Abstract
Purpose
To investigate the amelogenesis-inductive effects of surface structures at the nanoscale. For this purpose, variable nanostructured titanium dioxide (TiO2) surfaces were used as a framework to regulate the amelogenic behaviors of ameloblasts with the administration of retinoic acid (RA)/dexamethasone (DEX).
Materials and methods
TiO2 nanotubular (NT) surfaces were fabricated via anodization. Mouse ameloblast-like LS-8 cells were seeded and cultured on NT surfaces in the presence or absence of RA/DEX for 48 h. The amelogenic behaviors and extracellular matrix (ECM) mineralization of LS-8 cells on nanostructured Ti surfaces were characterized using field emission scanning electron microscope, laser scanning confocal microscope, quantitative polymerase chain reaction, MTT assay, and flow cytometry.
Results
TiO2 NT surfaces (tube size ~30 and ~80 nm) were constructed via anodization at 5 or 20 V and denoted as NT5 and NT20, respectively. LS-8 cells exhibited significantly increased spread and proliferation, and lower rates of apoptosis and necrosis on NT surfaces. The amelogenic gene expression and ECM mineralization differed significantly on the NT20 and the NT5 and polished Ti sample surfaces in standard medium. The amelogenic behaviors of LS-8 cells were further changed by RA/DEX pretreatment, which directly drove maturation of LS-8 cells.
Conclusion
Controlling the amelogenic behaviors of ameloblast-like LS-8 cells by manipulating the nanostructure of biomaterials surfaces represents an effective tool for the establishment of a systemic framework for supporting enamel regeneration. The administration of RA/DEX is an effective approach for driving the amelogenesis and maturation of ameloblasts.
Introduction
Dental caries have been ranked as the most common oral condition among 291 diseases.Citation1 A variety of factors, such as genetic disorders, microbial infections, and trauma, can lead to tooth decay and loss, which may be progressively aggravated by poor oral hygiene and negligence.Citation2–Citation4 Currently, dental treatments such as root canal therapy (RCT), conventional fix denture, and implant-supported denture are used to restore the affected tissues and their functionality. However, with these procedures after an RCT, teeth lose vitality and sensitivity, and become fragile over time.Citation5 Dental implants lack periodontal ligament; therefore, masticatory stresses and homeostasis of alveolar bone are not equivalent to that of natural dentition.Citation6 These shortcomings inspired the development of alternative methods for tooth regeneration such as the dental epithelium–mesenchymal complex, cell–tissues recombination, gene-modified tooth regeneration,Citation6–Citation8 and humanized chimeric tooth.Citation9 Although these studies reported promising results for regeneration of tooth tissues, no mature dental enamel was formed.
As the outmost layer of the human tooth, enamel is the most highly mineralized tissue in the human body and contains the highest percentage (>95%) of hydroxyapatite.Citation10,Citation11 Mature enamel does not contain any cells, blood vessels, or nerves and cannot repair or heal.Citation12,Citation13 It lacks an amelogenic micro-environment which provides crucial spatial structure and growth factors to support ameloblastic cellular growth and differentiation for enamel formation.Citation14,Citation15
Surface modifications at the nanoscale may represent a promising strategy for the construction of spatial structures that support tissue regeneration. Impressively, Dalby et al reported that slightly disordered nano-pits (~120 nm diameter) on polymethylmethacrylate induced osteogenic differentiation of mesenchymal stem cell (MSC).Citation16 This observation suggests that biomaterial surface topography plays an important role in regulating various host responses.Citation17,Citation18 Specifically, a TiO2 nanotubular (NT) surface array facilitated osteogenic differentiation and extracellular matrix (ECM) mineralization of osteoblastic lineage cells in vitro.Citation17,Citation19,Citation20 In addition, the nanostructure of the NT array closely matches the cross section of bone collagen.Citation21 The biomimetic structure of the NT array probably contributes to the functional alternation of osteoblastic lineage cells.Citation22 Notably, the spatial structures of the NT array mimic enamel rods.Citation23,Citation24 Therefore, we hypothesized that the NT array can be used as a framework to support ameloblasts for enamel regeneration.
In the current in vitro study, titanium (Ti) samples were treated with anodization and ultraviolet A/ultraviolet C (UVA/C) irradiation to fabricate super-hydrophilic TiO2 NT surfaces (NT5 and NT20) for enamel regeneration. The TiO2 NT surfaces were characterized for biological behavior to promote amelogenic differentiation of ameloblast-like cells (LS-8) on nanostructured Ti samples in standard medium or amelogenic differentiation medium. The aims of this study were to advance our understanding of the amelogenesis-inductive effects of surface structures at the nanoscale. For this purpose, variable nanostructured TiO2 surfaces were used as a framework to regulate the amelogenic behaviors of ameloblasts with the administration of retinoic acid (RA)/dexamethasone (DEX). The results obtained in this study may provide a systemic research framework for exploration and evaluation of enamel regeneration through surface nanotopography.
