 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In this study, zinc phthalocyanine (ZnPc) was loaded onto poly-ɛ-caprolactone (PCL) nanoparticles (NPs) using a solvent emulsification–evaporation method. The process yield and encapsulation efficiency were 74.2% ± 1.2% and 67.1% ± 0.9%, respectively. The NPs had a mean diameter of 187.4 ± 2.1 nm, narrow distribution size with a polydispersity index of 0.096 ± 0.004, zeta potential of −4.85 ± 0.21 mV, and spherical shape. ZnPc has sustained release, following Higuchi’s kinetics. The photobiological activity of the ZnPc-loaded NPs was evaluated on human lung adenocarcinoma A549 cells. Cells were incubated with free ZnPc or ZnPc-loaded NPs for 4 h and then washed with phosphate-buffered saline. Culture medium was added to the wells containing the cells. Finally, the cells were exposed to red light (660 nm) with a light dose of 100 J/cm2. The cellular viability was determined after 24 h of incubation. ZnPc-loaded NPs and free photosensitizer eliminated about 95.9% ± 1.8% and 28.7% ± 2.2% of A549 cells, respectively. The phototoxicity was time dependent up to 4 h and concentration dependent at 0–5 μg ZnPc. The cells viability decreased with the increase of the light dose in the range of 10–100 J/cm2. Intense lysis was observed in the cells incubated with the ZnPcloaded NPs and irradiated with red light. ZnPc-loaded PCL NPs are the release systems that promise photodynamic therapy use.
Introduction
Photodynamic therapy (PDT) is being actively exploited in many clinical applications such as cancer treatment, age-related macular degeneration, and infection.Citation1,Citation2 PDT is based on the administration of drugs known as photosensitizers that are taken up and/ or retained by neoplastic tissues.Citation3,Citation4 The photosensitizers alone are harmless and ideally have no effect on either healthy or abnormal tissues. After a predefined time interval that allows photosensitizer accumulation in the tumor tissue, the illumination of the tumor site with nonthermal light (600–800 nm) leads to the formation of an excited photosensitizer. The combined action of the excited triplet photosensitizer and molecular oxygen results in the formation of singlet oxygen (1O2), which is the main mediator of cellular death induced by PDT.Citation2 PDT has been shown to induce both apoptosis (slow process)Citation5 and necrosis (drastic process).Citation3 Among the most promising second-generation photosensitizers for PDT are the phthalocyanines. In this study, zinc phthalocyanine (ZnPc), a metallophthalocyanine, was used because of its very strong phototoxicity effect,Citation6 easy availability, and low cost. ZnPc is stable and produces 1O2 with high yield.Citation7 However, ZnPc is lipophilic and slightly soluble in water or in a physiologically acceptable vehicle. This hydrophobic characteristic hinders its systemic administration and restricts its clinical studies.
ZnPc is a dye and photosensitizer with molecular weight of 577.91 g/mol. It is a crystalline powder, lipophilic, and soluble in pyridine, dimethylsulfoxide, dimethylformamide, and dichloromethane (DCM).Citation8 The photosensitizer can be dissolved in dimethylsulfoxide and diluted with ethanol. However, it suffers aggregation in water with reduction of its photobiological activity.Citation9 It exhibits a high thermal stability, and <5% of its mass is lost at 500°C.Citation9 ZnPc has strong absorbance in the red region of the spectrum of visible light (λmax = 666 nm in ethanol). It has strong fluorescence emission in the range of 650–800 nm.Citation9 Methylene blue (MB) is a hydrophilic dye and photosensitizer.Citation10 In this study, it was used as a positive control of the photobiological activity. It exhibits strong absorbance in the red region (λmax = 656 nm in water) and fluorescence emission (λmax = 656 nm)Citation10 similar to ZnPc.
Nanoparticles (NPs) are promising delivery systems for use in PDT. These systems provide sustained release, avoid plasmatic fluctuations, decrease side effects, reduce the frequency of administration, and enhance the uptake of drugs by targeted tissue, increasing the treatment effectiveness.Citation2,Citation11–Citation14 Advances in nanobiotechnology have resulted in the development of several colloidal carrier systems such as NPs to achieve these multiple objectives.Citation2,Citation4 The encapsulation of hydrophobic drugs in NPs is a viable alternative in solving the solubility problem,Citation12 besides promoting targeted delivery and sustained release. The liposomes and the nanoemulsions are examples of drugs carrier. However, they have problems of physical stability. The liposomes can suffer disruption and have limited capacity to encapsulate lipophilic drugs.Citation15 Nanoemulsions can suffer creaming, flocculation, and coalescence.
NPs have excellent physical stability, provide protection of the actives, and sustain drugs release.Citation15 NPs have been used as a vehicle for the delivery of PDT agents.Citation2,Citation4,Citation16,Citation17 Several photosensitizers, many of which are hydrophobic, have been encapsulated in polyesters, such as poly(lactic-co-glycolic acid) and polylactic acid NPs, to overcome the problems associated with solubilization.Citation12 For example, meso-tetra(4-hydroxyphenyl) porphyrin,Citation18,Citation19 bacteriochlorophyll- a,Citation20 verteporfin,Citation21 MB,Citation22 hypericin,Citation23 and various phthalocyaninesCitation9,Citation24 have all been encapsulated in such polyesters. Several studies have proved that the NPs are promising delivery systems for photosensitizers. Generally, photosensitizers loaded in NPs induce better phototoxicity than free photosensitizers.Citation18,Citation23 Moreover, encapsulation in NPs decreases the aggregation of the photosensitizer in solutionCitation20 or in physiologically acceptable vehicles.
