?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The aim of this study was to prepare and evaluate mucoadhesive core-shell nanoparticles based on copolymerization of thiolated chitosan coated on poly methyl methacrylate cores as a carrier for oral delivery of docetaxel. Docetaxel-loaded nanoparticles with various concentrations were prepared via a radical emulsion polymerization method using cerium ammonium nitrate as an initiator. The physicochemical properties of the obtained nanoparticles were characterized by: dynamic light-scattering analysis for their mean size, size distribution, and zeta potential; scanning electron microscopy and transmission electron microscopy for surface morphology; and differential scanning calorimetry analysis for confirmation of molecular dispersity of docetaxel in the nanoparticles. Nanoparticles were spherical with mean diameter below 200 nm, polydispersity of below 0.15, and positive zeta potential values. The entrapment efficiency of the nanoparticles was approximately 90%. In vitro release studies showed a sustained release characteristic for 10 days after a burst release at the beginning. Ex vivo studies showed a significant increase in the transportation of docetaxel from intestinal membrane of rat when formulated as nanoparticles. Cellular uptake of nanoparticles was investigated using fluoresceinamine-loaded nanoparticles. Docetaxel nanoparticles showed a high cytotoxicity effect in the Caco-2 and MCF-7 cell lines after 72 hours. It can be concluded that by combining the advantages of both thiolated polymers and colloidal particles, these nanoparticles can be proposed as a drug carrier system for mucosal delivery of hydrophobic drugs.
Introduction
Oral drug delivery is the most acceptable method of drug delivery to the body. It is particularly important for cytotoxic medicines. These drugs are generally available as injectable dosage forms and their administration requires experienced personnel, patient’s presence at the hospital, and specific care, and thus poses many problems for the patients. Some of the advantages of oral chemotherapy include: increasing the drug efficacy and half-life, preventing excess increase in the blood concentration and the increase of drug toxicity, as well as decreasing the drug side effects in the body.Citation1,Citation2 For these reasons, many studies have been carried out in recent years on the administration of cytotoxic drugs in oral dosage form.Citation2–Citation6 Docetaxel (DTX) is a semisynthetic taxoid extract from Taxus baccata (European yew tree) and is one of the most effective drugs in chemotherapy.Citation7 The mechanism of this drug on cancer cells is through inhibiting microtubule depolymerization, and studies show that the cytotoxic effect of DTX on tumor cells is about two times that of paclitaxel.Citation8 DTX is used as an effective drug against breast, ovarian, lung, head, and neck cancersCitation9–Citation11 and is available only in the parenteral dosage form (Taxotere;® sanofi-aventis). Because of poor solubility of DTX in water, it is formulated as a solution with a high amount of Tween 80/ethanol. High concentration of solubilizers in its formula causes toxic effects and allergic reactions. Citation12 Like most effective drugs used in chemotherapy, it is not absorbable orally and has a very low bioavailability. In addition to its poor aqueous solubility, it is eliminated through the first pass extraction by the cytochrome P-450 processes and the action of efflux pump of p-glycoproteins (p-gp) in the liver and intestine.Citation13,Citation14 The use of surface-modified polymeric nanoparticle (NP) systems may be regarded as an effective means of overcoming these problems. NPs, due to their unique properties such as their small size and certain surface characteristics, can protect drugs from p-gp and cytochrome P450, protect drugs from the destructive factors in the gastrointestinal tract, and increase their permeability through the gastrointestinal barrier.Citation2 Considering the nature of these NPs, an interesting aspect in preparing surface-modified NPs is based on the synthesis of amphiphilic copolymers.Citation5,Citation15–Citation18 Synthesizing copolymers using polyalkylcyanoacrylate and a polysaccharide such as chitosan or dextran, as described by Chauvierre et al,Citation18 is a very favorable approach due to the simultaneous formation of NP and copolymer in a single stage. Here, NP formation is by a radical emulsion polymerization method, which is initiated by the creation of a free radical at the end of the polysaccharide chain, through an initiator like cerium ion in acidic medium; these free radicals cause the polymerization of cyanoacrylate monomers and the formation of a linear block copolymer. Since this reaction is carried out in an aqueous medium, the hydrophobic polymer tends to go inside the NPs at the same time that the particle is formed, and creates a hydrophobic core coated with a hydrophilic polysaccharide. This method results in the formation of a very stable NP suspension.Citation17–Citation19
One of the polymers which has attracted much interest is chitosan, due to its excellent characteristics for preparing surface-modified NPs. Chitosan is a nontoxic, biocompatible, and biodegradable polymer that is able to bind to mucus due to ionic interactions between its primary amino substructures and the sialic acid and sulfonic acid substructures of the mucus.Citation20
The mucoadhesive properties of chitosan are increased intensively by the immobilization of thiol groups on its structure.Citation21 The mucoadhesive properties of chitosan have been shown to improve 140-fold due to the immobilization of thiol groups on the polymer.Citation21 The reason for this is the formation of disulfide bonds between the thiolated polymer and cysteine-rich subdomains of the mucus gel layer.Citation22 The thiolated derivatives cause an increase in permeability through the intestine membrane by the mechanism of the regeneration of glutathione and inactivation of the protease enzymes in the gastrointestinal tract via absorbing and removing the bivalent cations from the medium.Citation6,Citation23 All these properties make the thiolated chitosan a very desirable material for increasing the absorption of peptides and NPs through the mucosa.Citation24 Due to these favorable characteristics of chitosan, many researchers in recent years have investigated the preparation and evaluation of NPs obtained from the copolymerization of acrylate derivatives, coated with chitosan or its derivatives.Citation17–Citation19,Citation25
In this study, the potential use of NPs prepared by the copolymerization of poly methyl methacrylate (pMMA) and chitosan–glutathione conjugates as a carrier for oral delivery of a hydrophobic drug (DTX) was investigated.
