extracellular matrix (). Neutrophils clearly phagocytized AgNPs. Interestingly, nanoparticles were also localized within myofibroblasts responsible for wound contraction (). Later during the tissue repair phase (day 14), we found AgNPs within the granulation tissue, both as intracellular and extracellular deposits. Nanoparticles were often localized in the areas surrounding new hair follicles ().
Figure 8 Silver nanoparticles detected in wounds treated with TA and unmodified AgNPs.
Notes: Representative microphotographs of the hematoxylin-stained wound sections at days 3 and 14 postinjury. Magnification 600×.
Abbreviations: AgNPs, silver nanoparticles; TAm, tannic acid-modified; UN, unmodified.
TA-modified AgNPs modulate expression of cytokines in wounds
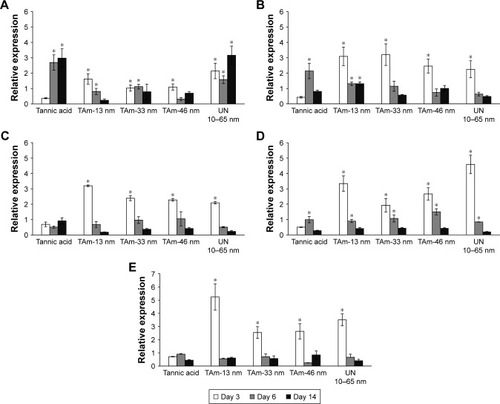
mRNA levels of TNF-α, TGF-β1, IL-1β, VEGF-α and PDGF-β were measured by qRT-PCR (). In general, mRNA expressions of TNF-α, TGF-β1, VEGF-α and PDGF-β were significantly increased in all AgNP groups at day 3 of wound repair in comparison to control wounds (p≤0.05) (). At day 6, mRNA levels of PDGF-β were significantly increased in all tested groups in comparison to the control group. Levels of mRNA of IL-1β were significantly increased at days 6 and 14 postinjury in wounds treated with TA, while unmodified 10–65 nm significantly increased mRNA of IL-1β at all tested time points in comparison to the control group (p≤0.05) (). TA-modified AgNPs upregulated IL-1β mRNA at day 3 from wounding, while TA-modified 13 and 33 nm AgNPs upregulated IL-1β mRNA at day 6 (p≤0.05) (). The highest level of mRNA of TNF-α was observed at days 6 and 14 in wounds treated with TA-modified 13 nm AgNPs (p≤0.05) ().
Figure 9 Cytokine expression changes in wounds subjected to treatment with TAm and unmodified AgNPs at days 3, 6 and 14 from injury.
Notes: mRNA levels of (A) IL-1β, (B) TNF-α, (C) VEGF-α, (D) PDGF-β, and (E) TGF-β1 are expressed as expression relative to control on the basis of the 2ΔΔCt method. mRNA levels were counted from 3 PCR reactions for each sample. N=3. *p≤0.05 versus untreated control.
Abbreviations: AgNPs, silver nanoparticles; IL, interleukin; PCR, polymerase chain reaction; PDGF-β, platelet-derived growth factor-β; TAm, tannic acid-modified; TGF-β1, transforming growth factor-β1; TNF-α, tumor necrosis factor-α; UN, unmodified; VEGF-α, vascular endothelial growth factor-α.
Discussion
Wound healing remains a challenging clinical problem, especially with chronic wounds or unpredictable complications. Wound dressings containing AgNPs can effectively protect an injury from bacterial infection and promote tissue regeneration during the wound healing process.Citation9,Citation10 Plant-based products have been used to treat wounds for centuries due to their antioxidant, anti-inflammatory and antimicrobial effects. TA has been shown to stabilize collagen and elastin in the extracellular matrix. This is achieved by inhibiting MMP collagenases while enhancing collagen cross-linking.Citation24,Citation25 Additionally, TA inhibits S. aureus, P. aeruginosa and E. coli biofilm formation in multiple biofilm models.Citation26–Citation28 Bacterial infection is one of the most serious complications impairing wound healing and tissue regeneration, which may lead to prolonged inflammatory responses, and hence it delays wound recovery. Here, we showed that AgNPs modified with TA inhibited bacterial growth more effectively than TA-modified or unmodified AgNPs (). Inhibition of bacterial growth was size-dependent () with the smallest AgNPs showing the strongest antibacterial activity toward E. coli, while higher sizes of TA-modified AgNPs showed better inhibitory activity toward S. aureus. The latter microorganism is the most common bacteria responsible for wound infection.Citation29 These results are in accordance with other studies showing size-related antibacterial effects of AgNPs.Citation30,Citation31 The small AgNPs show better inhibitory properties owing to the larger surface to volume ratio as compared to larger spherical AgNPs. The antimicrobial properties of AgNPs are attributed to direct interaction of nanoparticles with bacteria membrane and intracellular proteins as well as to the presence of biocidal ionic silver released from nanoparticle surfaces.Citation30–Citation32 Although TA does not inhibit bacterial growth, the presence of TA on the particles’ surface helps to increase interaction with the bacteria, since unmodified nanoparticles showed much lower MIC values ().