Biocompatible and biodegradable polymers can be used to produce NPs. Much attention has been focused on poly-ɛ-caprolactone (PCL), a semicrystalline polyester.Citation9,Citation25–Citation27 This polymer has been used in the development of release systems such as microparticles and NPs.Citation25–Citation27 PCL NPs have been used as delivery systems of isradipine,Citation28 primidone,Citation29 paclitaxel,Citation30 griseofulvin,Citation31 tamoxifen,Citation32 espironolactone,Citation33 vinblastine,Citation34 and docetaxel.Citation35
The aim of this study was to load ZnPc in PCL NPs to improve the photobiological activity of the photosensitizer. NPs were produced and characterized in terms of their size, charge, morphology, and encapsulation efficiency (EE). In vitro release studies were carried out to evaluate the photosensitizer release profile and kinetic study. The photobiological activity of the ZnPc-loaded NPs and free photosensitizer were assessed on human lung adenocarcinoma A549 cancer cells.
Material and methods
Materials
ZnPc (MW = 577.91), MB (MW = 373.90) (≥82%), hydrolyzed polyvinyl alcohol (PVA) (87%–89%) (MW = 13,000–23,000), 9,10-anthracenediyl-bis(methylene)dimalonic acid (ABMDMA), 3-(4,5-dimethyl-thiazol-2-yl)-2,5-biphenyl tetrazolium bromide (MTT), and PCL (MW = 42,500–65,000) were purchased from Sigma-Aldrich (St. Louis, MO). DCM and acetone were purchased from Merck (Darmstadt, Germany). Sodium dodecyl sulfate (SDS), NaCl, KCl, NaH2PO4, and Na2HPO4 were purchased from Vetec (Duque de Caxias, Brazil). Uranyl acetate was purchased from Reagem (Colombo, Brazil). The following cell culture items were purchased from Gibco Invitrogen (New York, NY): Dulbecco’s modified Eagle’s medium (DMEM), glutamine, fetal bovine serum (FBS), sodium bicarbonate, sodium hydroxide, and Pen Strep (penicillin 10,000 units/mL + streptomycin 10,000 μg/mL in aqueous solution).
NPs preparation and process yield (%)
NPs were prepared by the emulsification and solvent evaporation method. Briefly, ZnPc (3 mg) and PCL (97 mg) were dissolved in the DCM (8 mL). The organic solution was emulsified in 50 mL of aqueous solution of PVA (1.5%, w/w), for 5 min using an ultrasonicator UP100H (100 W, 30 kHz) (Hielscher, Teltow, Germany) in an ice bath. PVA is a biocompatible polymer and has the function of a surfactant. DCM was evaporated under reduced pressure using a rota vapor (Heidolph, Schwabach, Germany) at room temperature (28°C). The NPs were purified thrice by centrifugation at 20,000 g (Avanti J-25; Beckman Coulter, San Francisco, CA) for 20 min followed by resuspension in water to remove the surfactant. NPs were then transferred into glass vials, frozen in a liquid nitrogen bath, and lyophilized (Lyophilizer, FreeZone 4.5-L Benchtop Freeze Dry System; Labconco, Kansas City, MO). The lyophilized powder was stored at room temperature (28°C) before analysis.
The process yield (%, w/w) was calculated by EquationEq. 1(1) :
where Y(%) is the process yield, M1 is the mass of recovered lyophilized powder, and M2 is the mass of the formulation (PCL and ZnPc).
Characterization
NPs were characterized in terms of their size, polydispersity index (PI), morphology, surface charge, EE, and drug content (DC). The size and PI were determined by photon correlation spectroscopy using a Zetasizer® 5000 (Malvern Instruments, Malvern, UK). Before measurements, the lyophilized powders were diluted using purified water. The surface charge (zeta potential) was measured using the electrophoretic mode with the Zetasizer 5000. The lyophilized powder was dispersed in water. The pH of the suspension was measured at room temperature (28°C). Morphology was evaluated by transmission electron microscopy (TEM) (Morgagni 268; FEI, Hillsboro, OR). Lyophilized powder was dispersed in water and 5 μL was transferred to a grid. Uranyl acetate solution (3%) was added for fixing and contrast. After 30 sec, excess liquid was removed using filter paper (Whatman®; Maidstone, Kent, UK). Grids were placed in vacuum desiccators (Pyrex®; Corning, Lowell, MA) before analysis.
The drug extraction method from NPs was patronized for determination of the EE and DC. The lyophilized powder was added in hot acetone (45°C) for disruption of the NPs and dissolution of the photosensitizer. The organic solution was sonicated for 10 min using an ultrasonic bath (T14; Thornton, Vinhedo, São Paulo, Brazil). The organic solution was diluted in phosphate-buffered saline (PBS) containing 2% SDS to precipitate the polymer (PCL) and to extract the photosensitizer. The suspension was centrifuged, filtered through a membrane filter (0.45 μm, Millex-FH50 filter unit; Millipore, Billerica, MA) to remove the PCL, and quantified by fluorescence emission. The photosensitizer was diluted in PBS containing 2% SDS at concentrations of 20–200 ng/mL. The solutions were excited at 610 nm, and fluorescence emission was measured at 680 nm using a Jasco FP-750 fluorimeter (Jasco Corporation, Tokyo, Japan). The determination coefficient (R2) exceeded 0.999, with excellent linearity. Intraday and interday precision and accuracy of the method showed a variation coefficient and a relative error (E) not >2.3% and 3.1%, respectively.
EE was calculated from EquationEq. 2(2) :
where EE(%) is the encapsulation efficiency, M3 is the mass of ZnPc measured in lyophilized powder, and M4 is the mass of photosensitizer used in formulation.
DC in the NPs (μg ZnPc/mg NP) was calculated from EquationEq. 3(3) :
where DC is the drug content, M5 is the mass (μg) of ZnPc measured in lyophilized powder, and M6 is the mass (mg) of the lyophilized NP.