Materials and methods
Materials
DTX was obtained from Cipla (Mumbai, India). Chitosan (ChitoClear) with a medium molecular weight and degree of deacetylation of about 89% was purchased from Primex (Karmoy, Norway). L-Glutathione-reduced form (GSH), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) hydrochloride, N-hydroxysuccinimide (NHS), methyl methacrylate (MMA), ammonium cerium nitrate, sodium nitrite, hydrochloric acid, glacial acetic acid, sodium hydroxide, and potassium hydrogen phosphate were all purchased from Merck (Darmstadt, Germany). Fluoresceinamine (FA) isomer I and Ellman’s reagent, 5,50-dithiobis (2-nitro benzoic acid), were obtained from Sigma-Aldrich (St. Louis, MO). 3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) (MTT) was purchased from Sigma-Aldrich. Caco-2 and MCF-7 cell lines were obtained from the Pasteur Institute (Tehran, Iran). All other chemicals were of analytical grade.
Depolymerization of chitosan
Chitosan was selectively depolymerized following the method developed by Huang et al.Citation26 Briefly, 100 mL of a 2% (w/v) chitosan (400,000 g/mol) solution in 6% (v/v) acetic acid solution was prepared. Then the dissolved chitosan was depolymerized at room temperature under stirring with 10 mL of NaNO2 solutions in water at different concentrations (7.0, 2.7, and 1.6 g/L), to obtain the desired final molecular weight of 20,000, 50,000, and 100,000 g/mol, respectively. After 1 hour of reaction, precipitated chitosan was obtained by raising the pH to 9.0 by addition of 4N NaOH. The white-yellowish solid was filtrated, washed thoroughly with acetone, and re-dissolved in a minimum volume of acetic acid 0.1 N (40 mL).
Purification was carried out by subsequent dialyses against purified water (Sigma dialysis tubes, molecular weight cutoff, 12 kDa). The dialyzed product was lyophilized using a LyoTrap plus Freeze dryer (LTE Scientific, Oldham, UK), and the yellowish lyophilized product was then stored at 4°C until use. Products obtained were called Chito.20, Chito.50, and Chito100 depending on their theoretical molecular weight.
The average molecular weights of the prepared chitosans were determined by a viscometric method as described in our previous work.Citation5
Chitosan thiolation
Thiolated chitosan was prepared with covalent attachment of reduced glutathione to different molecular weight of chitosan according to the slightly modified method described by Atyabi et al and Kafedjiiski et al as follows:Citation5,Citation27
One gram of chitosan was dispersed in 50 mL demineralized water by stirring, then 10 mL HCl (1 mol/L) was added and finally dissolved by the addition of demineralized water to obtain a 1% (w/v) polymer solution. The pH was adjusted to 6.0 by the addition of NaOH (5 mol/L). Afterwards, 5 g of reduced glutathione in 10 mL demineralized water was added to the above solution under continuous stirring. EDC and NHS were then added in a final concentration of 200 mmol/L each. The pH was readjusted to 6.0 by adding NaOH (5 mol/L). The reaction mixture was incubated for 15 hours at room temperature under constant stirring. To eliminate unbound reagents, the resulting polymer conjugate was dialyzed using dialysis tubing (molecular weight cutoff 12 kDa), first against 5 mmol/L HCl, twice against 5 mmol/L HCl containing 1% NaCl, and finally twice against 1 mmol/L HCl. Controls were prepared in the same way but omitting EDC and NHS during the coupling reaction. Finally, the frozen aqueous polymer solutions were lyophilized at −50°C and 0.01 mbar (LyoTrap plus; LTE Scientific, UK) and stored at 4°C until further use.