Materials and methods
Nanostructured TiO2 surface fabrication and characterization
Nanoscale textured Ti samples were prepared using a multistep procedure. Grade 1 pure Ti (99.9%) sheets were donated by the Northwest Institute for Nonferrous Metal Research (NIN, Xi’an, People’s Republic of China). Disk-shaped samples (14×1 mm) were cut from a Ti sheet by machining. Nanostructured Ti samples were prepared as described previously.Citation25 In brief, samples were polished using SiC sandpaper (1,500–8,000 grit; Matador, Germany) and ultrasonically cleaned with acetone, ethanol, and deionized water in sequence for 15 min each. Ti samples were then anodized in an aqueous electrolyte solution containing 0.5 wt% hydrofluoric acid and 1 M phosphoric acid at 20°C for 1 h. A direct current power supply and a platinum cathode set at 5 or 20 V was used to fabricate the nanostructured, textured surfaces denoted P (polished Ti sample), NT5, and NT20, respectively.Citation26 The topography of the prepared Ti surfaces was assessed by an atomic force microscope (AFM, Dimension Icon, Bruker AXS Inc., Madison, WI, USA) and field emission scanning electron microscopy (FE-SEM, magnification of 100,000×, S-4800; Hitachi Ltd., Tokyo, Japan). The contact angles and hydrophilicity of the samples were assessed by a Contact Angle Meter (DSA1 System, Kruss, Germany). The contact angles were measured instantly after placing a droplet of water with 10 μL of each click and at an interval of 0.5 and 2.0 s with the Drop Shape Analysis (DSA) software. The elemental composition was analyzed by X-ray photoelectron spectroscopy (XPS, ESCALAB 250Xi; Thermo Fisher Scientific, Waltham, MA, USA). The prepared samples were sterilized by UVA/C irradiation (λ=365 nm (A)/254 nm (C); Philips, Poland) at a distance of 50 mm for 1 h and then placed in wells of 24-well plates (Corning, USA) prior to cell culture.
Culture of LS-8 cell line
Mouse immortalized ameloblast-like cells (LS-8) were acquired for the experiments (USC, Los Angeles, CA, USA). The presence of Tomes’ process-like structure suggests that LS-8 cells are ameloblasts in the secretory phase.Citation27 LS-8 cells were cultured in standard DMEM (Cellgro, USA) with 4.5 g/L glucose, 10% fetal calf serum (Thermo Fisher Scientific), and 1% penicillin/streptomycin complex (Cellgro) at 37°C with 5% CO2. The culture medium was changed every 2 days. After growing to sub-confluence, cells were digested, counted, and passaged into 75 cm2 culture flasks (~2×106 cells/flask). The study protocol, including the use of LS-8 cell lines, was reviewed and approved by the Medical Ethics Committee of the College of Stomatology, Xi’an JiaoTong University, Xi’an, People’s Republic of China (approval no. xjkqll[2018] No 012).
Morphological observation of LS-8 cells on nanostructured TiO2 surfaces
LS-8 cells were seeded onto the prepared Ti samples (2×104 cells/well) and cultured for 24, 48, 72, or 96 h either in standard medium (DMEM as described earlier) or amelogenic RA medium (standard medium containing 100 nM DEX [Sigma-Aldrich Co., St Louis, MO, USA] and 20 ug/mL retinacid [RA; USA Pharmacopeia, USA]). For laser scanning confocal microscopy (LSCM), samples were fixed in 4% paraformaldehyde and stained using rhodamine-labeled phalloidin (1 μg/mL; Sigma-Aldrich) and 4′,6-diamidino-2-phenylindole (DAPI, 1 μg/mL; Hoffman-La Roche Ltd., Basel, Switzer-land). After glycerol mounting, samples were observed by LSCM (FV1200; Olympus Corporation, Tokyo, Japan). LS-8 cell number and morphology were analyzed using Image Pro-Plus (Media Cybernetics, USA), with a scale bar of 50 μm.
LS-8 proliferation on different Ti surfaces
LS-8 cells were seeded onto the prepared Ti samples (2×104 cells/well) and cultured in a 24-well culture plate for 24, 48, 72, or 96 h in standard culture or RA medium. Proliferation of LS-8 cells was assessed by MTT assay as detailed previously.Citation26 Briefly, at the prescribed time points, the samples were rinsed thrice in PBS (Cellgro) and transferred to new 24-well culture plates. To each well was added 200 μL MTT-PBS solution (Amresco, USA), 800 μL serum- and phenol red-free DMEM, and the samples were incubated at 37°C for 4 h to allow formazen formation. The formazen was then dissolved with DMSO (1 mL/well; MP Biomedicals, USA). Optical density was measured at 490 nm (570 nm as reference) on a spectrophotometer (Biotek, USA).
