Abstract
Background
Hemozoin, a chemical analog of a malarial pigment, is a crystal composed of heme dimers that can act as a potent Th1-type adjuvant, which strongly induces antibody production. However, the clinical applications of malarial hemozoin have limitations due to biosafety concerns and difficulties in the manufacturing process. Based on the premise that an analog of the heme polymer might display immunostimulatory effects, a hemin-containing polymer was developed as a novel immunostimulator.
Materials and methods
To synthesize the copolymer containing hemin and N-isopropylacrylamide (NIPAM), this study employed a conventional radical polymerization method using 2,2′-azodiisobutyronitrile as the radical initiator; the synthesized copolymer was designated as NIPAM-hemin.
Results
NIPAM-hemin was soluble and showed no cytotoxicity in vitro. The NIPAM-hemin copolymer induced the production of interferon (IFN)-γ and interleukin (IL)-6 from peripheral blood mononuclear cells, although hemin and the NIPAM monomer individually did not induce the production of any cytokines. The production of IFN-γ induced by NIPAM-hemin was independent of toll-like receptor 9 and the NLRP3 inflammasome pathway.
Conclusion
Given that NIPAM-hemin induced IL-6 and IFN-γ production in immune cells without any cytotoxic effects, NIPAM-hemin has potential therapeutic applications as a Th1-type adjuvant.
Introduction
An adjuvant is a vaccine component that can improve the immune response to a greater extent than an antigen alone. Aluminum hydroxide crystal (alum) is the most commonly used adjuvant worldwide, but alum-containing vaccines occasionally cause local reactions such as swelling and pain at the injection site due to inflammation; in rare instances, this can lead to severe systemic symptoms.Citation1 Thus, there is a high demand for the development of a new adjuvant that is safe, affordable, and has high adjuvanticity with a variety of antigens.
Coban et alCitation2 reported that hemozoin, a biocrystal synthetized by the Plasmodium parasite, can act as a potent adjuvant molecule. Hemozoin is a heme crystal produced during malaria infection, and is responsible for the induction of a strong inflammatory response mediated by toll-like receptor 9 (TLR9) activation. A synthetic form of hemozoin known as β-hematin can be chemically synthesized from hemin chloride by acid catalysis,Citation3 and also exhibits adjuvant effects.Citation4,Citation5 Injecting house dust mite allergens with alum was shown to induce Th2 responses in vivo; however, Th1 responses were induced when the allergens were co-administered with β-hematin crystals.Citation6 These results showed that hemozoin and β-hematin can induce Th1 immune responses. However, the use of malarial hemozoin is limited by issues regarding its biosafety and mass production. Additionally, precise control of the size distribution of β-hematin by crystallization remains problematic, since the immunostimulatory effect of β-hematin depends on its crystal size.Citation6 Accordingly, a procedure for the controlled and optimized production of uniformly sized β-hematin is needed.
Hemin binds to TLR9 and changes its conformation in a manner similar to that when bound to β-hematin.Citation2 However, the hemin monomer shows no adjuvant effects.Citation2 Ohto et alCitation7 reported that the TLR9 signaling complex is formed by cooperative receptor dimerization. We predicted that the co-orientation of multiple hemin molecules is required for this conformational change in TLR9, which culminates in immune stimulation. Hemin contains two vinyl groups, and can polymerize radically with other acrylic monomers.Citation8 Therefore, polymers containing hemin can easily be synthe-sized, and aspects of its molecular structure and other specific properties can be optimized.
In this study, we synthetized a novel hemin-containing copolymer and evaluated its stimulatory effects in vitro. The copolymer was synthesized using hemin and N-isopropylacrylamide (NIPAM), and was highly soluble in aqueous solution. This hemin-containing copolymer induced interferon (IFN)-γ and interleukin (IL)-6 production from peripheral blood mono-nuclear cells (PBMCs) containing various immune cells, such as B cells, T cells, natural killer (NK) cells, macrophages, and dendritic cells. Our findings suggest that hemin-containing copolymers exhibit immunostimulatory activity, and could induce Th1-type responses, thereby acting as a potent type I adjuvant.
Materials and methods
Reagents
Hemin (ferriprotoporphyrin IX chloride) was purchased from Sigma Aldrich (St Louis, MO, USA). NIPAM and 2,2′-azodiisobutyronitrile (AIBN) were obtained from Wako Pure Chemical Industries (Osaka, Japan). The pNifty-Luc plasmid, which contains the firefly luciferase gene under the nuclear factor (NF)-κB-inducible element; the transfection reagent LyoVec; the alkaline phosphatase substrate QUANTI-Blue; and lipopolysaccharide (LPS) were purchased from InvivoGen (San Diego, CA, USA). The pGL4.74 [hRluc/TK] plasmid, which encodes the renilla luciferase gene, as well as a dual-luciferase reporter assay system, were purchased from Promega (Madison, WI, USA). A cell counting kit-8 (CCK-8) was used to assay cytotoxicity, and was purchased from Dojindo (Kumamoto, Japan). The phosphorothioated CpG oligodeoxynucleotide (ODN) 2006 PT (5′-TCGTCGTTTTGTCGTTTTGTCGTT-3′) was synthesized by Eurofins Genomics (Tokyo, Japan). Poly-NIPAM (molecular weight [MW]=66,400, weight average molecular weight [MW]/number average molecular weight [Mn]=1.40) was a kind gift from Dr Mitsuhiro Ebara, National Institute for Materials Science (NIMS).