The amount of thiol groups immobilized on chitosan–GSH conjugate was determined using Ellman’s reagent and the spectrophotometric method.Citation27–Citation29
Preparation of thiolated chitosan-pMMA NPs
The NPs were prepared by using a modified radical polymerization method. Conjugated chitosan (37.5 mg) with different theoretical molecular weight (20, 50, and 100 kDa) was dissolved in 4 mL nitric acid (0.2 mol/L) in a two-necked flask at 40°C, under gentle stirring and nitrogen bubbling. After 10 minutes, under vigorous magnetic stirring, a solution of 0.08 mol/L cerium IV ammonium nitrate (CAN) in 0.2 mol/L nitric acid and MMA was added to obtain a 5-mL solution. Nitrogen bubbling was continued for an additional 10 minutes. The reaction was allowed to continue at 40°C under gentle stirring for 40 minutes. After cooling to room temperature, the pH of the obtained suspension was adjusted to 4.5 by addition of sodium hydroxide (1 N) drop-wise. The NP suspension was then purified by dialysis (Sigma dialysis tubes, molecular weight cutoff, 12 kDa) against 1 L of acetic acid solution (16 μmol/L) in demineralized water, twice for 90 minutes and once overnight. The frozen NP suspensions were lyophilized at −50°C and 0.01 mbar and stored at 4°C until further use.
Determination of the thiol group content of thiolated chitosan-coated pMMA (Cht-GSH-pMMA) NPs
To quantify thiol group content of the NPs, iodine titration was performed as described by Bravo-Osuna et al and Kast and Bernkop-Schnürch.Citation24,Citation30 Twenty milligrams of the lyophilized NPs was hydrated in 6 mL of demineralized water. Following adjustment of pH to 2–3 with 1 mol/L HCl, the solution became clear. Then 500 μL of aqueous starch solution (1%) was added. The samples were titrated with an aqueous iodine solution (1 mmol/L) until a permanent light blue color was maintained.
Preparation of DTX- or FA-loaded NPs
The anticancer drug-loaded NPs were prepared using Chito. 20. After adding acidic solution of CAN, DTX was dissolved in 0.5 mL of methanol under stirring, and 0.25 mL MMA was added to obtain a clear solution. Under vigorous magnetic stirring, a solution of DTX was added in a two-necked flask. Nitrogen bubbling was continued for an additional 10 minutes. The added amount of DTX was in the range from 1% to 4% (w/w) based on the weight of MMA and thiolated chitosan. The reaction was allowed to continue at 40°C under gentle stirring for 40 minutes. After adjusting the pH of the obtained suspension to 4.5, the NP suspension was purified by dialysis. The amount of DTX in the obtained NPs was measured by high-performance liquid chromatography (HPLC). Isocratic reversed-phase HPLC was performed using a Knauer HPLC system (Knauer, Berlin, Germany) with a 5 μ Bondapak C18 column (Waters, Milford, MA). The mobile phase consisted of 75:25 (v/v) methanol/water and was delivered at a flow rate of 1.0 mL/min. Eluted compounds were detected at 227 nm using a Spectra100 UV-Vis detector. The FA-loaded NPs were prepared in the same way except for the addition of 0.25% (w/v) FA to the monomer (MMA) solution instead of DTX.
Size distribution of NPs
The mean diameter and size distribution of the NPs were determined by dynamic light scattering using Zetasizer® (Nano-ZS, Malvern, Instruments, Malvern, UK). All dynamic light scattering measurements were carried out at a wavelength of 633 nm at 25°C with an angle detection of 90°. The samples were diluted in acetic acid (16 μmol/L) in deionized water, three subsequent measurements were determined for each sample, and the result was expressed as mean size ± standard deviation.
Determination of zeta potential
The zeta potential measurements were performed by Laser Doppler Electrophoresis using Zetasizer (Nano-ZS). To maintain a constant ionic strength, the samples were diluted (1:50 v/v) in NaCl 1 mmol/L (pH 6.5).Citation3 Each sample was measured three times.
Differential scanning calorimetry (DSC)
DSC was performed to investigate the physical status of DTX in the NPs. DSC scans of DTX, empty and DTX-loaded NPs were performed on a Mettler DSC 823e equipped with Mettler STARe system software for the data acquisition. The samples were scanned at a speed of 5°C/min in a 30°C–250°C temperature range.
Scanning electron microscopy
The surface morphology of the NPs was evaluated by using a scanning electron microscope (SEM XL 30; Philips, Eindhoven, The Netherlands). NP suspensions were successively diluted in deionized water to 1/50 (v/v). The dilutions were spread on an aluminum disc and dried at room temperature before the analysis. The dried NPs were then coated with a thin layer of gold metal using a sputter coater (SCD 005; Bal-Tec, Pfaffikon, Switzerland).
Transmission electron microscopy (TEM)
Transmission electron microscopy (TEM; CEM 902A; Zeiss, Oberkochen, Germany) was used to examine the structure and the topography of the NPs. NP suspensions were diluted in demineralized water to 1/100 (v/v), and prior to observation, the NPs were negatively stained with a solution of 1% phosphotungstic acid.
Drug loading and entrapment efficiency
The entrapment efficiency (EE) of the process was determined indirectly upon separation of the NPs by ultracentrifugation at 25,000 rpm, 8°C for 30 minutes from the aqueous medium containing free DTX. The amount of free DTX in the supernatant was measured using HPLC. The EE of DTX NPs was calculated as the ratio of DTX loaded into the NPs with respect to the total amount of DTX used for preparation of the original mixture as follows:
where DTXt is the total amount of DTX used for preparation of the original mixture and DTXf is the free DTX amount recovered in the supernatant. All samples were measured in triplicate.