Apoptosis and necrosis of LS-8 cells on different Ti surfaces
LS-8 cells were seeded on Ti samples at 1×105 cells/well and cultured for 24 h to allow cell attachment. Ti samples were then cultured in standard or RA medium for 48 h. LS-8 cells on prepared Ti surfaces were resuspended using 0.25% trypsin (Cellgro). The apoptosis/necrosis of LS-8 cells was assessed using the Annexin V/propidium iodide (PI) kit (Seven Seas, People’s Republic of China) as described by the manufacturer. Using the FACS Aria II system (BD Biosciences, San Jose, CA, USA), Annexin V−/PI− cells were considered viable, annexin V+/PI− cells were considered apoptotic cells, and Annexin V+/PI+ cells were considered necrotic. Quantitative polymerase chain reaction (qPCR) was used to detect the expression of the apoptosis-related genes – B-cell lymphoma-2 (BCL-2); BCL2-associated X protein (BAX); and caspase 3 (CASP3). Total RNA was isolated from cells using the RNAiso Plus system (Takara, Japan). Aliquots of 1 μg total RNA were translated to cDNA using the Prime-ScriptTM RT reagent kit (Takara). qPCR was conducted using FastStart Universal SYBER Green Master (Hoffman-La Roche Ltd.) on a CFX96™ PCR System (Bio-Rad Laboratories Inc., Hercules, CA, USA). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a housekeeping gene. The primers used are listed in .
Table 1 Primers used for real-time PCR
Amelogenic gene expression of LS-8 cells on different Ti surfaces
We used qPCR to detect the expression of enamel formation-related genes, such as bone morphogenetic protein-2 (BMP-2), runt-related transcription factor 2 (RUNX2), sonic hedgehog (SHH), amelogenin X-linked (AMELX), ameloblastin (AMBN), enamelin (ENAM), kallikrein related-peptidase 4 (KLK-4), matrix metallopeptidase 20 (MMP-20), amelotin (AMTN), and odontogenic ameloblast-associated protein (ODAM). As described in Apoptosis and necrosis of LS-8 cells on different Ti surfaces section, cells were seeded on Ti samples at a concentration of 1×105 cells/well and cultured for 24 h. Then, half of the Ti samples were cultured in standard medium for 3 or 7 days, whereas the other half of the Ti samples were treated with RA medium for 48 h; this was followed by culturing with standard medium for an additional 1 or 5 days (3 or 7 days of culture, in total). The total RNA was isolated and translated to cDNA. qPCR was undertaken with GAPDH as a housekeeping gene.
ECM mineralization of LS-8 cells on different Ti surfaces
As described in Apoptosis and necrosis of LS-8 cells on different Ti surfaces section, cells were seeded on Ti samples at a concentration of 1×105 cells/well and cultured for 24 h. Then, half of the Ti samples were cultured in standard medium for 7 or 28 days, and the other half of Ti samples were treated with RA medium for 48 h and then cultured in standard medium for an additional 5 or 26 days (7 or 28 days of culture, in total). Samples cultured for 7 days were fixed in 4% paraformaldehyde and stained with the alkaline phosphatase (ALP) staining kit (Sigma-Aldrich) according to the manufacturer’s instructions. Samples cultured for 28 days were fixed in 60% isopropanol for 1 min. After rehydration in distilled water for 3 min, cells were stained with 1 wt% alizarin red S (Sigma-Aldrich) for 3 min at room temperature. Images were taken by a stereoscopic microscope (Leica Microsystems, Wetzlar, Germany). To quantify the red-stained mineralized nodules, the stain was solubilized within 10% cetylpyridinum chloride in 10 mM sodium phosphate, and absorbance values were measured at 620 nm using a spectrophotometer (Biotek) and with a protocol similar to that used for the MTT assay.
Statistical analysis
Experiments were repeated three times, with four replicates in each group. All data were analyzed using SPSS 19.0 (IBM Corporation, Armonk, NY, USA) and are expressed as mean ± SD (for data fitting normal distribution) or mean (for data not fitting normal distribution) for continuous variables. Significant differences between groups were identified using one-way analysis of variance (ANOVA) followed by Student–Newman–Keuls post hoc test for parametric data (expressed as bar charts with mean values and error bars) or Kruskal–Wallis test followed by Dunn’s multiple comparison test for non-parametric data (expressed in scatter plots with mean values). Differences were considered statistically significant when p<0.01.
Results
Surface characterization of TiO2-nanostructured surfaces on Ti samples
The surface topography of the prepared samples (P, NT5, and NT20) was visualized by AFM and FE-SEM from a vertical and a lateral view (). As a result of anodization at 5 and 20 V, uniformly distributed NT structures with tube size of ~30 and ~80 nm and tube lengths of ~100 nm (NT5) and 650 nm (NT20) were formed on Ti samples. In contrast, the P exhibited a smooth surface. The roughness of nanostructured Ti surfaces was measured by AFM. Anodization increased the roughness significantly, and the roughness of all samples was gradually increased as a factor of anodization voltage (). Surface elements were analyzed by XPS. The relative atomic concentrations of Ti, O, and C, and the Ti:O ratios were similar on P, NT5, and NT20 surfaces (; ).
Table 2 Surface roughness of nanostructured Ti surfaces
Table 3 Elements composition of nanostructured Ti surfaces
Figure 1 Surface characterization of nanostructured Ti surfaces.