In vitro releasing studies and kinetic
A known amount of NPs (50 mg NP containing 1 mg of ZnPc) was dispersed in 60 mL of PBS containing 0.5% SDS, with pH 7.4, kept at 37°C in a thermostatic bath (M214M2; Quimis, Diadema, São Paulo, Brazil). The acceptor solution was stirred with a magnetic stirrer at a constant rate of 300 rpm using a stirrer (M15; Marte, Santa Rita do Sapucaí, Minas Gerais, Brazil). At given time intervals, six samples (n = 6) of 3 mL were withdrawn and centrifuged at 20,000 g for 20 min. The precipitates were resuspended in 3 mL of fresh medium and placed in the respective dissolution vessels. The released photosensitizer was quantified by fluorescence emission. The in vitro release profile was obtained by correlating time (h) versus drug release (%).
After 240 h of release studies, the acceptor solution containing the NPs was removed from the dissolution vessel and centrifuged thrice at 20,000 g for 20 min to recover the nanostructured system. The NPs were lyophilized, and the photosensitizer content was determined by fluorescence emission. The extraction and quantification of the photosensitizer of the lyophilized NPs have been described in the ‘Characterization’ section.
The release data were fitted using the mathematical models of zero order, first order, Higuchi, Hixon–Crowell, square root of mass, three seconds root of mass, and Baker–Lonsdale.Citation36,Citation37 Equations of the mathematical modeling are shown in . The data were fitted, and the linear regression was assessed. The correlation coefficient (r) and R2 were compared to determine the mathematical model that best fits the release data.
Table 1 Mathematical models used for linearization of the photosensitizer release profile data
In vitro photobiological activity
The human lung adenocarcinoma cell line (A549)Citation38 was chosen for evaluation of the photobiological activity. The cells were thawed and placed in DMEM supplemented with 10% FBS. Penicillin (100 units/mL) and streptomycin (100 μg/mL) were added to the culture medium. The medium was maintained at 37°C in a 5% CO2 atmosphere for 48 h. Cell concentrations were determined by the exclusion test of trypan blue using a Neubauer counting chamber. Aliquots of 1 × 105 cells/mL were placed into 96-well dishes containing 250 μL of culture medium. The cell culture was incubated for 24 h at 37°C in a 5% CO2 atmosphere before examination of the toxicity and phototoxicity of the free photosensitizer, and the photosensitizer was loaded in NPs.
An NP suspension was prepared in culture medium (DMEM) at 1 mg/mL. The cells were washed with PBS and incubated with 250 μL of the NP suspension (dose = 5 μg of ZnPc) at 37°C in a 5% CO2 atmosphere for 4 h. After incubation, the cells were washed twice with PBS and fresh culture medium (250 μL) was added in each well. Finally, after cellular uptake of the free ZnPc or ZnPc-loaded NPs, the cells were exposed to red light (660 nm) for 90 sec, with a light dose of 100 J/cm2 (Photon Lase I; DMC, São Carlos, São Paulo, Brazil). After light exposure, the cell culture was incubated for 24 h at 37°C in a 5% CO2 atmosphere, and the cellular viability was determined by MTT assay. The NP suspension without photosensitizer (empty NPs), control (PBS) solution, and photosensitizer solution were also assessed in the phototoxicity studies. The dark toxicity was also assessed with the same samples. The photobiological activity of the light was evaluated. The cell culture was exposed to red light (660 nm) with light dose of 0–100 J/cm2, and cell viability was determined by the MTT assay. Briefly, after removing the cell medium, 50 μL of MTT solution (1 mg/mL) was added to each well and incubated for 3 h.Citation39 Then, the formazan crystals were dissolved with 100 μL of 10% SDS in 0.01 M HCl in each well.Citation39 The absorbance was determined at 595 nm by a microplate reader. For each sample, the cellular viability was calculated from the data of 10 wells (n = 10) and expressed as a percentage, compared with the untreated cells (100%). Comparison of the mean optical density between the untreated (100%) and treated cells 24 h after illumination allowed the evaluation of the phototoxicity.
MB was used as a positive control of the photobiological activity. The influence of different parameters on the in vitro phototoxicity was investigated by varying the time (1–6 h), concentration of photosensitizer (0–10 μg), and light dose (0–100 J/cm2).
Morphological evaluation
Aliquots of 1 × 105 cells/mL were placed in 24-well dishes containing culture medium. A coverslip was placed on the bottom of the well for growth of cells in monolayer. The cell culture was incubated for 24 h at 37°C in a 5% CO2 atmosphere before phototoxicity assay. The cells were washed with PBS and incubated with NPs suspension (dose = 5 μg of ZnPc) (at 37°C in a 5% CO2 atmosphere) for 4 h. After incubation, the cells were washed twice with PBS. Culture medium was added in each culture plate. Finally, the cells were exposed to red light (660 nm) for 90 sec (light dose of 100 J/cm2) and incubated for 24 h (at 37°C in a 5% CO2 atmosphere) before microscopic analysis. The empty NPs and control solution (PBS) were also assessed.
For microscopic analysis, the cells were fixed in Bouin’s liquid for 5 min and washed with alcohol (70%, v/v) and water to remove the fixative solution. The cells were stained with Giemsa’s liquid for 2 h.Citation40 Then, the cells were treated and dehydrated with glacial acetic acid, acetone, acetone–xylene (7:3, v/v), acetone–xylene (1:1, v/v), acetone–xylene (3:7, v/v), and xylene. The coverslips were dried and mounted on glass slides for observation under light microscope (DML30; Leica, Bannockburn, IL). The morphology was evaluated for detection of changes in the cytoplasm and nucleus.
1O2 detection by photobleaching
The 1O2 generation was determined by photobleaching of the chemical probe ABMDMA. It is a water-soluble derivative of anthracene that can react with 1O2 to yield an endoperoxide, which causes a decrease in the ABMDMA absorption.Citation41 The reaction was monitored spectrophotometrically by recording the decrease in absorption at 400 nm (wavelength of maximum absorption). In this study, 0.15 mM of the sodium salt of ABMDMA in water was mixed with 5 μg of the encapsulated ZnPc or free ZnPc. The empty NPs were used as control. The solution was irradiated with red light (660 nm) (Photon Lase I; DMC) in an open quartz cell with continuous stirring. The absorbance measurements followed by irradiation were carried out every 60 sec.