Drug loading (DL) was calculated as follows:
In vitro drug release study
Drug release from DTX-loaded NPs was studied by incubating the NPs in phosphate-buffered solution (PBS), at pH 7.4, at 37°C. Two milligrams of NPs was dispersed in 5 mL of release medium (PBS of pH 7.4 containing 0.1% w/v Tween 80) in a dialysis tube (Sigma dialysis tubes, molecular weight cutoff, 12 kDa), and the closed dialysis bag immersed in 20 mL release medium in a centrifuge tube. Tween 80 was used to increase the solubility of DTX in the buffer solution to maintain sink condition. The tube was placed in a shaker bath at 37°C and shaken horizontally at 100 cycles/min. At given time intervals, 15-mL samples were withdrawn and replaced with the same volume of fresh medium. The samples were filtered through a 0.22-μm filter and were analyzed for the amount of DTX using HPLC.
In vitro cytotoxicity of DTX-loaded NPs
Human colon adenocarcinoma cells (Caco2) and MCF-7 cells were used for the evaluation of cell viability of DTX-loaded NPs. Separately, Caco-2 and MCF-7 cells were incubated in 96-well transparent plates (Costar, Chicago, IL) at 1 × 104 cells/well (100 μL). After 12 hours the old medium was removed, and the cells were incubated in the medium containing DTX or DTX-loaded NPs. Because of the cytotoxic effects of Tween 80,Citation4,Citation31 DTX was dissolved in dimethyl sulfoxide (DMSO) instead of Tween 80/ethanol/saline as solvent. DTX was dissolved in DMSO at a proper concentration and diluted 100-fold with growth medium. DTX-loaded NPs were then dispersed into the purified water, and after dilution with growth medium were added to each well at equivalent drug concentrations ranging from 0.1 to 1000 ng/mL. After 72 hours incubation, 20 μL of MTT (3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazonium-bromide) solution (5 mg/mL) was added to each well of the plate. After 4 hours, 100 μL DMSO was added to each well, and the absorbance of the solution was measured at 570 nm and reference wavelength at 690 nm by a microplate reader (Anthos 2020; Anthos Labtec Instruments, Wals, Austria).
Caco-2 cell uptake of fluorescent NPs
For qualitative uptake studies, the Caco-2 cells were seeded in the chambered glass system (Lab-Tek; Nunc International Co., Naperville, IL). Cells were washed four times after incubation with FA-loaded NPs for 2 hours and then fixed by a cold mixture of methanol/acetone (50:50 v/v) for15 minutes at room temperature. The cells were washed twice with PBS and mounted in mounting medium consisting of Na2HPO4 and acetic acid (pH 5.5)/glycerol (50:50 v/v) to be observed by fluorescence microscope (λext: 540 nm and λem: 580 nm; BX40; Olympus, Tokyo, Japan). The fluorescent images were taken by DP70 digital imaging system (Olympus) and analyzed by Olysia imaging software (Olympus).
Ex vivo study
The everted sac method was chosen for measurement of transportation of DTX across the intestine barrier. It was carried out according to the slightly modified method that was described by Barr and Riegelman,Citation32 as follows.
First, a section of about 5 cm of the jejunum was removed from a male rat under ketamine (50 mg/kg) and chlorpromazine (10 mg/kg) anesthesia and washed with Krebs–Ringer bicarbonate solution of pH = 7.4. This section was then gently inverted with a glass rod, and a tube was inserted in one side of the section and tied securely with tape. The other side of the intestine was tied, and 1 mL Krebs–Ringer bicarbonate solution was poured through the hypodermic needle in the tube. The gut sac was placed in a medium saturated with 95% O2, 5% CO2, and contained the test sample in Krebs–Ringer bicarbonate solution at 37°C. The test samples used include: (1) DTX (1 mg) as Taxotere®, and (2) Chito.20-GSH-DTX 4% NPs (equivalent to 1 mg of DTX). In absorption studies, an O2 and CO2 mixture was bubbled into the intestinal mucosa to obtain intestinal peristaltic movement. At certain periods of time, 0.5-mL samples were drawn from inside the intestine and replaced with the same volume of fresh medium. The amount of transported DTX in the samples was measured by the HPLC method.
Results and discussion
Chitosan depolymerization and characterization
Several studies have demonstrated that the molecular weight of chitosan is very important to NP properties such as particle size,Citation33 paracellular permeability,Citation34 and immunological response.Citation35 Due to high molecular weight and thus high viscosity and poor solubility at physiological pH range of the commercial chitosan, the use of low-molecular-weight chitosan is preferred in particular drug delivery systems.Citation36
Depolymerization of chitosan was carried out via an oxidative process by NaNO2 as a simple and reproducible method. Molecular weights of depolymerized chitosan determined by capillary viscometry measurement are shown in .