Notes: (A) Representative atomic force microscopy images showing the topography of the polished Ti (P) and nanostructured TiO2 NT surfaces via anodization at 5 or 20 V (NT5 and NT20, respectively) TiO2 surfaces. Scanning range=500×500×50 nm. (B) Representative field emission scanning electron microscopy images showing the morphology and topography of P, NT5, and NT20 surfaces from the vertical and lateral views (insets). Magnification=100,000×; scale bar=100 nm. (C) Representative images showing the water contact angle on modified Ti surfaces. Scale bar=1 mm; (D) contact angle analysis. *p<0.01 vs P; #p<0.01 vs NT5.
Abbreviation: NT, nanotubular.
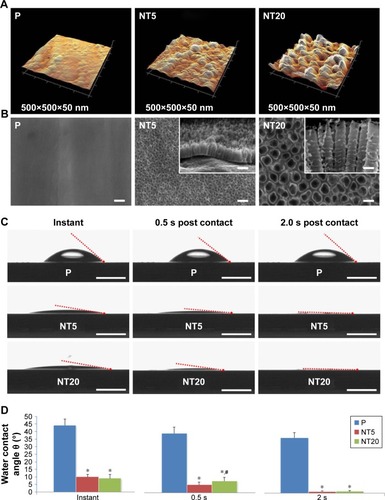
Based on contact-angle measurements (instant, 0.5 s, and 2.0 s post-contact), the hydrophilicities of NT5 and NT20 were similar and were both significantly higher (p<0.01) than that of the P surfaces (). The instant contact angles for NT5 (9.94°±1.72°) and NT20 (9.04°±2.58°) were significantly lower (p<0.01) than that of P surfaces (44.11°±4.19°, ). The contact angles for NT5 and NT20 were significantly reduced to 1.16°±0.23° and 1.28°±0.37°, respectively, within 2 s.
Morphological of LS-8 cells on nanostructured TiO2 surfaces
The morphology of LS-8 cells on modified Ti samples was investigated via F-actin/DAPI staining and semi-quantitative image analysis. LS-8 cell morphology differed remarkably between surfaces with a different topography ( and ). In standard medium, compared to P surfaces, NT5 and NT20 enhanced the spread and the number of cells at all time points. Cells on NT5 and NT20 showed plenty of pseudopods compared to smooth edge on P surfaces (). In addition, cell confluence was observed earlier in NT5 and NT20 samples (72 h) than in P surfaces, where it was achieved at 96 h (). These findings are supported by cell counting analysis (). Cell numbers were significantly lower on P surfaces than on NT5 and NT20 regardless of culturing time (p<0.01), whereas, the number of cells in the NT5 and NT20 groups did not differ significantly after 24 h.
Figure 2 Morphology of LS-8 cell attachment on nanostructured Ti surfaces in standard or retinoic acid (RA) medium.
Notes: (A and C) Representative images showing the morphology of LS-8 cells attached to the prepared Ti surfaces in standard or RA medium. Magnification=400×; scale bar=50 μm. (B and D) Image semi-quantitative analysis of attached LS-8 cells in standard or RA medium. *p<0.01.
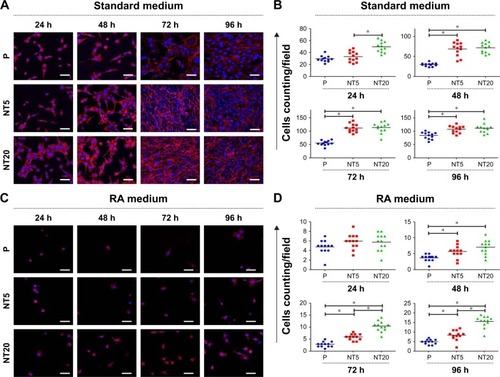
In RA medium, significantly fewer cells were observed in all groups (). However, slightly more cells were observed on NT5 and NT20 surfaces than on P surfaces (). This difference in cell number was not observed at 24 h in the RA medium. At 48 h, the number of cells on NT5 and NT20 surfaces was similar, but significantly higher than on P surfaces (p<0.01). At 72 and 96 h, the number of cells on NT20 surfaces was significantly higher than all other samples (p<0.01; ). Notably, fewer pseudopods with smooth edges were observed on all prepared Ti surfaces in the RA medium than in standard medium ().
LS-8 cellular proliferation on different Ti surfaces
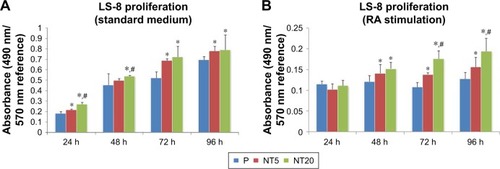
The proliferation of LS-8 cells on modified Ti surfaces was assessed by measuring the relative absorbance of media at 490/570 nm (MTT assay). In the standard medium, the absorbance of all samples was gradually increased as a factor of time, and NT5 and NT20 showed significantly higher (p<0.01) absorbance than the P surfaces (). In RA medium, the absorbance of all Ti surfaces was remarkably lower at any time than in the standard medium (). The absorbance data suggested that RA medium reduced cell proliferation. NT20 significantly promoted proliferation of LS-8 cells (p<0.01) in both media compared to NT5 and P ().