Cell culture
The 293XL/hTLR9 and 293XL/null cell lines (InvivoGen) were cultured in high-glucose (4.5 g/L) Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS), 100 U/mL penicillin, 100 μg/mL streptomycin, and 10 μg/mL blasticidin at 37°C in a humidified incubator containing 5% CO2. The murine macrophage cell line RAW-Blue (InvivoGen), which stably expresses an NF-κB/activator protein 1 (AP-1)-inducible secreted embryonic alkaline phosphatase (SEAP) reporter gene, was maintained in high-glucose DMEM supplemented with 10% (v/v) FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in a humidified incubator containing 5% CO2. Another murine macrophage cell line, RAW 264 (RCB0535), was purchased from Riken BioResource Center (Tsukuba, Japan). RAW 264 cells were cultured in DMEM containing 10% (v/v) heat-inactivated FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in a humidified incubator containing 5% CO2. The human-derived THP-1 monocyte cell line THP1-XBlue (InvivoGen) was cultured in RPMI 1640 supplemented with 10% (v/v) heat-inactivated FBS, 10 mM HEPES, 1.0 mM sodium pyruvate, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in a humidified incubator containing 5% CO2.
Radical polymerization of the NIPAM-hemin copolymer
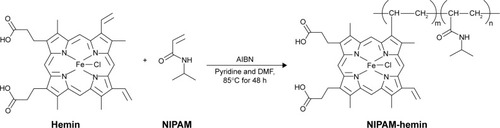
The NIPAM-hemin copolymer was synthesized from NIPAM and hemin by conventional radical polymerization. Hemin (1.5 g, 2.30 mmol), NIPAM (26.0 g, 0.230 mol), AIBN (0.389 g, 2.37 mmol), and pyridine (5 mL) were dissolved in 20 mL N,N-dimethylformamide. The solution was degassed and purged with argon for 20 min. The polymerization reaction was carried out in an oil bath at 85°C for 48 h. The reaction was stopped by cooling and exposing the mixture to air. Non-polymerized hemin was removed from the copolymer by extraction with methanol, and the copolymer was precipitated by adding excess diethyl ether. The precipitate was dissolved in methanol and separated using silica gel chromatography. The final product was obtained by evaporation and dissolved in sterilized deionized water for further analysis.
Characterization of the NIPAM-hemin copolymer
Gel permeation chromatography (GPC) was conducted to determine the molecular weight of the NIPAM-hemin copolymer. The copolymer was pumped through sequential columns containing Shodex SB-804 HQ and SB-802.5 HQ resin (Showa Denko, Tokyo, Japan), and poly(sodium 4-styrenesulfonate) was used to construct a standard calibration curve.
NIPAM-hemin (50 μg/mL), hemin (5 μg/mL), poly-NIPAM (100 μg/mL), and NIPAM (100 μg/mL) were each dissolved in dimethyl sulfoxide (DMSO), and absorption spectra were measured using a U-2900 spectrophotometer (Hitachi, Tokyo, Japan). To obtain infrared (IR) spectra, KBr pellets were prepared from dried samples of each reagent, and Fourier-transform infrared (FTIR) spectroscopy was performed using an IRTracer-100 (Shimadzu, Kyoto, Japan) with 20 scans per spectrum, a resolution of 4 cm−1, and a spectral range of 400–4,000 cm−1.
The iron content of the polymer was determined by inductively coupled plasma optical emission spectrometry (ICP-OES) on an Agilent 720-ES (Agilent Technologies, Santa Clara, CA, USA) by treating 12.5 mg NIPAM-hemin with dilute nitric acid, sulfuric acid, and perchloric acid, followed by heating in hydrochloric acid.
In vitro cytotoxicity assay
The 293XL/null cells and RAW 264 cells were seeded in 96-well plates at a density of 5,000 cells/well and incubated at 37°C in a humidified incubator containing 5% CO2 for 24 h. The medium was then replaced with fresh high-glucose DMEM supplemented with 10% (v/v) heat-inactivated FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. NIPAM-hemin copolymer was added to the medium at a final concentration of 0, 0.25, 0.5, 0.75, 1.0, or 2.0 mg/mL. After an additional 24 or 48 h incubation, the medium was replaced with 100 μL fresh medium, and 10 μL CCK-8 solution was added to each well, followed by incubation for 1 or 3 h at 37°C in a humidified incubator containing 5% CO2. The absorbance at 450 nm (A450) of each well was measured using a microplate reader (MTP-880Lab; Corona Electric, Ibaraki, Japan). An in vitro cytotoxicity assay for poly-NIPAM was also conducted using the same procedure described above.