Table 1 Molecular weights of depolymerized chitosan and thiolated chitosan characterization (n = 3)
Thiolated chitosan preparation and quantification
summarizes the amounts of free thiol groups, disulfide bonds, and total sulfhydryl groups immobilized on chitosan-GSH conjugates. The free thiol content and total sulfhydryl groups of different molecular weights of chitosan is very similar to the results obtained by Bravo-Osuna et al.Citation39 The results indicated that when the molecular weight of chitosan increased, the difficulty of the thiol-bearing agent to reach the amino groups of chitosan was increased, and fewer sulfhydryl groups could be fixed on the polymer.
NP preparation and characterization
The mean hydrodynamic diameter, polydispersity index (PDI), zeta potential, and thiol content of NPs prepared using different molecular weights of chitosan-GSH in a fixed amount of MMA and CAN are presented in .
Table 2 Mean hydrodynamic diameter, polydispersity index, zeta potential, and free thiol of nanoparticles made by different molecular weight of chitosan-GSH
The mean size of particles obtained by different molecular weights of thiolated chitosan, with values varying from 153 to 264 nm, demonstrated an incremental increase in accordance with the molecular weight of chitosan. This result is very similar to the results obtained by Bravo-Osuna et al.Citation39 The zeta potential of particles was positive, indicating that the negative surface charge of noncoated pMMACitation40 was completely covered by the cationic chitosan. According to the significant effect of particle size and type of surface coating on the cellular uptake of polymeric NPsCitation41 in this study, chitosan with low molecular weight (20,000) was used for preparation of DTX NPs. Smallest NPs with most free thiol on their surface were produced using Chito.20.
DTX-loaded NPs characterization
The size, PDI, and zeta potential of DTX-loaded NPs prepared by Chito.20-GSH and different amounts of DTX in a fixed amount of MMA and CAN are seen in .
Table 3 Characterization of nanoparticles made by Chito.20 and varying concentrations of DTX
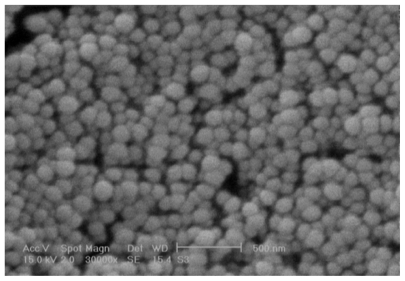
As can be seen in , the zeta potential of the NPs was positive due to their chitosan coating with free amino groups.Citation33 This is favorable for making electrostatic bonds with negatively charged mucosa and preventing elimination through the alimentary canal. There is an inverse relationship between the concentration of entrapped DTX and zeta potential value of particles. The SEM image of the NPs shows that they are spherical (). The figure also confirms the size of the NPs measured by Zetasizer shown in .
shows the TEM image of DTX-loaded NPs. The core and coat of NPs can be recognized in this figure.
As given in , all NPs have a desired PDI less than 0.2 indicating a homogenous size distribution. Commonly, the PDI is an index of stability of all the nanosuspensions.
Entrapment efficiency and drug loading of NPs
The EE and DL of the NPs is shown in . The EE of the NPs was determined with an indirect method after isolation of NPs by centrifuging them as described above. Ideally, a successful nanoparticulate system should have a high DL capacity. As seen in , the EE of Chito.20-GSH-DTX 1%, 2%, 3%, and 4% NPs are 92.4, 91.1, 88.9, and 93.6%, respectively. The maximum EE was related to NPs with 4% DTX. This high EE might be due to the tendency of DTX as a hydrophobic molecule to enter the hydrophobic core (pMMA) of the NPs.
The DL of Chito.20-DTX NPs was between 8.5% and 20.4%. A high DL capacity was expected, due to the hydrophobicity of DTX.
Physical status of DTX in the NPs
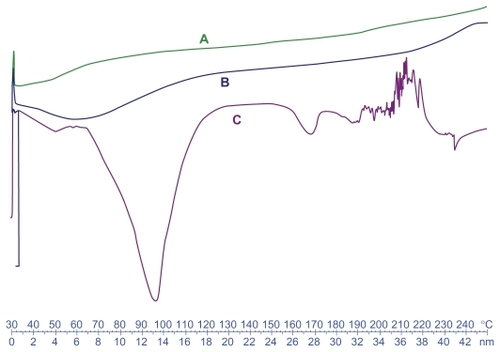
DSC studies were performed to investigate the physical status of DTX in the NPs. shows the DSC thermograms of free DTX, unloaded, and drug-loaded NPs. Free DTX showed a sharp endothermic melting peak at about 169°C related to DTX. However, no melting peak could be detected for the NP formulations. This shows that DTX in the NPs is in an amorphous or disordered crystalline phase, miscible in polymeric NPs.
In vitro drug release study
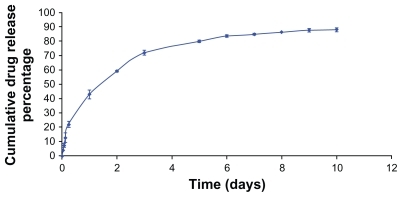
The in vitro release profile of the DTX-loaded NPs is shown in . It shows a sustained Fickian release profile, suggesting that the hydrophobic core acts as a barrier against the release of entrapped DTX from the polymeric matrix into the aqueous solution.