Apoptosis/necrosis of LS-8 cells on different Ti surfaces
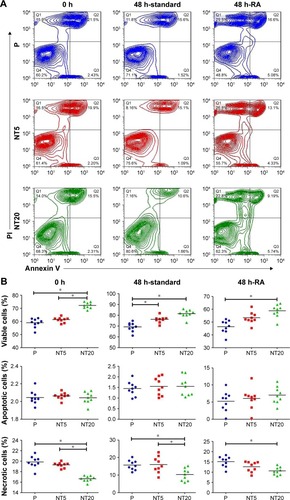
The apoptosis/necrosis of LS-8 cells was assessed by flow cytometry after staining with Annexin V/PI. In standard medium, NT20 significantly increased the proportion of viable cells (>70%) but reduced the fraction of necrotic cells to ~16.5% (). Cells incubated on NT5 and P surfaces displayed similar trends (). The RA medium directly reduced cell viability and induced accumulation of cellular debris on all groups of Ti surfaces (). Nevertheless, cells incubated with NT20 were more viable in the presence of RA medium.
Figure 4 Apoptosis/necrosis of LS-8 cell on nanostructured Ti surfaces in standard or RA media.
Notes: (A) Representative images of flow cytometry; (B) analysis of the proportion of viable cells (upper row), apoptotic cells (middle row), and necrotic cells (lower row). *p<0.01.
Abbreviation: RA, retinoic acid.
To clarify the role of the surface nanostructure and RA medium in apoptosis, the expression of apoptosis-related genes BAX, BCL-2, and CASP3 mRNA was measured. However, the surface nanostructure did not appear to affect apoptosis or necrosis of LS-8 cells in either media because expressions of BAX, BCL-2, and CASP3 mRNA were not affected by NT5 and NT20 conditions (even between the Ti surface and culture plate), but only by RA stimulation ().
Amelogenic gene expression in LS-8 cells on different Ti surfaces
The expression of amelogenic genes in various cultures were analyzed by qPCR ( and ). BMP-2 and SHH expression was enhanced on nanostructured Ti surfaces – both in the presence/absence of RA medium pretreatment. (). RUNX2, KLK4, and ODAM expression did not differ between all prepared Ti surfaces in standard medium but was dramatically elevated on NT5 and NT20 under RA medium stimulation (). The expression of BMP-2, SHH, AMELX, AMBN, ENAM, and MMP-20 was significantly enhanced on NT5 and NT20 surfaces in the standard medium. However, such regulative effects were weakened or even reversed under the stimulation of RA medium (). AMTN expression was extremely low (>40 cycles) on all prepared Ti surfaces (). Besides, RA medium pretreatment suppressed the expression of BMP-2, AMELX, AMBN, ENAM, and MMP-20, but enhanced the expression of RUNX2, SHH, KLK4, and ODAM on polished Ti surfaces ().
Figure 5 Expression of amelogenic genes in LS-8 cells cultured on nanostructured Ti surfaces in standard culture medium.
Notes: (A) BMP-2, (B) RUNX2, (C) SHH, (D) AMELX, (E) AMBN, (F) ENAM, (G) KLK4, (H) MMP-20, and (I) ODAM. *p<0.01 vs P; #p<0.01 vs NT5. Dotted line, gene expression of LS-8 cells on culture plate.
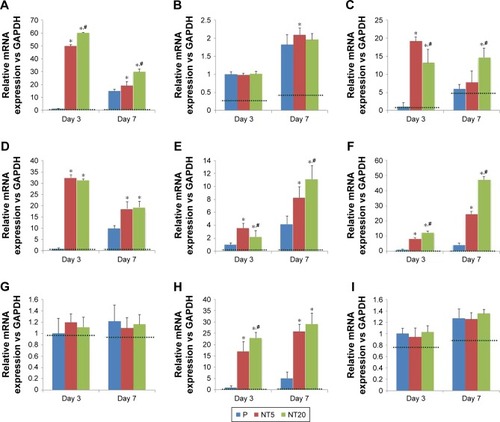
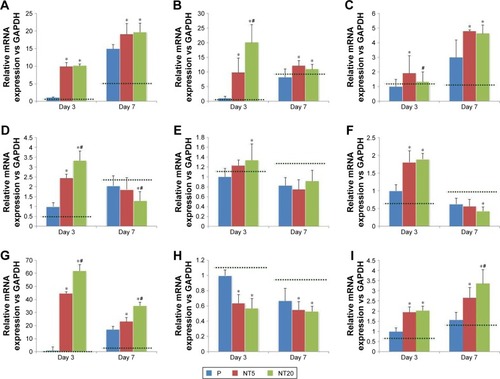
Figure 6 Expression of amelogenic genes in LS-8 cells cultured on nanostructured Ti surfaces with RA medium pretreatment.
Notes: (A) BMP-2, (B) RUNX2, (C) SHH, (D) AMELX, (E) AMBN, (F) ENAM, (G) KLK4, (H) MMP-20, and (I) ODAM. *p<0.01 vs P; #p<0.01 vs NT5. Dotted lines, gene expression of LS-8 cells on culture plate.