Statistical analysis
The encapsulation method, process yield, size, PI, surface charge, EE, DC, and 1O2 detection by photobleaching were accomplished in triplicate (n = 3). The in vitro release studies and kinetics were carried out to six determinations (n = 6). The cellular viability was calculated from the data of 10 wells (n = 10) and expressed as a percentage, compared with the untreated cells (100%). All results were expressed as the means ± standard deviation. The statistical comparisons of the photobiological activity were performed using Student’s t-test. The differences were considered significant at a level of P < 0.05.
Results
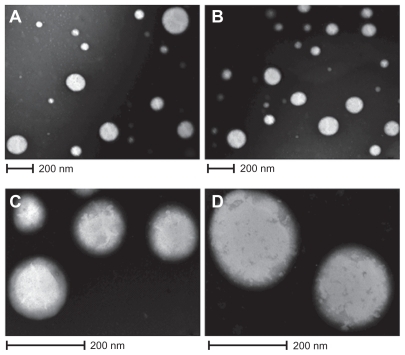
The process yields of the empty NPs and ZnPc-loaded NPs were 73.5% ± 1.8% and 74.2% ± 1.2% (w/w), respectively. The EE of ZnPc in PCL NPs was about 67.1% ± 0.9%. Besides, the ZnPc content of NPs was 20.1 ± 0.07 μg/mg. The ZnPc-loaded NPs had an average size of 187.4 ± 2.1 nm. Compared with empty NPs (179 ± 1.5 nm), photosensitizer-loaded NPs had a larger size. IP of the empty NPs and ZnPc-loaded NPs was of 0.102 ± 0.02 and 0.096 ± 0.004, respectively. The empty NPs and ZnPc-loaded NPs exhibited negative zeta potential of −3.4 ± 0.08 and −4.85 ± 0.21 mV, respectively. The morphology of the particles was examined by TEM and is shown in . The ZnPc-loaded NPs were spherical (). shows the PCL NPs, which were isolated and magnified. are photomicrographs of empty NPs.
Figure 1 Photographs of TEM. A) and C) ZnPc-loaded PCL nanoparticles; B) and D) empty nanoparticles.
Abbreviations: TEM, transmission electron microscopy; ZnPc, zinc phthalocyanine; PCL, poly-ɛ-caprolactone.
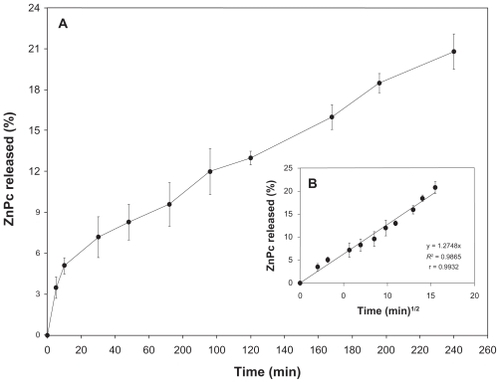
The in vitro release studies are shown in . ZnPc has a sustained release. Besides, 22% of the photosensitizer was released from the NPs in 240 h.
Figure 2 A) In vitro releasing profile of the ZnPc from nanostructured system. B) Graphic representation of the Higuchi model obtained after regression. Mean and SD of n = 6 determinations.
Abbreviations: ZnPc, zinc phthalocyanine; SD, standard deviation.
After in vitro release studies, the remaining content (%) of ZnPc in the PCL NPs was assessed to compare with released photosensitizer in the acceptor solution.
Table 2 Released drug in the acceptor solution and remaining content of photosensitizer in the nanoparticles
Several mathematical models were used to investigate the mechanism of drug release from PCL NPs. Thus, linear regression of the release data was performed using the mathematical models of zero order, first order, Higuchi, Hixon–Crowell, square root of mass, three seconds root of mass, and Baker–Lonsdale. The R2 and r values obtained after linear regression are shown in .
Table 3 Determination coefficient (R2) and correlation coefficient (r) obtained after linear regression of the release data
Kinetic models were established to describe the release kinetics and understand the mechanism of drug release from delivery systems.Citation36,Citation37 As shown in and , the Higuchi model gave the highest value of R2 and r, indicating that this mathematical model was the most suitable for describing the release of the photosensitizer from PCL NPs. These results suggest that the release of ZnPc from PCL NPs is controlled by diffusion.
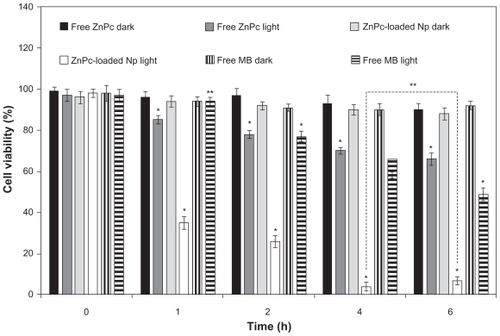
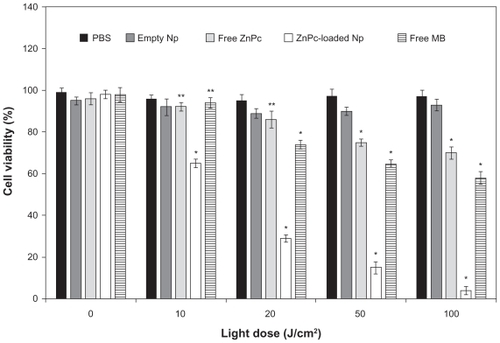
The photobiological activity at different incubation times of free ZnPc, free MB, and ZnPc-loaded NPs is shown in . No toxicity was observed in the dark. The phototoxicity was time dependent up to 4 h. No significant difference (P > 0.05) in the phototoxicity of the encapsulated ZnPc was observed for the incubation time of 4 and 6 h. Thus, the incubation time of 4 h was chosen for other phototoxicity studies (influence of the light dose and concentration). The cell viability of the free ZnPc slowly decreased in the range 1–6 h. The photobiological activity of the MB was observed after 2 h of incubation with activity maximum in 6 h. ZnPc-loaded NPs exhibited phototoxicity significantly higher than free photo-sensitizers (P < 0.05) ().