Fluorescent microscopy image
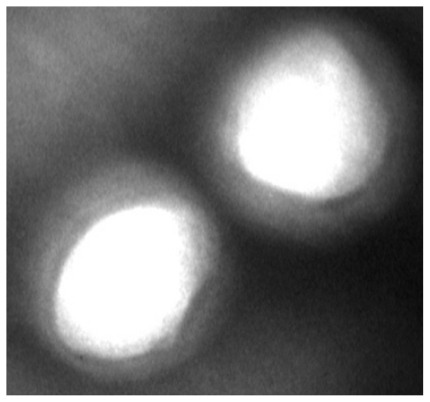
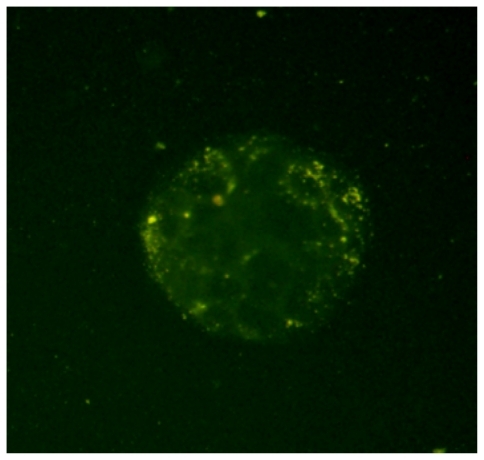
shows the fluorescent microscopy image of Caco-2 cells after 2 hours incubation with FA-loaded NPs. As can be seen in this figure, after 2 hours incubation, the FA-loaded NPs have been internalized by the Caco-2 cells, which are located in the cytoplasm around the nucleus.
Figure 5 Fluorescence microscopic images of Caco-2 cells after 2 hours incubation with fluoresceinamine (FA)-loaded nanoparticles at 37°C. As shown in the image, the yellow fluorescence of the FA-loaded nanoparticles is distributed in the cytoplasm, indicating the uptake of nanoparticles by the cells.
In vitro cell viability of NPs
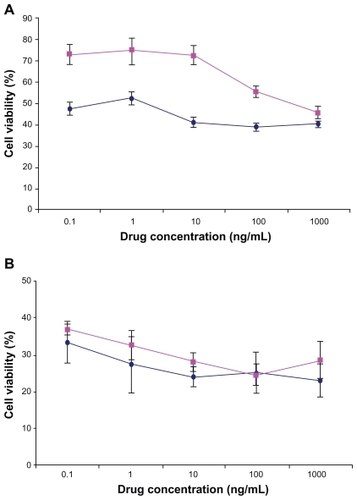
shows the viability of Caco-2 and MCF-7 cells after 72 hours incubation time with DTX-loaded NPs in comparison with free DTX drug at the same concentration (0.1, 1.0, 10, 100, and 1000 ng/mL). In our previous study, it was shown that the blank NPs did not show any cytotoxic effect on the Caco-2 and MCF-7 cells.Citation44 The results in this study indicated that the drug entrapped in thiolated Chito.20 NPs had more cytotoxic effect than the free drug for Caco-2 cells at most concentrations (P < 0.05) and had equal cytotoxicity for MCF-7 after 72 hours. Based on the result of in vitro release studies (), after 72 hours, approximately 70% of the drug is released from the NPs. Thus, more cytotoxicity effect of the NPs is not only related to the DTX released from the NPs but probably also because of cell uptake of the NPs and/or better penetration of NPs into the cells. The site of action of DTX is the cytoplasm, and NPs act as intracellular depots that slowly release the loaded therapeutic agent into the cellular cytoplasm. This increase of cytotoxic effects of NPs after a period of time is in agreement with previous reports.Citation45,Citation46
Figure 6 Viability of Caco-2 cells (A) and MCF-7 (B) after 72 hours cell culture with DTX-loaded Chito.20-GSH nanoparticles (♦) in comparison with DTX (■) at different concentrations (n = 6). Percentage survival was assessed by MTT assay. Data are mean ± standard deviation (n = 6).
Abbreviations: DTX, docetaxel; GSH, glutathione; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide).
Ex vivo study
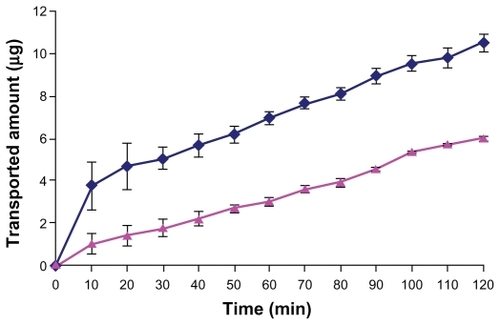
In this study, we used the everted intestinal sac method for measuring the transporting of DTX from the intestinal barrier. shows the amount of DTX transported across the intestinal barrier. As seen in the figure, after 120 minutes, the amount of DTX transported from the intestinal barrier with Chito.20-GSH NPs was significantly higher than free DTX. Consequently, on the basis of these results, it was hypothesized that transportation of DTX across the intestine membrane is low, and the mucoadhesive NPs can increase DTX transport by opening tight junctions and bypassing the efflux pump of p-gp.