Abbreviation: RA, retinoic acid.
ECM mineralization of LS-8 cells on different Ti surfaces
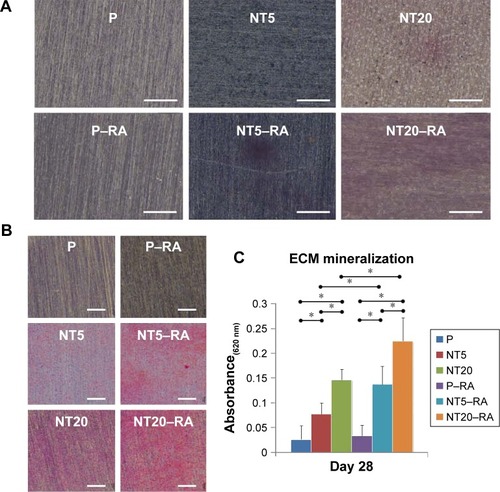
The ECM mineralization of LS-8 cells on modified surfaces with/without RA medium was evaluated by staining of ALP and mineralized nodules. In the absence of RA medium, modified Ti surfaces (NT5 and NT20) slightly promoted ALP synthesis compared to P (). In contrast, in RA medium, ALP synthesis was improved on NT5 and NT20, whereas no obvious enhancement of ALP production was observed on P surfaces. ECM mineralization was increased on both NT5 and NT20 in standard medium and was further enhanced on NT surfaces in the presence of RA medium (). In addition, mineralized nodule formation was not increased on P surfaces following the addition of RA medium. Conclusively, the highest ECM mineralization was obtained on NT20 in RA medium, which remained significantly higher (p<0.01) than on any other surface ().
Figure 7 Extracellular matrix (ECM) mineralization of LS-8 cells on nanostructured Ti surfaces in standard or RA medium.
Notes: (A) Alkaline phosphatase (ALP) synthesis on modified surfaces in standard medium (upper row) and RA medium (lower row). (B) The formation of mineralized nodules on modified surface in standard medium (left column) and RA medium (right column), with the semi-quantitative analysis (C). Magnification=100×; scale bar=400 μm. *p<0.01.
Abbreviation: RA, retinoic acid.
Discussion
This study was conducted to assess the effects of nanoscale TiO2 surfaces on amelogenesis and study the amelogenic behaviors of ameloblasts stimulated by RA and DEX. The behavior and amelogenic differentiation of ameloblast-like cells (LS-8) on nanostructured TiO2 NT surfaces was characterized using various techniques. In addition to the LS-8 cell line, the ameloblast-lineage cell line (ALC) is also widely used. Sarkar et al compared the expression profiles of LS-8 and ALC cells and found that LS-8 cells expressed greater levels of genes that define secretory-stage activities (AMELX, AMBN, ENAM, and MMP20), whereas ALC expresses greater levels of genes that define maturation-stage activities (ODAM and KLK4). In addition, no AMTN expression was noted in either ameloblast-like cell line.Citation28 Therefore, we chose LS-8 cells in this study to investigate whether the amelogenic differentiation and maturation of LS-8 cells can be manipulated by surface nanostructure and RA/DEX medication. It is well known that cells are sensitive to surface features and may respond selectively to the surface topography of biomaterials.Citation29–Citation31 We fabricated NT surfaces that share similar chemical composition, protein adsorption, and dramatically high hydrophilicity (from instant to 2.0 s after water contact), but featured nanostructures of different scales.Citation25,Citation32 Nanoscale modifications increase the surface area and energy that are likely to enhance cellular interactions and protein absorption.Citation33,Citation34 In addition, surface nanostructures could alter the focal adhesion and cytoskeleton reorganization of cells, including osteoblasts, fibroblasts, and various stem cells.Citation35,Citation36 Such morphologic alterations mediated by surface nanostructures are linked to the functional status of various kinds of cells,Citation37–Citation41 particularly osteogenic differentiation and mineralization behaviors of bone mesenchymal stem cells (bMSCs).Citation37,Citation42,Citation43 To exclude the influence of the crystal phase of TiO2 on cellular responses,Citation22 samples were not annealed. Thus, the TiO2 layer on Ti samples (P, NT5, and NT20) was still amorphous (therefore, no typical signals were detected by XRD, data not shown). With samples sharing a similar chemical composition () and crystal phase, the different cellular responses can be attributed to the impact of surface nanostructures on prepared Ti samples.
In this study, we showed that LS-8 cells were more evenly distributed and produced more pseudopodia on NT5 or NT20 surfaces than on P surface in standard medium. LS-8 cells on NT surfaces proliferated faster, and easily achieved confluence through enhanced interaction (, and ). Similar to ameloblasts in the secretory stage, a large number of LS-8 cells facilitated ECM production, aiding the formation of a niche to support their amelogenic differentiation. However, in RA medium, the proliferation of LS-8 cells was significantly suppressed and fewer cells and pseudopodia were observed on all modified Ti surfaces. Moreover, LS-8 cells in six-well culture plates showed a decrease in proliferation, suggesting that the recession of proliferation may be associated with LS-8 cell maturation with exogenous RA and DEX, independent of different surface chemical compositionsCitation44,Citation45 and nanostructure.