Stimulation of human PBMCs with NIPAM-hemin
Commercially available frozen PBMCs were purchased from Cellular Technology Limited (Shaker Heights, OH, USA). PBMCs were thawed according to the manufacturer’s instructions, and were then seeded in a 96-well plate at a density of 1.0×106 cells/well, and stimulated with 500 μg/mL NIPAM-hemin, hemin, NIPAM, or poly-NIPAM (MW=66,400 Da). After incubation at 37°C for 48 h in a humidified incubator containing 5% CO2, the supernatants were collected. The levels of IFN-α, IFN-γ, IL-6, and IL-1β secreted into the medium were determined using Ready-Set-Go! ELISA kits (eBiosciences, San Diego, CA, USA) according to the manufacturer’s instructions.
TLR reporter assay
The 293XL/hTLR9 and 293XL/null cells were seeded at a density of 7.5×104 cells/well in a 96-well plate and transfected with pNifty-Luc and pGL4.74[hRluc/TK] using LyoVec transfection reagent. After culturing at 37°C for 24 h, the medium was replaced with fresh DMEM containing 10% (v/v) heat-inactivated FBS. Thereafter, NIPAM-hemin, hemin, NIPAM, or poly-NIPAM (MW=66,400 Da) were added at a final concentration of 500 μg/mL, and the cells were incubated for an additional 24 h at 37°C in 5% CO2. All compounds, except hemin, were dissolved in sterilized deionized water. Hemin was dissolved in DMSO. Water or DMSO without the compounds of interest were added to the culture as a negative control. CpG ODN 2006 PT was added to the medium at a final concentration of 0.5 μM as a positive control. The expression level of each luciferase was determined according to the manufacturer’s instructions for the dual-luciferase reporter assay system.
RAW-Blue cells and THP1-XBlue cells were seeded separately in 96-well plates at a density of 1.0×105 cells/well and incubated for 24 h. The medium was replaced with medium containing 10% (v/v) heat-inactivated FBS before stimulation. NIPAM-hemin, hemin, NIPAM, or poly-NIPAM (MW=66,400 Da) were individually added to the medium at a final concentration of 500 μg/mL, and the cells were incubated for an additional 24 h. The supernatants were then collected, and NF-κB/AP-1 activation linked to SEAP was determined using QUANTI-Blue reagent according to the manufacturer’s instructions. LPS (500 μg/mL) was used as a positive control.
Statistical analysis
All data are shown as the mean±SD. Statistical significance was evaluated using Student’s t-test. A value of P<0.05 was considered statistically significant.
Results
Characterization of the NIPAM-hemin copolymer
The hemin-containing copolymer NIPAM-hemin was synthesized by the radical polymerization of hemin and NIPAM monomers. shows the process of NIPAM-hemin synthesis. A dark-brown product was obtained after precipitation in diethyl ether and separation by silica gel chromatography. The final product was easily soluble in water, methanol, and DMSO.
The molecular weight of NIPAM-hemin was measured using GPC. The Mn and Mw of NIPAM-hemin were 1.5×103 and 3.2×103 Da, respectively, providing a polydispersity index of 2.1 (Mw/Mn).
The presence of hemin in the copolymer was confirmed by UV-vis absorption spectroscopy. shows the UV-vis absorption spectrum of NIPAM-hemin dissolved in DMSO. NIPAM-hemin showed a Soret peak at 398 nm, and a peak at 570 nm in the Q-band region, similar to those of hemin solution. This result indicated that the copolymer contained hemin. ICP-OES measurements were conducted to estimate the proportion of hemin in the copolymer. We found that 1 mg polymer contained 11.5 μg iron. Since the number of iron atoms is equal to the number of hemin molecules, the molar ratio of hemin to NIPAM in the copolymer was estimated to be 1:37.
Figure 1 (A) UV-vis and (B) FTIR spectra for NIPAM-hemin (red), hemin (blue), NIPAM (green), and poly-NIPAM (MW=66,400 Da) (purple).
Abbreviations: FTIR, Fourier-transform infrared; NIPAM, N-isopropylacrylamide; hemin, ferriprotoporphyrin IX chloride; MW, molecular weight.
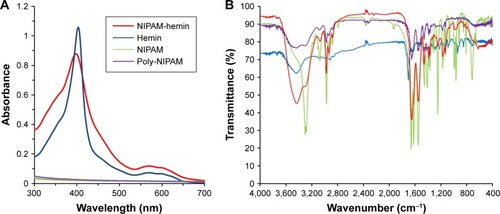
To confirm the presence of hemin in copolymer, the FTIR spectra were also determined (). The spectra of NIPAM-hemin showed peaks at 1,458 cm−1, 1,547 cm−1, and 1,651 cm−1, consistent with the −CH3, C−N, and C=O bonds of the NIPAM monomer, respectively.Citation9 Compared to the NIPAM spectra, the 1,622 cm−1 band was missing from the NIPAM-hemin spectra, indicating that the C=C bond of NIPAM was used for polymerization. These results indicated that each monomer was copolymerized using the vinyl group of each monomer. Peaks corresponding to hemin (1,703 cm−1) were not observed in the spectra of NIPAM-hemin because the proportion of hemin monomers in the copolymer was low compared to that of NIPAM. To confirm the limitations of FTIR analysis of NIPAM-hemin, we examined whether a mixture of hemin and poly-NIPAM would show clear peaks of the hemin monomer. The mixture, which contained hemin and NIPAM adjusted to a molar ratio of 1:37, did not show any clear peaks from hemin ().