Conclusion
In this work we applied mucoadhesive thiolated chitosan pMMA NPs as a carrier for oral delivery of DTX. DTX was loaded into the pMMA thiolated chitosan NPs using a modified radical polymerization method. This method has some advantages for obtaining a drug carrier, such as elimination of organic solvent and surfactants and preparation of NPs under mild conditions. The size of the NPs was small and size distribution was narrow. EE was high (around 90%), and in vitro release studies showed the potential of NPs for extended release of DTX in medium solution. It seems that the penetration ability of DTX-loaded NPs prepared with thiolated chitosan into the cancer cells causes more cytotoxic effect of NPs versus free drug after 72 hours. Such hydrophobic and mucoadhesive NPs seem to be very promising vehicles for oral delivery of hydrophobic drugs such as taxanes.
Acknowledgments
The authors would like to thank Nanotechnology Research Centre of Tehran University of Medical Sciences. The authors are grateful to Dr A Kebriaeezadeh, managing director of OSVAH pharmaceutical company, for his support and encouragement of this study, and also thank Mrs S Tavajjohi for her kind assistance in cell culture experiments.
Disclosure
The authors report no conflicts of interest in this work.
References
- AjaniJATakiuchiHRecent developments in oral chemotherapy options for gastric carcinomaDrugs199958Suppl 3859010711846
- FengSSDongYPoly(D,L-lactide-co-glycolide)/montmorillonite nanoparticles for oral delivery of anticancer drugsBiomaterials2005266068607615894372
- ArangoaMAPonchelGOrecchioniAMRenedoMJDucheneDIracheJMBioadhesive potential of gliadin nanoparticulate systemsEur J Pharm Sci20001133334111033077
- ArechabalaBCoiffardCRivallandPCoiffardLJMde Roeck-HoltzhauerYComparison of cytotoxicity of various surfactants tested on normal human fibroblast cultures using the neutral red test, MTT assay and LDH releaseJ Appl Toxicol19991916316510362266
- AtyabiFAghaei MoghaddamFDinarvandRZohuriaan-MehrMJPonchelGThiolated chitosan coated poly hydroxyethyl methacrylate nanoparticles: synthesis and characterizationCarbohydr Polym2008745967
- Bernkop-SchnürchAKastCEChemically modified chitosans as enzyme inhibitorsAdv Drug Deliv Rev20015212713711718936
- MangatalLAdelineMTGuénardDGuéritte-VoegeleinFPotierPApplication of the vicinal oxymination reaction with asymmetric induction to the hemisynthesis of taxol and analoguesTetrahedron19894541774190
- KellandLRAbelGComparative in vitro cytotoxicity of taxol and Taxotere against cisplatin-sensitive and -resistant human ovarian carcinoma cell linesCancer Chemother Pharmacol1992304444501356649
- EiermannWDocetaxel – maximising outcomes towards cure in early breast cancerBreast200615Suppl 3S1316
- EscobarPFRosePGDocetaxel in ovarian cancerExpert Opin Pharmacother20056152719272616316310
- Iwao-KoizumiKMatobaRUenoNPrediction of docetaxel response in human breast cancer by gene expression profilingJ Clin Oncol200523342243115659489
- Van ZuylenLVerweijJSparreboomARole of formulation vehicles in taxane pharmacologyInvest New Drugs200119212514111392447
- ShouMMartinetMKorzekwaKRKrauszKWGonzalezFJGelboinHVRole of human cytochrome P450 3 A4 and 3 A5 in the metabolism of taxotere and its derivatives: enzyme specificity, inter-individual distribution and metabolic contribution in human liverPharmacogenetics1998853914019825831
- MalingreMMBeijnenJHSchellensJHMOral delivery of taxanesInvest New Drugs200119215516211392449
- PeracchiaMTVauthierCPassiraniCCouvreurPLabarreDComplement consumption by poly(ethylene glycol) in different conformations chemically coupled to poly(isobutyl 2-cyanoacrylate) nanoparticlesLife Sci19976177497619252249
- LemarchandCGrefRCouvreurPPolysaccharide-decorated nanoparticlesEur J Pharm Biopharm20045832734115296959
- Bravo-OsunaISchmitzTBernkop-SchnürchAVauthierCPonchelGElaboration and characterization of thiolated chitosan-coated acrylic nanoparticlesInt J Pharm200631617017516580797
- ChauvierreCLabarreDCouvreurPVauthierCNovel polysaccharide-decorated poly(isobutyl cianoacrylate) nanoparticlesPharm Res2003201786179314661923
- ChauvierreCLabarreDCouvreurPVauthierCRadical polymerization of alkylcyanoacrylates initiated by the redox system dextrancerium (IV) under acidic aqueous conditionsMacromolecules20033660186027