We have previously reported that TA-modified AgNPs show little cytotoxicity to both mouse and human keratinocytes in vitro, but an increased toxicity was found in mouse monocytes, which showed efficient internalization of TA-modified AgNPs.Citation18,Citation19 Furthermore, toxicity was also size dependent with TA-modified AgNPs sized <20 nm inducing more cytotoxicity and inflammatory reaction than TA-modified AgNPs >20 nm.Citation18,Citation19 TA-modified AgNPs showed little toxicity to L929 fibroblasts, and this was concentration-dependent and can be accounted for by the small internalization of nanoparticles (). Interestingly, TA-modified AgNPs altered mitochondrial functionality in murine fibroblasts, but this did not seem to lead to cell death ( and ). Similar results were shown by Rigo et alCitation33 for 10–15 nm AgNPs in human fibroblasts.
Fibroblasts play an important role in the wound healing process, by synthesizing and secreting skin collagens; newly formed collagens can fill tissue defects, provide a scaffold for the migration of epidermal cells and regulate cell migration and proliferation.Citation34 Nanosilver has been shown to increase wound closure through the promotion of proliferation and migration of keratinocytes and can drive differentiation of fibroblasts into myofibroblasts, thereby promoting wound contraction.Citation35,Citation36 Here, the therapeutic efficacy of TA-modified AgNPs in wound healing was assessed using the scratch assay as an in vitro model system and the incisional wound model in mice. Our results from the in vitro model show that TA-modified AgNPs increase HaCaT keratinocyte migration above that observed in control cultures, while TA-modified and unmodified AgNPs increased migration insignificantly (). These results are in contrast to the study by Zanette et al,Citation37 showing that a relatively short time of contact with AgNPs causes a long-lasting inhibition of cell growth in HaCaT keratinocytes, not associated with consistent AgNPs’ internalization. When applying nanoparticles in the mouse incisional wound model we observed different morphological changes during the inflammation and re-epithelialization phase, depending on the size of TA-modified AgNPs. A faster wound closure, increased re-epithelialization and an increased number of myofibroblasts were observed in mice treated with TA-modified 33 and 46 nm AgNPs as compared with control mice. However, TA-modified 13 nm AgNPs showed delayed wound healing even at the proliferation and remodeling phase. We have not observed better wound healing with uncoated AgNPs. Other authors showed that not only uncoated AgNPs sized 5–15 nm but also those with a diameter of ~20 nm accelerated re-epithelialization, enhanced migration of fibroblasts and reduced neutrophil and macrophage infiltration at the wound site in rodent models.Citation36,Citation38,Citation39
Histological evaluations revealed that TA-modified AgNPs sized 13 nm, and to a lesser degree, 10–65 nm unmodified AgNPs, maintained neutrophil infiltration at the wound site up to 14 days. In contrast, TA-modified 33 nm AgNPs significantly accelerated neutrophil disappearance during the remodeling phase. In common experimental models using healthy mice, neutrophil accumulation peaks around day 1 and returns to uninjured levels by days 5–10 after skin wounding.Citation40 Neutrophils act as the first-line defense against microbial invasion and foreign debris, which is cleared by phagocytosis. However, the abundance of neutrophils may also lead to the reduced levels of growth factors and production of destructive radical oxygen species and a further delay in healing.Citation40,Citation41 This was actually observed here for TA-modified 13 nm AgNPs. Additionally, application of TA-modified 13 nm AgNPs onto the stratified ear skin in the LLNA test also led to the local irritation and inflammation, as shown by the presence of Gr-1-positive cells, representing neutrophils and inflammatory monocytes (). This shows that nanoparticle size can strongly influence its pro- or anti-inflammatory potential. We have previously shown that TA-modified 13 nm AgNPs are more toxic to mouse monocytes than TA-modified AgNPs of higher sizes.Citation18,Citation19 We can therefore conclude that the increased inflammatory reaction observed in wounds exposed to TA-modified 13 nm AgNPs may result from the toxic reaction induced in phagocytizing neutrophils and inflammatory monocytes. Monocytes/macrophages of the inflammatory phase appear to orchestrate the transition to the proliferative phase of healing. Depletion of monocytes/macrophages during the inflammatory phase reduces granulation tissue formation and cell proliferation in mouse skin wounds.Citation40 In this study, TA-modified 13 nm AgNPs maintained a high number of macrophages within the wound during all tested periods, while unmodified AgNPs induced accumulation of macrophages in the late phase of wound healing. Interestingly, both TA-modified 33 and 46 nm AgNPs maintained a high number of macrophages later during healing. This accumulation of macrophages may result from the presence of nanoparticles long after wounding, as we found AgNPs also within the dermis, mostly extracellularly (). Rigo et alCitation33 tested for the presence of nanoparticles in human burn wound treated with Acticoat™ Flex 3 containing AgNPs. The healed skin at day 7 showed a great number of agglomerates of nanoparticles in the upper part of the dermis, and these agglomerates were detected even at 17-days post-burning.Citation33 Therefore, AgNPs can affect the wound also at later phases – such as remodeling due to their constant presence in the wound.