Dynamic light scattering (DLS) measurements were then performed to check for aggregation of NIPAM-hemin in water. No nano-size aggregates of NIPAM-hemin were observed by DLS. In contrast, poly-NIPAM formed particles of around 200 nm in diameter under the same measurement conditions as those used for NIPAM-hemin, as shown in .
NIPAM-hemin showed no cytotoxic effects and induced the production of IFN-γ and IL-6
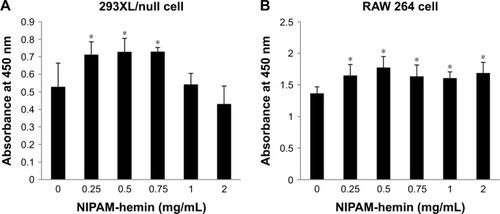
The cytotoxic effects of NIPAM-hemin copolymer were first evaluated in 293XL/null and RAW 264 cells using a water-soluble tetrazolium salt-based cell proliferation assay. shows the effect of NIPAM-hemin copolymer concentration on cell proliferation. Tetrazolium salt shows an increased A450 when reduced by dehydrogenases in visible cells. Therefore, the effects of NIPAM-hemin on cell viability were estimated by measuring changes in the A450. After incubation for 24 h, the A450 was increased by adding NIPAM-hemin to 293XL/null cells () and RAW 264 cells () at a concentration of 0.25, 0.5, and 0.75 mg/mL. However, no significant decrease in the A450 was observed at any concentration. We increased the incubation time to 48 h to check the cytotoxicity of NIPAM-hemin in RAW 264 cells. No significant decrease was observed after incubation for 48 h (). Using the same experiment, we confirmed that poly-NIPAM showed no cytotoxicity in either of the cell lines (). These data showed that NIPAM-hemin has no detectable cytotoxic effects on mammalian cells in vitro.
Figure 2 Cytotoxicity assay for NIPAM-hemin in (A) 293XL/null and (B) RAW 264 cells. Cells were incubated with NIPAM-hemin for 24 h in 96-well plates; cell viability was estimated by measuring the absorbance at 450 nm.
Notes: Data are expressed as the mean±SD (n=5). *P<0.05 vs no treatment group.
Abbreviations: NIPAM, N-isopropylacrylamide; hemin, ferriprotoporphyrin IX chloride.
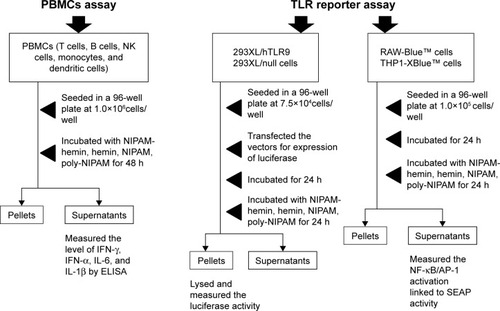
shows the experimental flow for the evaluation of the immunostimulatory effects of NIPAM-hemin. First, we investigated the potential immunostimulatory effects of NIPAM-hemin on human PBMCs, which consist of lymphocytes (T cells, B cells, and NK cells), monocytes, and dendritic cells. shows the IFN-γ, IL-6, and IL-1β activity levels in PBMCs treated with NIPAM-hemin copolymer, hemin, NIPAM monomer, or poly-NIPAM. PBMCs stimulated with NIPAM-hemin copolymer or poly-NIPAM produced IFN-γ and IL-6. The levels of IFN-γ induced by NIPAM-hemin copolymer was almost 11-fold higher than that induced by poly-NIPAM. In contrast, there were no significant differences in IL-6 levels in cells stimulated with NIPAM-hemin copolymer or poly-NIPAM. Neither hemin nor NIPAM monomer induced the production of IFN-γ or IL-6.
Figure 3 NIPAM-hemin induced the production of IFN-γ and IL-6, and had a lesser effect on the induction of IL-1β, in PBMCs. PBMCs were stimulated with NIPAM-hemin, hemin, NIPAM, or poly-NIPAM (MW=66,400). The levels of (A) IFN-γ, (B) IL-6, and (C) IL-1β were determined after 48 h. The concentration of each stimulant was 500 μg/mL. Hemin was dissolved in DMSO, while the other compounds were dissolved in sterilized deionized water.
Notes: Data are expressed as the mean±SD (n=3). *P<0.05; #IFN-γ, IL-6, and IL-1β were produced at levels lower than the detectable minima of 3.91 pg/mL, 1.95 pg/mL, and 2.34 pg/mL, respectively.
Abbreviations: NIPAM, N-isopropylacrylamide; hemin, ferriprotoporphyrin IX chloride; IFN-γ, interferon-γ; IL, interleukin; PBMCs, peripheral blood mononuclear cells; MW, molecular weight; DMSO, dimethyl sulfoxide.