Figure 3 Influence of the incubation time (h) in the phototoxicity of free ZnPc or ZnPc-loaded nanoparticles. The cells were incubated with 5 μg (250 μL) of free ZnPc and ZnPc-loaded nanoparticles at different times, washed, and irradiated at a light dose of 100 J/cm2. Dark toxicity was studied for all samples. The MTT assay was performed 24 h after light exposure. Each data point represents the mean (±SD) of n = l0 determinations.
Notes: *Significant difference (P < 0.05), **No significant difference (P > 0.05).
Abbreviations: ZnPc, zinc phthalocyanine; MTT, 3-(4,5-dimethyl-thiazol-2-yl)-2,5-biphenyl tetrazolium bromide; MB, methylene blue; SD, standard deviation.
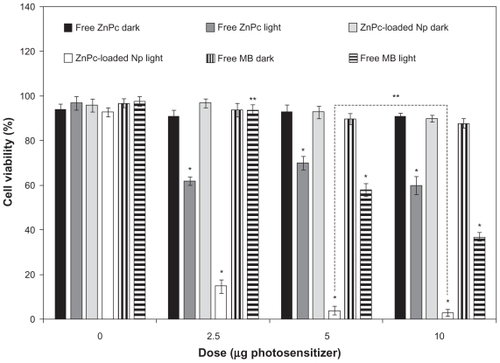
The photobiological activity of different concentrations of ZnPc-loaded NPs was evaluated in cell cultures and compared with the free photosensitizers. MB was used as control. No toxicity was observed in the dark. In the assays without photosensitizer, ZnPc-loaded NPs and free photosensitizer were replaced by empty NPs and PBS, respectively. The cell viability decreased with the increase in drug concentration (). No significant difference (P > 0.05) in the photobiological activity of the encapsulated ZnPc was observed for the concentrations of 5 and 10 μg/mL. The phototoxicity of the free ZnPc was not concentration dependent (). The phototoxicity of the MB was observed only in 5 and 10 μg/mL. ZnPc-loaded NPs exhibited phototoxicity significantly higher than free photosensitizers (P < 0.05) ().
Figure 4 Influence of the photosensitizer concentration in the photobiological activity of free ZnPc, free MB, or ZnPc-loaded nanoparticles. The cells were incubated with 250 μL of free photosensitizer (ZnPc or MB) and ZnPc-loaded nanoparticles for 4 h, washed, and irradiated at a light dose of 100 J/cm2. Dark toxicity was studied for all samples. The MTT assay was performed 24 h after light exposure. Each data point represents the mean (±SD) of n = 10 determinations.
Notes: *Significant difference (P < 0.05), **No significant difference (P > 0.05).
Abbreviations: ZnPc, zinc phthalocyanine; MB, methylene blue; MTT, 3-(4,5-dimethyl-thiazol-2-yl)-2,5-biphenyl tetrazolium bromide; SD, standard deviation.
The influence of the light dose (J/cm2) on the photobiological activity was evaluated using 5 μg of ZnPc (volume of 250 μL) and incubation time of 4 h. No toxicity was observed in the dark (without irradiation). Photobiological activity of the ZnPc-loaded NPs was observed at 10 J/cm2. The light dose increase was responsible for the improvement of the biological effect. The free ZnPc exhibited photobiological activity in the light dose of 50–100 J/cm2. Phototoxicity of the MB was observed at 20–100 J/cm2. Besides, ZnPc-loaded NPs exhibited a phototoxic effect significantly higher than free photosensitizers (P < 0.05) ().
Figure 5 Influence of light dose on phototoxicity of free ZnPc and ZnPc-loaded nanoparticles. The cells were incubated for 4 h, at an equivalent drug dose of 5-pg ZnPc (250 μL), washed, and irradiated with red light (660 nm). MTT assay was performed 24 h after light exposure. Each data point represents the mean (±SD) of n = 10 determinations.
Notes: *Significant difference (P < 0.05), **No significant difference (P > 0.05).
Abbreviations: ZnPc, zinc phthalocyanine; MTT, 3-(4,5-dimethyl-thiazol-2-yl)-2,5-biphenyl tetrazolium bromide; SD, standard deviation.
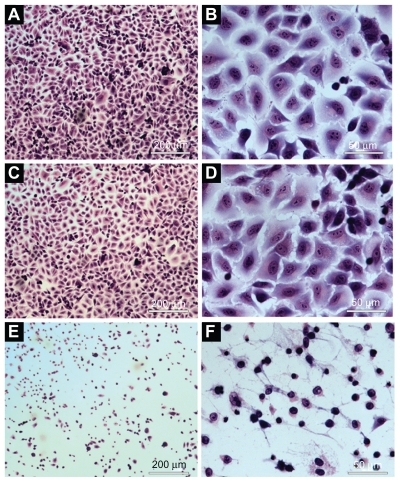
No morphological change was observed in cells treated with saline (control group) () and empty NPs (no photosensitizer) () after illumination with visible light (red light, 660 nm, for 90 sec, with light dose of 100 J/cm2). The cells incubated with NPs containing ZnPc and irradiated with visible light showed morphological changes. Morphological alterations of the cell induced by photobiological action of ZnPc included the following changes: cytoplasmic condensation with significant reduction of cell volume, rarified cytoplasmic matrix, extensive cell lysis, and loss of plasma membrane extensions ().
Figure 6 Morphological evaluation of the A549 cells using light microscope: A) and B) cells incubated with PBS (control). C) and D) cells incubated with empty nanoparticles suspension. E) and F) cells incubated with ZnPc-loaded nanoparticles. (A), (C), and (E) were observed at magnification of ×50 (scale 200 μm). (B), (I), and (F) were observed at magnification of ×400 (scale 50 μm).