- HassanEEGalloJMA simple rheological method for the in vitro assessment of mucin-polymer bioadhesive bond strengthPharm Res199074914951694990
- RoldoMHornofMCalicetiPBernkop-SchnürchAMucoadhesive thiolated chitosans as platforms for oral controlled drug delivery: synthesis and in vitro evaluationEur J Pharm Biopharm200457111512114729087
- LeitnerVMGuggiDKraulandAHBernkop-SchnürchANasal delivery of human growth hormone: in vitro and in vivo evaluation of a thiomer/glutathione microparticulate delivery systemJ Control Release2004100879515491813
- Bernkop-SchnürchAKastCEGuggiDPermeation enhancing polymers in oral delivery of hydrophilic macromolecules: thiomer/GSH systemsJ Control Release20039395103
- Bravo-OsunaITeutonicoDArpiccoSVauthierCPonchelGCharacterization of chitosan thiolation and application to thiol quantification onto nanoparticle surfaceInt J Pharm20073401217318117606340
- CuiFQianFYinCPreparation and characterization of mucoadhesive polymer-coated nanoparticlesInt J Pharm200631615416116567070
- HuangMKhorELimLYUptake and cytotoxicity of chitosan molecules and nanoparticles: effects of molecular weight and degree of deacetylationPharm Res200421234435615032318
- KafedjiiskiKFögerFWerleMBernkop-SchnürchrchASynthesis and in vitro evaluation of a novel chitosan–glutathione conjugatePharm Res20052291480148816132360
- GuggiDBernkop-SchnürchAImproved paracellular uptake by the combination of different types of permeation enhancersInt J Pharm200528814115015607266
- Bernkop-SchnürchASchwarzVSteiningerSPolymers with thiol groups: a new generation of mucoadhesive polymersPharm Res19991687688110397608
- KastCEBernkop-SchnürchAThiolated polymersthiomers: development and in vitro evaluation of chitosan-thioglycolic acid conjugatesBiomaterials2001222345235211511031
- Cheon LeeSKimCChan KwonIChungHYoung JeongSPolymeric micelles of poly(2-ethyl-2-oxazoline)-block-poly([var epsilon]-caprolactone) copolymer as a carrier for paclitaxelJ Control Rel2003893437446
- BarrWHRiegelmanSIntestinal drug absorption and metabolism. I. Comparison of methods and models to study physiological factors of in vitro and in vivo intestinal absorptionJ Pharm Sci19705921541635411337
- Bravo-OsunaIPonchelGVauthierCTuning of shell and core characteristics of chitosan-decorated acrylic nanoparticlesEur J Pharm Sci200730214315417157487
- ChenM-CWongH-SLinK-JThe characteristics, biodistribution and bioavailability of a chitosan-based nanoparticulate system for the oral delivery of heparinBiomaterials200930346629663719767097
- VilaASanchezAJanesKLow molecular weight chitosan nanoparticles as new carriers for nasal vaccine delivery in miceEur J Pharm Biopharm200457112313114729088
- LinYHChenCTLiangHFNovel nanoparticles for oral insulin delivery via the paracellular pathwayNanotechnology20071810111
- JanesKAAlonsoMJDepolymerized chitosan nanoparticles for protein delivery: preparation and characterizationJ Appli Polym Sci2003881227692776
- MaoSShuaiXUngerFSimonMBiDKisselTThe depolymerization of chitosan: effects on physicochemical and biological propertiesInt J Pharm2004281124554
- Bravo-OsunaIPonchelGVauthierCTuning of shell and core characteristics of chitosan-decorated acrylic nanoparticlesEur J Pharm Sci20073014315417157487
- PassiraniCBarrattGDevissaguetJ-PLabarreDInteractions of nanoparticles bearing heparin or dextran covalently bound to poly(methyl methacrylate) with the complement systemLife Sci19986287757859489513
- FengSSWinaKYEffects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugsBiomaterials2005262713272215585275
- FlorenceATNanoparticle uptake by the oral route: fulfilling its potential?Drug Discov Today Technol2005217581
- NorrisDASinkoPJEffect of size, surface charge, and hydrophobicity on the translocation of polystyrene microspheres through gastrointestinal mucinJ Appl Polym Sci19976314811492
- AkhlaghiSPSaremiSOstadSNDinarvandRAtyabiFDiscriminated effects of thiolated chitosan-coated pMMA paclitaxel-loaded nanoparticles on different normal and cancer cell linesNanomedicine20106568969720172052
- EsmaeiliFDinarvandRGhahremaniMHOstadSNEsmailyHAtyabiFCellular cytotoxicity and in-vivo biodistribution of docetaxel poly(lactide-co-glycolide) nanoparticlesAnticancer Drugs201021435219809300
- ZhaoJKimJEReedELiQQMolecular mechanism of antitumor activity of taxanes in lung cancerInt J Oncol20052724725615942666