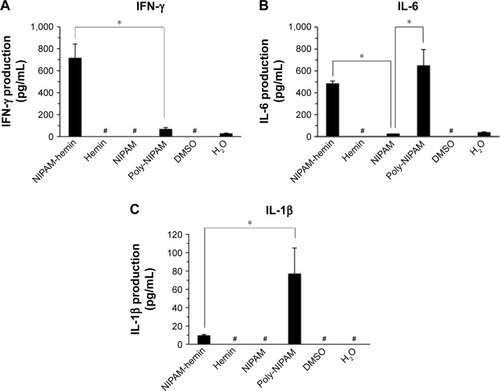
The IL-1β activity levels were also examined to determine whether NIPAM-hemin activates the NLRP3 inflammasome. Poly-NIPAM induced IL-1β production at 80 pg/mL, whereas NIPAM-hemin induced only 10 pg/mL IL-1β. The IFN-α production levels in all samples were below the detection limit of 7.81 pg/mL. These results indicate that NIPAM-hemin copolymer considerably stimulated the production of IFN-γ.
NIPAM-hemin copolymer did not activate TLRs
As noted above, NIPAM-hemin induced the production IFN-γ, an indicator of the Th1 immune response. TLRs are often involved in the induction of the Th1 response.Citation10 Moreover, the role of TLR9 in the activation of the immune response by natural hemozoin (heme crystal) has been shown in vivo.Citation2
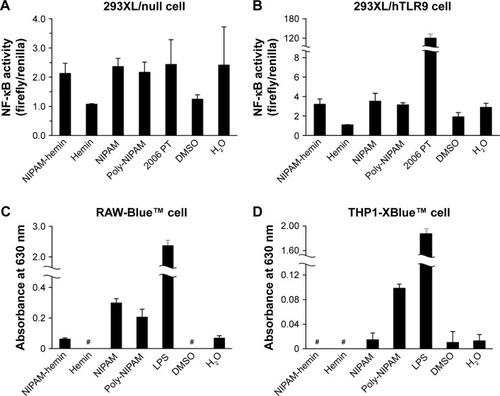
Therefore, we examined whether TLR9 was involved in cytokine production using a human TLR9-expressing reporter cell line. shows the results of the NF-κB activation assay. NIPAM-hemin did not induce NF-κB activation in 293XL/hTLR9, which stably expresses a TLR9, whereas phosphorothioated CpG ODN (2006 PT) induced NF-κB activation by more than 60-fold ().
Figure 4 NIPAM-hemin did not induce NF-κB signaling via most TLRs in reporter cells. (A) 293XL/null cells, (B) 293XL/hTLR9 cells, (C) RAW-Blue cells, and (D) THP1-XBlue cells were incubated with 500 μg/mL NIPAM-hemin, hemin, NIPAM, or poly-NIPAM (MW=66,400 Da). LPS (500 μg/mL) or 2006 PT (0.5 μM), agonists for TLR4 and TLR9, respectively, were added to the cells as positive controls. Hemin was dissolved in DMSO, while the other compounds were dissolved in sterilized deionized water.
Notes: Data are expressed as the mean±SD (n=5). #Absorbance not detected.
Abbreviations: NIPAM, N-isopropylacrylamide; hemin, ferriprotoporphyrin IX chloride; NF-κB, nuclear factor-κB; LPS, lipopolysaccharide; MW, molecular weight; DMSO, dimethyl sulfoxide.
Scheme 1 The process of NIPAM-hemin synthesis.
Abbreviations: AIBN, 2,2′-azodiisobutyronitrile; DMF, N,N-dimethylformamide; Hemin, ferriprotoporphyrin IX chloride; NIPAM, N-isopropylacrylamide.
Scheme 2 The evaluation of the immunostimulatory effects of NIPAM-hemin.
Abbreviations: NIPAM, N-isopropylacrylamide; hemin, ferriprotoporphyrin IX chloride; IFN-γ, interferon-γ; IL, interleukin; NK, natural killer; PBMCs, peripheral blood mononuclear cells; ELISA, enzyme-linked immunosorbent assay; TLRs, toll-like receptors; SEAP, secreted embryonic alkaline phosphatase.
To determine the role of other TLRs in this process, we used two types of immune cell-derived reporter cell lines, RAW-Blue and THP1-XBlue. Both cell lines express most TLRs and show different responses against TLR agonists. RAW-Blue cells expressed all TLRs (TLR1–4 and 6–9) except TLR5 at the mRNA level, and showed a weak response to TLR3 agonists. THP1-XBlue cells expressed all TLRs, including TLR5, at the mRNA level, and showed a weak response to agonists for TLR3, 7, and 9. NIPAM-hemin did not induce NF-κB activation in either cell line (). These results indicate that in vitro cytokine production by NIPAM-hemin might be mediated by receptors other than those for the TLR family.
Discussion
The aim of the present study was to develop a novel heme-based immunostimulator that induces the Th1 immune response. We synthesized a hemin-based copolymer containing hemin and NIPAM at a ratio of 1:37 (hemin:NIPAM). The copolymer had no effects on cell viability. NIPAM-hemin induced IFN-γ and IL-6 production in human PBMCs, although neither of the monomers induced the production of these factors.