Abbreviations: PBS, phosphate-buffered saline; ZnPc, zinc phthalocyanine.
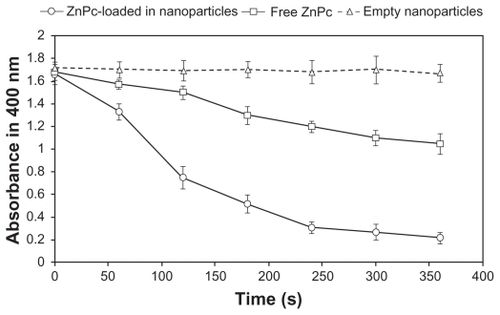
As shown in , the chemical measurements showed a significant decrease in the absorbance at 400 nm for ZnPc-loaded NPs with ABMDMA after irradiation, suggesting a high efficiency in the generation of 1O2 (). For the free ZnPc, the photobleaching caused by 1O2 was lower than that of ZnPc-loaded NPs. There was no decrease in the absorbance for a sample containing ABMDMA and empty NPs ().
Figure 7 Photobleaching of ABMDMA by singlet oxygen generated by encapsulated ZnPc and free ZnPc. Empty nanoparticles were used as control. The change in ABMDMA absorption at 400 nm was measured as a function of the irradiation time. Each data point represents the mean (±SD) of n = 3 determinations.
Abbreviations: ABMDMA, 9,10-anthracenediyl-bis(methylene)dimalonic acid; ZnPc, zinc phthalocyanine; SD, standard deviation.
Discussion
NPs have been used as a possible method to target and control drug delivery, and prevent the side effects associated with undesirable biodistribution of the free form of drugs.Citation11,Citation15 Moreover, there are many options for modifying the biodistribution of NPs by appropriate selection of the polymer, surface modification (hydrophilic coating), and particle size.Citation11 Among the morphofunctional features of solid tumors are the abnormal function of the lymphatic vasculature, which causes fluid retention, and the increased permeability of blood vessel walls. Both these characteristics lead to a higher retention of NPs in the tumor area in comparison with normal tissues, resulting in a passive selectivity of the system.Citation42 Thus, nanometric size, which is directly related to the technique of NPs obtention, is crucial for the desired tissue penetration.Citation40
The size of the NPs obtained depends on many factors such as the stirring speed, solution viscosity, organic/aqueous phase ratio, and surfactant concentration in the external aqueous phase. In this study, the use of solvent emulsification-evaporation method (SEEM) associated with sonication produced the photosensitizer-loaded NPs of appropriate size. NPs containing photosensitizer were produced with success using PCL by SEEM. The encapsulation method produced particles in nanometric scale (<200 nm) with narrow size distribution (IP < 0.1), spherical shape, and excellent EE (>60%). The low release depends on the nature of both the polymer and the encapsulated molecule.Citation38 ZnPc presented a low and sustained release. The release data were fitted using the mathematical models of zero order, first order, Higuchi, Hixon–Crowell, square root of mass, three seconds root of mass, and Baker–Lonsdale. Zero-order kinetics can be used to describe the drug release from several types of delivery systems such as matrix tablets for drugs with low solubilityCitation44 and osmotic systems where drug release would be directly proportional to time. The first-order model describes the release profile from the delivery systems containing drugs dispersed in porous matrices where drugs would be released at rates proportional to the amount of drug remaining in the interior of the delivery system.Citation36 Hixon and Crowell recognized that the particle regular area is proportional to the cubic root of its volume.Citation45 This relationship between area and volume plus the release and remaining amount of the drug can influence the release kinetics. The shape factor for cubic or spherical particles should be kept constant if the particles are dissolved in an equal manner on all sides. When this model is used, it is assumed that the release rate is limited by the drug particle dissolution rate and not by the diffusion that might occur through the polymeric matrix.Citation45 The Baker–Lonsdale model was developed from the Higuchi model and describes drug controlled release from a spherical matrix.Citation46 This mathematical model has been used for linearization of release data from several formulations of microparticles and NPs. The mathematical models of squared root and three seconds root of mass were also tested in the release kinetic study.Citation36,Citation37 The Higuchi model has been based on the Fick’s law where the release occurs by the diffusion of drugs within the delivery system.Citation36,Citation47 In this case, the released amount of the drug is proportional to the square root of time. The Higuchi model gave the highest value of R2 and r, indicating that this mathematical model was the most suitable for describing the release of the photosensitizer from PCL NPs (). These results suggest that the release of ZnPc from PCL NPs is controlled by diffusion. From these results, the mechanism of the photosensitizer release from PCL NPs can be considered as follows: 1) Water penetrates into polymeric matrix of the NPs through pores, slowly dissolving the photosensitizer. 2) ZnPc is released by diffusion into acceptor solution. 3) The photosensitizer is hydrophobic and accumulates into SDS micelles.
The in vitro phototoxicity studies showed that the ZnPc-loaded NPs were more phototoxic than the free ZnPc (–). The photobiological activity is highly dependent on the photosensitizer’s cellular uptake mechanism and its subcellular localization. In aqueous medium, a free lipophilic photosensitizer is generally absorbed by diffusion across the plasmatic membrane (lipophilic) leading to a low intracellular concentration. Moreover, lipophilic photosensitizers such as ZnPc tend to aggregate in aqueous medium with reduction of its photodynamic activity.Citation48 Due to its instability in water, the photobiological activity of the free ZnPc was not concentration dependent (). The MB was less phototoxic than the ZnPc-loaded NPs (P < 0.05) (–). MB is hydrophilic and has difficulty in crossing the lipophilic membrane of the cells, leading to a low intracellular concentration. However, NPs are able to enter the cells by endocytosis, releasing the photosensitizer in the cell cytoplasm. The intracellular localization of the photosensitizer, an important factor in PDT, can induce different damage in the cells. Due to its hydrophobic characteristics, intracellular ZnPc is able to accumulate in the cytoplasmic membrane, lysosomal compartment, and mitochondria. Intracellular generation of 1O2 and additional free radicals can act as potent cytotoxic agents.