All stages of the wound-healing process are regulated by different growth factors, chemokines and cytokines.Citation42 Pro-inflammatory cytokines produced by macrophages such as TNF-α and IL-1β can protect against potential invasion of various microorganisms and help to promote proliferative response.Citation42,Citation43 However, persistent inflammatory response after wounding delays healing and increases complications. In this study, TA-modified AgNPs showed an advantageous profile of IL-1β and TNF-α production when compared to control, TA-modified and unmodified AgNPs. Both IL-1β and TNF-α were upregulated early after wounding (up to day 3, except for 13 nm TA-modified AgNPs) and decreased at later times. Previous reports showed that AgNPs were capable of inhibiting the inflammatory reaction to promote wound healing.Citation39,Citation44 We also showed previously that TA-modified but not unmodified AgNPs downregulated inflammatory response in human keratinocytes subjected to TNF-α and LPS.Citation19 Apart from creating a pro-inflammatory microenvironment, macrophages also release growth factors including VEGF-α, TGF-1β and PDGF-β, which promote keratinocyte and fibroblast migration, proliferation and angiogenesis.Citation42
Here, we found that both TA-modified and unmodified AgNPs induced expression of VEGF-α, TGF-1β and PDGF early during wound healing (proliferative phase), but this effect could be contributed to the AgNPs rather than TA modification and did not depend on the AgNPs’ size. Other authors also showed that AgNPs act efficiently during the early stages of wound healing by upregulating the expression levels of cytokines associated with the TGF-1β/Smad signaling pathway, such as TGFβ1.Citation45,Citation46 TGFβ1 plays an important role in almost all stages of wound healing and scar formation.Citation46 Gurunathan et alCitation47 have demonstrated that AgNPs inhibited VEGF-induced cell proliferation, migration and capillary-like tube formation of bovine retinal endothelial cells as well as effectively inhibited formation of new blood microvessels induced by VEGF. Here, we observed an opposite effect, with both TA-modified and unmodified AgNPs inducing VEGF upregulation early during wound healing.
Despite the fact that all sizes of TA-modified AgNPs induced upregulation of cytokines involved in wound healing, small-sized TA-modified AgNPs can elicit strong inflammatory response not only during wound healing but also when applied to stratified skin.
Conclusion
Taking into account not only the ability of AgNPs to penetrate skin and interact with the skin immune system but also the immunomodulatory effects of TA, we can assume that the synthesis of AgNPs with TA allows preparation of a new type of nanomaterials. TA-modified AgNPs sized >26 nm not only show a good biocompatibility, an effective antibacterial activity and anti-inflammatory properties but can also efficiently accelerate wound healing. Therefore, TA-modified AgNPs can be potentially used as an ideal wound dressing for cutaneous wounds.