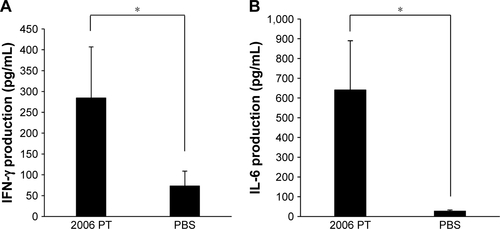
Alum, emulsions, and lipids are used as vaccine adjuvants for the activation of both humoral immunity and cellular immunity, as summarized in .Citation11 Recently, CpG ODNs were the subject of much interest as a potential new vaccine adjuvant. TLRs, a type of innate immune receptor, play an important role in cellular immunity.Citation10 CpG ODN, a ligand of TLR9, is involved in the production of several cytokines, including IL-6, IL-12, tumor necrosis factor (TNF)-α, and IFN-γ.Citation12 These cytokines activate immune cells and ultimately stimulate the differentiation of T helper cells into Th1 cells. CpG ODNs can be categorized into two major types: D/A type, and K/B type. D/A type CpG ODNs contain a palindromic CpG motif and a poly-G tail, and activate plasmacytoid dendritic cells to produce both Type I IFNs (IFN-α and IFN-β) and Type II IFNs (IFN-γ).Citation13 K/B type CpG ODNs comprise multiple CpG motifs and activate B cells to produce inflammatory cytokines such as IL-6, IL-12, and TNF-α, resulting in antibody production.Citation14 D/A type CpG ODNs form higher order structures and, thus, form aggregates and precipitates, thereby hampering their clinical use.Citation15 Only type K/B CpGs, which marginally induce type I and type II IFNs, hold promise for clinical applications. In the current study, NIPAM-hemin induced the production of IL-6 and large amounts of IFN-γ, a type II IFN; however, hemin alone did not induce the production of IFN-γ or IL-6 (). The levels of IFN-γ and IL-6 induced by 2006 PT, which is known to be a major immunostimulator, were about 300 pg/mL and 600 pg/mL, respectively (). The levels of IL-6 and IFN-γ induced by NIPAM-hemin were comparable to those induced by CpG ODN 2006 PT. IFN-γ is the major molecule involved in the differentiation of T helper cells to Th1 cells, resulting in IgG2 production. Therefore, we expected NIPAM-hemin to be a successful Th1 adjuvant.
Table 1 The type of major adjuvants used for the development of vaccines
NIPAM-hemin did not decrease cell viability, even though hemin is known to be toxic to several cell types.Citation16,Citation17 One of the causes of hemin-induced cytotoxicity is the hydrophobicity of the heme group, which interacts with lipids and leads to peroxidation.Citation16 NIPAM is soluble and binds to the non-soluble hemin to form a soluble copolymer. Fang et alCitation18 demonstrated that soluble pegylated hemin does not show cytotoxic effects in vitro. Therefore, the non-toxicity of NIPAM-hemin in vitro can be explained by the reduced hydrophobicity of the polymer.
β-hematin is chemically synthesized from hemin. Natural hemozoin consists of a heme dimer. β-hematin induces the production of cytokines such as IL-6, IL-1α, and IL-1β in vivo.Citation19 Recent studies have suggested that β-hematin activates the NLRP3 inflammasome, showing effects similar to those of particles such as alum, asbestos, and silica.Citation20–Citation22 The NLRP3 inflammasome plays an important role in the production of the inflammatory cytokine IL-1β.Citation23 IL-1β is known to be a major inducer of IL-6 production in monocytes.Citation24 These studies have also indicated that the activation of the NLRP3 inflammasome could be a common immune response induced by particles or crystals. Conversely, Coban et alCitation2 reported that the adjuvant effect of β-hematin is independent of the NLRP3 inflammasome. The implications of the effect of β-hematin on the activation of the NLRP3 inflammasome are not yet fully understood. In this study, poly-NIPAM (MW=66,400 Da), a polymer of NIPAM, formed particles of around 200 nm in size and stimulated IL-1β production (80 pg/mL) (). Gómez et alCitation25 reported that silica particles of around 200 nm in size (10 μg/mL), another activator of the NLRP3 inflammasome, also induced the production of IL-1β (200–500 pg/mL) in PBMCs. On the other hand, we observed no particle formation by NIPAM-hemin, and reduced production of IL-1β (10 pg/mL). These results suggest that the induction of IL-6 and IFN-γ in PBMCs by NIPAM-hemin occurs independent of the NLRP3 inflammasome.
The activation of natural hemozoin is mediated by TLR9, and is dependent on MyD88. Natural hemozoin induces the production of monocyte chemoattractant protein 1 (MCP-1), TNF-α, IL-12, p40, and IL-6 in spleen cells via TLR9 mediation.Citation26 Moreover, Temizoz et alCitation27 reported that TLR9 and stimulator of interferon gene (STING) agonists induce IL-12 and IFN-α, respectively, which synergistically regulate IFN-γ production in NK cells in human PBMCs.Citation27 NIPAM-hemin also induces the production of IFN-γ; thus, we expected to observe NF-κB activation by NIPAM-hemin in TLR9-expressing cells. However, our study demonstrated that NIPAM-hemin did not activate NF-κB via any TLRs, including TLR9, in vitro (). Although hemin does not exhibit an adjuvant effect, it binds to the extracellular domains of recombinant human TLR9, and causes a conformational change.Citation2 Moreover, TLR9 forms a symmetrical TLR-CpG ODN complex with 2:2 stoichiometry, in which each dimer binds a CpG ODN.Citation7 Based on these previous reports, we predicted that the binding of ligand molecules to each dimer of TLR9 might be necessary for TLR9 activation. The mechanism of TLR9 activation by hemozoin remains unclear. However, hemozoin consists of a heme dimer; thus, two hemes will be closely situated. Consequently, multiple adjacent heme molecules might be important for the formation of the active form of TLR9. ICP-OES measurements showed that the copolymer contained a low ratio of hemin to NIPAM. It is unlikely that multiple hemins would be in close proximity to each other, as the hemin concentration in the polymer was low. This may explain why we did not observe NF-κB activation via TLR9 by NIPAM-hemin. However, β-hematin has an adjuvant effect independent of TLR9, as does NIPAM-hemin; therefore, future investigations are needed to assess the IFN-γ production pathway induced by NIPAM-hemin.