The proportion of total apoptotic/necrotic cells () was all increased after 48 h treatment in RA medium. The expression of BAX, BCL-2, and CASP3 mRNA was upregulated on treatment with RA medium (). BAX is known to be a component of the intrinsic apoptosis pathway, and CASP3 is the key executioner of both the intrinsic and extrinsic apoptosis pathways.Citation46–Citation48 Higher levels of BAX and CASP3 mRNA were observed to be associated with increased LS-8 apoptosis/necrosis under RA/DEX stimulation and the upregulated expression of BCL-2 (an inhibitor of apoptosis;Citation49 ).Citation45 Although the mechanism remains controversial, BCL-2 can either elicit or suppress apoptosis according to the status of cellular differentiation.Citation50,Citation51 Overexpression of BCL-2 induced osteocyte and bMSC apoptosis during differentiation.Citation52–Citation54 Our results are consistent with those of previous studies.Citation45,Citation52–Citation54 Thus, BCL-2 may be involved in apoptosis during the amelogenic differentiation of LS-8 cells. However, the role of BAX, BCL-2, and caspase-3 in the apoptosis and amelogenic differentiation of LS-8 cells with RA/DEX stimulation needs further clarification. Intriguingly, the alteration of BAX, BCL-2, and CASP3 expression may be controlled by RA/DEX stimulation directly rather than by the Ti surface nanostructures. At 0 h, the number of viable cells on the NT20 surface exceeded that on the P and NT5 surfaces, perhaps because the proliferation on the NT20 surface was promoted during the 24 h pre-culture as described in section 2.4.
In our previous work, TiO2 NT surfaces promoted proliferation, osteogenic gene expression, collagen synthesis, and mineralized nodule formation of osteoblastic lineage cells, including osteoblasts and bMSCs.Citation25,Citation26 Ameloblasts are similar to osteoblastic lineage cells in terms of their role in the formation of mineralized tissues. Therefore, we hypothesized that TiO2 NT surfaces may enhance the amelogenic behavior of LS-8 cells. In standard medium, NT5 and NT20 significantly enhanced amelogenic gene expression. However, such promotion was partially altered in the RA medium. Notably, RUNX2, KLK4, and ODAM were expressed similarly in standard medium on all Ti surfaces (except RUNX2 at Day 7) but were highly expressed on NT5 and NT20 surfaces with RA/DEX medication. In contrast, the MMP-20 expression was increased on NT5 and NT20 surfaces in standard medium but was suppressed with RA/DEX medication. A similar impact of RA/DEX on the expression of BMP-2, SHH, AMELX, AMBN, and ENAM was also observed on modified Ti surfaces.
BMP-2 and RUNX2 are crucial for various tooth development stages.Citation55 BMP-2 is constantly expressed during the differentiation of odontoblasts and ameloblasts in embryonic and postnatal tooth development.Citation56 SHH is another crucial regulator for germ growth/morphogenesis and tissue–tissue interactions that participates in the proliferation and differentiation of dental epithelial stem cells, and controls the fate of dental epithelium as an autocrine signal.Citation56,Citation57 Together with SHH, BMP-2/SHH signaling is very important for the fate of dental epithelial stem cells during organogenesis, and partially contributes to the different growth potential of postnatal teeth.Citation58,Citation59 On the other hand, RUNX2 is essential for later stages of tooth formation, during the formation of mineralized tooth tissues, and functions as a regulator by binding to the ameloblastin promoter region.Citation60–Citation62
The morphological formation of the tooth crown is precisely controlled by a balance of proliferation and differentiation of dental epithelium-derived ameloblasts.Citation63 Amelo-blasts support and regulate enamel formation by producing enamel organic matrix biomolecules such as amelogenin, ameloblastin, enamelin, and enamel matrix proteases, including KLK4 and MMP-20.Citation64–Citation67 Although containing very little protein (<5%), enamel matrix proteins are crucial for the formation and maturation of tooth enamel.Citation12 Enamel matrix proteins are highly expressed in the secretory stage of enamel development.Citation68 In the present study, we analyzed three enamel matrix proteins including amelogenin X-linked (AMELX), ameloblastin (AMBN), and enamelin (ENAM). Amelogenin and enamelin are the two main kinds of protein produced during the development of postnatal enamel in mammals.Citation69 Amelogenin is mainly secreted by ameloblasts and regulates the organization of crystallographic structure and thickness of enamel.Citation70 Besides, ameloblastin is produced by ameloblasts and comprises 5%–10% of all enamel proteins. Although not fully understood, ameloblastin is thought to be a regulator of enamel crystal elongation and enamel mineralization.Citation71 As a cell-adhesion molecule, AMBN is indispensable for the attachment of ameloblasts to the enamel matrix and polarity.Citation14
Enamel matrix proteins are secreted by ameloblasts during the secretory stage of amelogenesis and then are decomposed by enamel-specific proteases during the maturation stage.Citation72 KLK4 and MMP-20 are the two main enamel-specific proteases that are secreted at different stages of amelogenesis. MMP (a zinc-dependent peptidase) decomposes many ECM proteins, and plays an important role in early amelogenesis.Citation73,Citation74 MMP-20 expression is mostly elevated during the late secretory/transitional stages of enamel development and is dramatically decreased during the maturation stage.Citation75 KLK4 expression was enhanced in the transition stage and remained elevated during the maturation stage.Citation76 The decreased expressions of BMP-2, AMELX, AMBN, ENAM, and KLK4 mRNA and increased expression of RUNX2, SHH, KLK4, and ODAM mRNA suggested that LS-8 cells were differentiated into the maturation stage in the presence of RA medium on P ().Citation28,Citation45 Such alteration in the mRNA expression of enamel matrix proteins and proteases has been reported previously by Yang et alCitation45 and Suzawa et al.Citation77 Notably, MMP-20 expression dropped significantly on treatment with RA medium (), which is partially inconsistent with a previous report.Citation45 Compared to Yang et al’s study, LS-8 cells in this study were cultured for an additional 1 or 5 days after 48 h treatment with RA/DEX. Therefore, the divergence of MMP-20 expression may be attributed to the different maturation stages of ameloblasts.