Author contributions
Piotr Orlowski made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data. Magdalena Zmigrodzka made substantial contributions to acquisition of data and analysis of data. Emilia Tomaszewska made substantial contributions to acquisition of data and analysis of data. Katarzyna Ranoszek-Soliwoda made substantial contributions to acquisition of data and analysis of data. Malgorzata Antos-Bielska made substantial contributions to acquisition of data. Janusz Szemraj made substantial contributions to acquisition of data. Grzegorz Celichowski made substantial contributions to design, and analysis and interpretation of data. Jaroslaw Grobelny made substantial contributions to design, and analysis and interpretation of data. Malgorzata Krzyzowska made substantial contributions to conception and design, interpretation of data, drafting the article, agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy of any part of the work are appropriately investigated and resolved. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
This work was supported by the National Science Centre Poland grant no 2014/13/B/NZ5/01356. We would like to thank Aleksandra Fruba and Karolina Bien for excellent technical help.
Disclosure
The authors report no conflicts of interest in this work.
References
- SchremlSSzeimiesRMPrantlLLandthalerMBabilasPWound healing in the 21st centuryJ Am Acad Dermatol201063586688120576319
- MartinPNunanRCellular and molecular mechanisms of repair in acute and chronic wound healingBr J Dermatol2015173237037826175283
- ChoKHParkJEOsakaTParkSGThe study of antimicrobial activity and preservative effects of nanosilver ingredientElectrochim Acta2005515956960
- ClintonACarterTChronic wound biofilms: pathogenesis and potential therapiesLab Med201546427728426489671
- SadeghiBGarmaroudiFSHashemiMComparison of the antibacterial activity on the nanosilver shapes: nanoparticles, nanorods and nanoplatesAdv Powder Technol20122312226
- LareseFFD’AgostinFCroseraMHuman skin penetration of silver nanoparticles through intact and damaged skinToxicology20092551–2333718973786
- KimJSKukEYuKNAntimicrobial effects of silver nanoparticlesNanomedicine2007319510117379174
- MoronesJRElechiguerraJLCamachoAThe bactericidal effect of silver nanoparticlesNanotechnology200516102346235320818017
- TianJWongKKHoCMTopical delivery of silver nanoparticles promotes wound healingChemMedChem20072112913617075952
- ZhangSLiuXWangHPengJWongKKSilver nanoparticle-coated suture effectively reduces inflammation and improves mechanical strength at intestinal anastomosis in miceJ Pediatr Surg201449460661324726122
- HaslamENatural polyphenols (vegetable tannins) as drugs: possible modes of actionJ Nat Prod19965922052158991956
- AkiyamaHFujiiKYamasakiOOonoTIwatsukiKAntibacterial action of several tannins against Staphylococcus aureusJ Antimicrob Chemother200148448749111581226
- BakondiEBaiPErdelyiKSzaboCGergelyPViragLCytoprotective effect of gallotannin in oxidatively stressed HaCaT keratinocytes: the role of poly(ADP-ribose) metabolismExp Dermatol200413317017814987257
- FernandezOCapdevilaJZDallaGMelchorGEfficacy of Rhizophora mangle aqueous bark extract in the healing of open surgical woundsFitoterapia2002737–856456812490213
- BuzziniPArapitsasPGorettiMAntimicrobial and antiviral activity of hydrolysable tanninsMini Rev Med Chem20088121179118718855732
- Ranoszek-SoliwodaKTomaszewskaESochaEThe role of tannic acid and sodium citrate in the synthesis of silver nanoparticlesJ Nanopart Res20171927328824288
- CataldoFUrsiniOAngeliniGA green synthesis of colloidal silver nanoparticles and their reaction with ozoneEur Chem Bull2013210700705
- OrlowskiPKrzyzowskaMZdanowskiRAssessment of in vitro cellular responses of monocytes and keratinocytes to tannic acid modified silver nanoparticlesToxicol In Vitro20132761798180823727252
- OrlowskiPSoliwodaKTomaszewskaEToxicity of tannic acid-modified silver nanoparticles in keratinocytes: potential for immunomodulatory applicationsToxicol In Vitro201635435427216470
- TomaszewskaESoliwodaKKadziolaKDetection limits of DLS and UV-vis spectroscopy in characterization of polydisperse nanoparticles colloidsJ Nanomaterials20132013313081
- SalvioliSArdizzoniAFranceschiCCossarizzaAJC-1, but not DiOC6(3) or rhodamine 123, is a reliable fluorescent probe to assess delta psi changes in intact cells: implications for studies on mitochondrial functionality during apoptosisFEBS Lett1997411177829247146