In addition to IFN-γ production, it was clear that NIPAM-hemin stimulated the production of IL-6, which stimulates B cell maturation.Citation28 Further studies are needed to determine how NIPAM-hemin acts as an adjuvant, for example, by injecting it into mice along with an antigen, followed by measuring the amount of IgG isotypes (IgG2a or IgG1).
Conclusion
In this work, we synthesized a hemin-containing immunostimulator by radical polymerization of hemin with NIPAM. Importantly, NIPAM-hemin does not affect cell viability, and induced the production of large amounts of IFN-γ in human PBMCs. The NIPAM-hemin copolymer shows potential as a new adjuvant.
Acknowledgments
The authors would like to thank Ms Satomi Kohara, Ms Satomi Magae, Ms Yuko Matsumoto, and Mr Kazuya Tsukagoshi for technical assistance with the experiments. We are deeply grateful to Dr Ebara (NIMS, Japan) who provided poly-NIPAM, and to Dr Satoshi Kawada and Mr Akira Ishitoya in materials analysis station (NIMS, Japan) for ICP-OES measurement.
This work was conducted in NIMS Molecule & Material Synthesis platform, supported by Nanotechnology Platform Program of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. This work was supported by Japan Society for the Promotion of Science Kakenhi grant number 15K06589. We thank Editage, a division of Cactus Communications, for editing language service.
Supplementary materials
Figure S1 FTIR spectra of a mixture of hemin monomer and poly-NIPAM (MW=66,400 Da).
Notes: The molar ratio of hemin and NIPAM in poly-NIPAM was adjusted to 1:37. The broken line shows the peaks attributed to the hemin monomer.
Abbreviations: FTIR, Fourier-transform infrared; hemin, ferriprotoporphyrin IX chloride; MW, molecular weight; NIPAM, N-isopropylacrylamide.
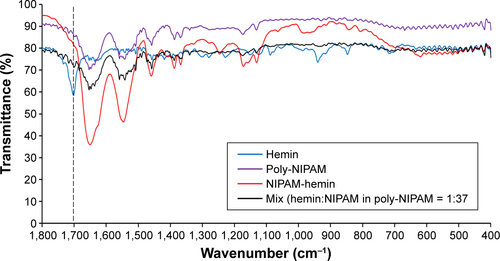
Figure S2 Poly-NIPAM (MW=66,400 Da) formed particles at 37°C in water.
Note: Particle size was determined by DLS measurement.
Abbreviations: NIPAM, N-isopropylacrylamide; MW, molecular weight; DLS, dynamic light scattering.
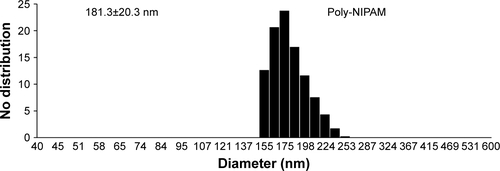
Figure S3 NIPAM-hemin was not cytotoxic to RAW 264 cells after incubation for 48 h. Cells were incubated with NIPAM-hemin for 48 h in 96-well plates; cell viability was estimated by measuring the absorbance at 450 nm.
Notes: Data are expressed as the mean±SD (n=5). *P<0.05 vs no treatment group.
Abbreviations: NIPAM, N-isopropylacrylamide; hemin, ferriprotoporphyrin IX chloride.
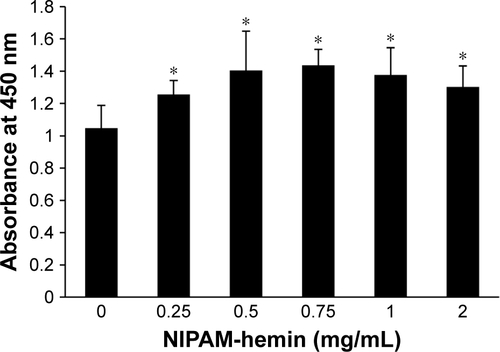
Figure S4 Poly-NIPAM was not cytotoxic to (A) 293XL/null and (B) RAW 264 cells. Cells were incubated with poly-NIPAM for 24 h in a 96-well plate and cell viability was estimated by measuring the absorbance at 450 nm.
Notes: Data are expressed as the mean±SD (n=5). *P<0.05 vs no treatment group.
Abbreviation: NIPAM, N-isopropylacrylamide.