The 1O2 is the main toxic species generated by phtha-locyanines during PDT.Citation49 The photobleaching studies () are in agreement with the photobiological activity results (–). ZnPc-loaded NPs generated 1O2 more efficiently than free ZnPc (). The free ZnPc suffers aggregation in water leading to a low 1O2 generation. However, encapsulation in NPs protected the ZnPc from the aggregation in aqueous medium. Due to slow release (), many ZnPc molecules can remain adsorbed on the surface of the NPs. The adsorbed photosensitizer can be activated by red light leading to a strong 1O2 generation. The photobleaching data are consistent with those obtained by Zhao et al.Citation50 The 1O2 generation was improved after encapsulation of the lipophilic photosensitizer (silicon phthalocyanine (SiPc)) in silica NPs. SiPc is a lipophilic photosensitizer. It aggregates in aqueous solution, which reduces its therapeutic efficacy and limits its clinical use.Citation50 Zhao et al have encapsulated SiPc using silica NPs, which not only improves the aqueous stability but also increases its 1O2 generation and photobiological activity compared with free SiPc.Citation50 SiPc-loaded silica NPs generated photo-induced 1O2 more efficiently than free SiPc.Citation50
The results obtained from in vitro phototoxicity studies demonstrated that the phototoxicity of ZnPc against the A549 cells occurred after 24 h of light exposure because the cellular damage resulting from the photochemical reaction is not immediately lethal. This phenomenon could be due to an apoptotic response, which is a slow process.Citation5 Cell death by necrosisCitation3 may occur on a smaller scale. Necrosis is a drastic process of death that results in cell lysis.
Conclusion
Nanotechnology is bringing new perspectives to a number of fields of human knowledge. Its applications in cancer treatment by PDT are being investigated largely with excellent results. The nanoencapsulation was suitable for the preparation of PCL NPs because the release system has nanometric size, narrow distribution, regular shape, and sustained release. Achieving optimal effect of PDT on tumor cells requires that the photosensitizer becomes closely associated to the subcellular organelles. Encapsulation of ZnPc in the PCL NPs improved its stability in aqueous medium and the in vitro phototoxicity. The photobiological activity was influenced by photodynamic parameters such as incubation time, light dose, and drug concentration. Based on all the photobiological measurements, it can be concluded that the ZnPc-loaded PCL NPs are promising delivery systems for use in PDT.
Acknowledgments
We thank the financial support of Conselho Nacional d Desenvolvimento Científico e Tecnológico (CNPq) and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ).
Disclosure
The authors report no conflict of interest in this work.
References
- AllisonRRDownieGHCuencaRHuXHChildsCJHSibataCHPhotosensitizers in clinical PDTPhotodiagnosis Photodyn Ther200412742
- BechetDCouleaudPFrochotCViriotMLGuilleminFBarberi-HeyobMNanoparticles as vehicles for delivery of photodynamic therapy agentsTrends Biotechnol2008261161262118804298
- CastanoAPDemidovaTNHamblinMRMechanisms in photodynamic therapy: Part three – Photosensitizer pharmacokinetics, biodistribution, tumor localization, and modes of tumor destructionPhotodiagnosis Photodyn Ther20052291106
- ChatterjeeDKFongLSZhangYNanoparticles in photodynamic therapy: an emerging paradigmAdv Drug Deliv Rev200860151627163718930086
- ZhouCShunjiCJinshengDJunlinLJoriGMilanesiCApoptosis of mouse MS-2 fibrosarcoma cells induced by photodynamic therapy with Zn (II)-phthalocyanineJ Photochem Photobiol B19963332192238683397
- BonnettRPhotosensitizers of the porphyrin and phthalocyanine series for photodynamic therapyChem Soc Rev199526371933
- SavolainenJvan der LindenDDijkhuizenNHerekJLCharacterizing the functional dynamics of zinc phthalocyanine from femtoseconds to nanosecondsJ Photochem Photobiol A Chem2008196199105
- ZanfolimAAVolpatiDOlivatiCAJobAEConstantinoCJLStructural and electric-optical properties of zinc phthalocyanine evaporate in films: temperature and thickness effectsJ Phys Chem2010114281229012299
- Ricci-JúniorEMarchettiJMPreparation, characterization, photocytotoxicity assay of PLGA nanoparticles containing zinc (II) phthalocyanine for photodynamic therapy useJ Microencapsul200623552353816980274
- WainwrightMPhoenixDARiceLBurrowSMWaringJIncreased cytotoxicity and phototoxicity in the methylene blue series via chromophore methylationJ Photochen Photobiol B1997403233239
- HansMLLowmanAMBiodegradable nanoparticles for drug delivery and targetingCurr Opin Solid State Mater Sci200264319327
- CamerinMMagaraggiaMSoncinMThe in vivo efficacy of phthalocyanine–nanoparticle conjugates for the photodynamic therapy of amelanotic melanomaEur J Cancer201046101910191820356732
- MurthySKNanoparticles in modern medicine: state of the art and future challengesInt J Nanomedicine20072212914117722542
- De JongWHBormPJDrug delivery and nanoparticles: applications and hazardsInt J Nanomedicine20083213314918686775
- SoppimathKSAminabhaviTMKulkarniARRudzinskiWEBiodegradable polymeric nanoparticles as drug delivery devicesJ Control Release2001701212011166403
- UngunBPrud’hommeRKBudijonSJNanofabricated upconversion nanoparticles for photodynamic therapyOpt Express2009171808619129875
- AllisonRRMotaHCBagnatoVSSibataCHBio-nanotechnology and photodynamic therapy–state of the art reviewPhotodiagnosis Photodyn Ther200851192819356632