- LiangCCParkAYGuanJLIn vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitroNat Protoc20072232933317406593
- SasmonoRTEhrnspergerACronauSLMouse neutrophilic granulocytes express mRNA encoding the macrophage colony-stimulating factor receptor (CSF-1R) as well as many other macrophage-specific transcripts and can transdifferentiate into macrophages in vitro in response to CSF-1J Leukoc Biol200782111112317438263
- ZhangHZhuSJWangDWeiYJHuSSIntramyocardial injection of tannic acid attenuates postinfarction remodeling: a novel approach to stabilize the breaking extracellular matrixJ Thorac Cardiovasc Surg2009137121622219154928
- NatarajanVKrithicaNMadhanBSehgalPKPreparation and properties of tannic acid cross-linked collagen scaffold and its application in wound healingJ Biomed Mater Res B Appl Biomater2013101456056723255343
- PayneDEMartinNRParzychKRRickardAHUnderwoodABolesBRTannic acid inhibits Staphylococcus aureus surface colonization in an IsaA-dependent mannerInfect Immun201381249650423208606
- HancockVDahlMVejborgRMKlemmPDietary plant components ellagic acid and tannic acid inhibit Escherichia coli biofilm formationJ Med Microbiol201059pt 449649819959627
- TrentinDSSilvaDBAmaralMWTannins possessing bacteriostatic effect impair Pseudomonas aeruginosa adhesion and biofilm formationPLoS One201386e6625723776646
- PercivalSLHillKEWilliamsDWHooperSJThomasDWCostertonJWA review of the scientific evidence for biofilms in woundsWound Repair Regen201220564765722985037
- RazaMAKanwalZRaufASabriANRiazSNaseemSSize- and shape-dependent antibacterial studies of silver nanoparticles synthesized by wet chemical routesNanomaterials (Basel)20166474
- ZilleAFernandesMMFranceskoASize and aging effects on antimicrobial efficiency of silver nanoparticles coated on polyamide fabrics activated by atmospheric DBD plasmaACS Appl Mater Interfaces2015725137311374426057400
- ChoiODengKKKimNJRossLSurampalliRYHuZQThe inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growthWater Res200842123066307418359055
- RigoCFerroniLToccoIActive silver nanoparticles for wound healingInt J Mol Sci20131434817484023455461
- PastarIStojadinovicOYinNCEpithelialization in wound healing: a comprehensive reviewAdv Wound Care201437445464
- LiuXLeePYHoCMSilver nanoparticles mediate differential responses in keratinocytes and fibroblasts during skin wound healingChemMedChem20105346847520112331
- KwanKHLiuXToMKYeungKWHoCMWongKKModulation of collagen alignment by silver nanoparticles results in better mechanical properties in wound healingNanomedicine20117449750421272666
- ZanetteCPelinMCroseraMSilver nanoparticles exert a long-lasting antiproliferative effect on human keratinocyte HaCaT cell lineToxicol In Vitro20112551053106021501681
- NeibertKGopishettyVGrigoryevAWound-healing with mechanically robust and biodegradable hydrogel fibers loaded with silver nanoparticlesAdv Healthc Mater20121562163023184797
- WenLZengPZhangLHuangWWangHChenGSymbiosis theory-directed green synthesis of silver nanoparticles and their application in infected wound healingInt J Nanomedicine2016112757276727358563
- DoviJVHeLKDiPietroLAAccelerated wound closure in neutrophil-depleted miceJ Leukoc Biol200373444845512660219
- MirzaRDiPietroLAKohTJSelective and specific macrophage ablation is detrimental to wound healing in miceAm J Pathol200917562454246219850888
- WernerSGroseRRegulation of wound healing by growth factors and cytokinesPhysiol Rev200383383587012843410
- RoderoMPKhosrotehraniKSkin wound healing modulation by macrophagesInt J Clin Exp Pathol20103764365320830235
- WongKKCheungSOHuangLFurther evidence of the anti-inflammatory effects of silver nanoparticlesChemMedChem2009471129113519405063
- LiCWWangQLiJSilver nanoparticles/chitosan oligosaccharide/poly(vinyl alcohol) nanofiber promotes wound healing by activating TGFβ1/Smad signaling pathwayInt J Nanomedicine20161137338626855575
- TangBZhuBLiangYAsiaticoside suppresses collagen expression and TGF-β/Smad signaling through inducing Smad7 and inhibiting TGF-βRI and TGF-βRII in keloid fibroblastsArch Dermatol Res2011303856357221240513
- GurunathanSLeeKJKalishwaralalKSheikpranbabuSVaidyanathanREomSHAntiangiogenic properties of silver nanoparticlesBiomaterials200930316341635019698986