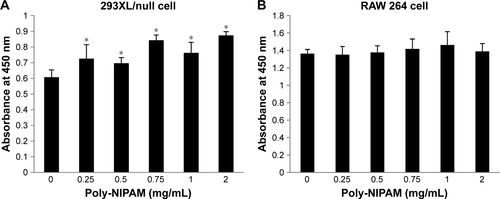
Figure S5 The levels of IFN-γ and IL-6 production in PBMCs after incubation with 2006 PT. PBMCs were stimulated with 2006 PT (0.5 μM); the amounts of (A) IFN-γ and (B) IL-6 were determined after incubation for 48 h.
Notes: Data are expressed as the mean±SD (n=6–9). *P<0.05.
Abbreviations: IFN-γ, interferon-γ; IL, interleukin; PBMCs, peripheral blood mononuclear cells.
Disclosure
The authors report no conflicts of interest in this work.
References
- LindbladEBAluminium compounds for use in vaccinesImmunol Cell Biol200482549750515479435
- CobanCIgariYYagiMImmunogenicity of whole-parasite vaccines against plasmodium falciparum involves malarial hemozoin and host TLR9Cell Host Microbe201071506120114028
- SlaterAFSwiggardWJOrtonBRAn iron-carboxylate bond links the heme units of malaria pigmentProc Natl Acad Sci U S A19918823253291988933
- OnishiMKitanoMTaniguchiKHemozoin is a potent adjuvant for hemagglutinin split vaccine without pyrogenicity in ferretsVaccine201432253004300924721532
- UrakiRDasSCHattaMHemozoin as a novel adjuvant for inactivated whole virion influenza vaccineVaccine201432415295530025108216
- CobanCYagiMOhataKThe malarial metabolite hemozoin and its potential use as a vaccine adjuvantAllergol Int201059211512420414048
- OhtoUShibataTTanjiHStructural basis of CpG and inhibitory DNA recognition by Toll-like receptor 9Nature2015520754970270525686612
- GaoYChenJRedox reaction of hemin-immobilized polyallylamine–polystyrene latex suspensionsJ Electroanal Chem20055781129136
- JadhavSABrunellaVMilettoIBerlierGScalaroneDSynthesis of poly(N-isopropylacrylamide) by distillation precipitation polymerization and quantitative grafting on mesoporous silicaJ Appl Polym Sci20161334444181
- NeteaMGVan der MeerJWSutmullerRPAdemaGJKullbergBJFrom the Th1/Th2 paradigm towards a toll-like receptor/T-helper biasAntimicrob Agents Chemother200549103991399616189071
- LeeSNguyenMTRecent advances of vaccine adjuvants for infectious diseasesImmune Netw2015152515725922593
- ChuRSTargoniOSKriegAMLehmannPVHardingCVCpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunityJ Exp Med199718610162316319362523
- KrugARothenfusserSHornungVIdentification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cellsEur J Immunol20013172154216311449369
- HartmannGWeeratnaRDBallasZKDelineation of a CpG phosphorothioate oligodeoxynucleotide for activating primate immune responses in vitro and in vivoJ Immunol200016431617162410640783
- KerkmannMCostaLTRichterCSpontaneous formation of nucleic acid-based nanoparticles is responsible for high interferon-α induction by CpG-A in plasmacytoid dendritic cellsJ Biol Chem200528098086809315591070
- HigdonANBenavidesGAChackoBKHemin causes mitochondrial dysfunction in endothelial cells through promoting lipid peroxidation: the protective role of autophagyAm J Physiol Heart Circ Physiol20123027H1394H140922245770
- ZhouYFZhangCYangGHepcidin protects neuron from hemin-mediated injury by reducing ironFront Physiol2017833228588503
- FangJQinHSekiTTherapeutic potential of pegylated hemin for reactive oxygen species-related diseases via induction of heme oxygenase-1: results from a rat hepatic ischemia/reperfusion injury modelJ Pharmacol Exp Ther2011339377978921890508
- GriffithJWSunTMcIntoshMTBucalaRPure hemozoin is inflammatory in vivo and activates the NALP3 inflammasome via release of uric acidJ Immunol200918385208522019783673
- DuewellPKonoHRaynerKJNLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystalsNature201046472931357136120428172
- HornungVBauernfeindFHalleASilica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilizationNat Immunol20089884785618604214
- KusakaTNakayamaMNakamuraKIshimiyaMFurusawaEOgasawaraKEffect of silica particle size on macrophage inflammatory responsesPLoS One201493e9263424681489
- HeYHaraHNúñezGMechanism and regulation of NLRP3 inflammasome activationTrends Biochem Sci201641121012102127669650
- TosatoGJonesKDInterleukin-1 induces interleukin-6 production in peripheral blood monocytesBlood1990756130513102310829
- GómezDMUrcuqui-InchimaSHernandezJCSilica nanoparticles induce NLRP3 inflammasome activation in human primary immune cellsInnate Immun201723869770829113588
- CobanCIshiiKJKawaiTToll-like receptor 9 mediates innate immune activation by the malaria pigment hemozoinJ Exp Med20052011192515630134
- TemizozBKurodaEOhataKTLR9 and STING agonists synergistically induce innate and adaptive type-II IFNEur J Immunol20154541159116925529558
- RincónMAnguitaJNakamuraTFikrigEFlavellRAInterleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cellsJ Exp Med199718534614699053446