- KonanYNBertonMGurnyRAllemannEEnhanced photodynamic activity of meso-tetra(4-hydroxyphenyl)porphyrin by incorporation into sub-200 nm nanoparticlesEur J Pharm Sci2003183424124912554067
- KonanYNChevallierJGurnyRAllemannEEncapsulation of p-THPP into nanoparticles: cellular uptake, subcellular localization and effect of serum on photodynamic activityPhotochem Photobiol200377663864412870850
- GomesAJLunardiLOMarchettiJMLunardiCNTedescoACPhotobiological and ultrastructural studies of nanoparticles of poly(lactic-co-glycolic acid)-containing bacteriochlorophyll-a as a photosensitizer useful for PDT treatmentDrug Deliv200512315916416025845
- Konan-KouakouYNBochRGurnyRAllemannEIn vitro and in vivo activities of verteporfin-loaded nanoparticlesJ Control Release20051031839115710502
- TangWXuHKopelmanRPhilbertMAPhotodynamic characterization and in vitro application of methylene blue-containing nanoparticle platformsPhotochem Photobiol200581224224915595888
- Zeisser-LabouebeMLangeNGurnyRDelieFHypericin-loaded nanoparticles for the photodynamic treatment of ovarian cancerInt J Pharm20063261217418116930883
- AllemannERousseauJBrasseurNKudrevichSVLewisKvan LierJEPhotodynamic therapy of tumours with hexadecafluoro zinc phthalocyanine formulated in PEG-coated poly(lactic acid) nanoparticlesInt J Cancer19966668218248647656
- NairLSLaurencinCTBiodegradable polymers as biomaterialsProg Polym Sci20073289762798
- SinhaVRBansalKKaushikRKumriaRTrehanAPoly-ɛ-caprolactone microspheres and nanosperes: an overviewInt J Pharm2004278112315158945
- KumariAYadavSKYadavSCBiodegradable polymeric nanoparticles based drug delivery systemsColloids Surf B Biointerfaces201075111819782542
- vergerMLFluckigerLKimYIHoffmanMMaincentPPreparation and characterization of nanoparticles containing an antihypertensive agentEur J Pharm Biopharm19984621371439795032
- FerrantiVMarchaisHChabenatCOrecchioniAMLafontOPrimidone-loaded poly-ɛ-caprolactone nanocapsules: incorporation efficiency and in vitro release profilesInt J Pharm1999193110711110581427
- KimSYLeeYMTaxol-loaded block copolymer nanospheres composed of methoxy poly(ethylene glycol) and poly(epsilon-caprolactone) as novel anticancer drug carriersBiomaterials200122131697170411396872
- ZiliZSfarSFessiHPreparation and characterization of poly-ɛ-caprolactone nanoparticles containing griseofulvinInt J Pharm20052941226126715814226
- ShenoyDBAmijiMMPoly(ethylene-oxide)-modified poly(ɛ-caprolactone) nanoparticles for targeted delivery of tamoxifen in breast cancerInt J Pharm20052931226127015778039
- BlouzaILCharcossetCSfarSFessiHPreparation and characterization of spironolactone-loaded nanocapsules for pediatric useInt J Pharm200632512124131
- PrabuPChaudhariAADharmarajNKhilMSParkSYKimHYPreparation, characterization, in-vitro drug release and cellular uptake of poly(caprolactone) grafted dextran copolymeric nanoparticles loaded with anticancer drugJ Biomed Mater Res200990411281136
- ZhengDLiXXuHLuXHuYFanWStudy on docetaxel-loaded nanoparticles with high antitumor efficacy against malignant melanomaActa Biochim Biophys Sin200941757858719578721
- CostaPSousa LoboJMModeling and comparison of dissolution profilesEur J Pharm Sci200113212313311297896
- Barzegar-JalaliMAdibkiaKValizadehHKinetic analysis of drug release from nanoparticlesJ Pharm Pharm Sci200811116717718471378
- LieberMSmithBSzakalANelson-ReesWTodaroGA continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cellsInt J Cancer19761716270175022
- MosmannTRapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assaysJ Immunol Methods1983651255636361138
- VeigaVFNimrichterLTeixeiraCAExposure of human leukemic cells to direct electric current: generation of toxic compounds inducing cell death by different mechanismsCell Biochem Biophys2005421617415673929
- LindigBARodgersMAJSchaapAPDetermination of the lifetime of singlet oxygen in D2 using 9,10-anthracenedipropionic acid, a water-soluble probeJ Am Chem Soc19801021755905593
- AlexisFRheeJWRichieJPRadovic-MorenoAFLangerRFarokhzadOCNew frontiers in nanotechnology for cancer treatmentUrol Oncol2008261748518190835
- KongGBraunRDDewhirstMWHyperthermia enables tumor-specific nanoparticle delivery: effect of particle sizeCancer Res200060164440444510969790
- TanakaNImaiKOkimotoKDevelopment of novel sustained-release system, disintegration-controlled matrix tablet (DCMT) with solid dispersion granules of nilvadipineJ Control Release200510823386395
- HixsonAWCrowellJHA simple model based on first order kinetics to explain release of highly waterInd Eng Chem Res193123923931
- BakerRWLonsdaleHSControlled Release of Biologically Active AgentsTanquaryACLaceyRENew York, NYPlenum Press1974
- HiguchiTMechanism of sustained-action medication: theoretical analysis of rate of release of solid drugs dispersed in solid matricesJ Pharm Sci1963521145114914088963
- IseleUSchieweckKKesslerRvan HoogevestPCapraroHGPharmacokinetics and body distribution of liposomal zinc phthalocyanine in tumor-bearing mice: influence of aggregation state, particle size, and compositionJ Pharm Sci19958421661737738795
- AllenCMSharmanWMvan LierJECurrent status of phthalocyanines in the photodynamic therapy of cancerJ Porphyr Phthalocyanines20015161169
- ZhaoBYinJJBilskiPJChignellCFRobertsJEHeYYEnhanced photodynamic efficacy towards melanoma cells by encapsulation of Pc4 in silica nanoparticlesToxicol Appl Pharmacol2009241216317219695274