Besides the regulative effects of RA medium, the surface nanostructure can further manipulate the amelogenic behaviors of ameloblasts. TiO2 NT surfaces, especially NT20 surfaces, improved the amelogenic functions of ameloblasts – both in standard and RA media – as confirmed by macroscopic observation of ALP synthesis and mineralized nodule formation (). According to the pattern of amelogenic gene expression observed in different culture media, it was clear that the regulatory effect of the TiO2 NT surface was mild and unlikely to directly drive maturation of LS-8 cells. However, the combination of surface nanostructure and short-term RA/DEX medication could promote enamel matrix molecule secretion and maturation of ameloblasts, following enhanced mineralized enamel formation in the macroscopic view without any exogenous mineralization inducer. In addition, the timing and dose of RA/DEX treatment should be carefully determined, as improper or excessive RA/DEX stimulation reduces LS-8 cell viability and even impairs amelogenesis and, thereby, may result in enamel defects in vivo.Citation78
Future improvements to our research include methods to fabricate the biomimetic surface nanostructure of biomaterials with additional chemical–biological modifications, to regulate maturation of ameloblasts via precise biomolecules and other bioactive compounds. Furthermore, primary dental epithelium and ameloblasts should be employed in further research as they possess the full range of cellular functions.
Conclusion
Nanotube arrays of various tube sizes and morphological features can be constructed on Ti surfaces via anodization. The surface nanostructures can effectively induce amelogenic differentiation of LS-8 cells. Anodized Ti surfaces may further enhance the amelogenic functions of LS-8 cells in combination with precise administration of RA/DEX. The current study established a systemic framework suitable for enamel maturation and regeneration based on biomaterial surface nanotopography.
Acknowledgments
This work was supported financially by the National Natural Science Fund (grant nos. 91442108, 81501599, 81530051, and 81571531) and funded by the China Postdoctoral Science Foundation (grant no. 2016M593009). Special thanks to Prof Malcolm L Snead of USC for kindly donating the LS-8 cell line.
Supplementary materials
Figure S1 Elements analysis on nanostructured Ti surfaces via X-ray photoelectron spectroscopy (XPS) scanning.
Abbreviation: CPS, counts per second; ev, electron volt.
Figure S2 Morphology of LS-8 cells cultured on nanostructured Ti surfaces in standard medium (A) and RA medium (B), the arrows indicate pseudopods of LS-8 cells. Magnification=400×; scale bar=25 μm.
Abbreviation: RA, retinoic acid; P, polished Ti surface.
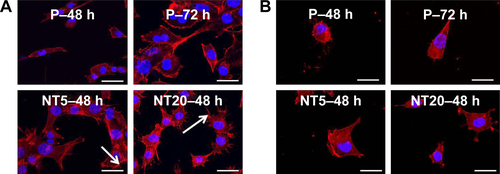
Figure S3 The apoptosis-related genes expression of LS-8 cells on nanostructured Ti surfaces in standard medium and RA medium at 24 and 48 h. (A) BAX, (B) BCL-2, and (C) CASP3. *p<0.01.
Abbreviation: NS, no significance.
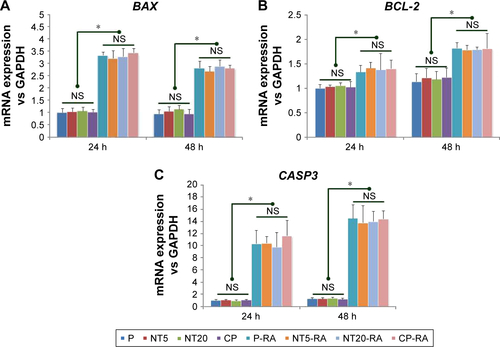
Figure S4 The alternation of amelogenic genes expression in LS-8 cells in response to RA medium 48-h pretreatment: (A) amelogenic genes expression on polished Ti surface in presence/absence of RA medium. (B) AMTN expression on nanostructured Ti surfaces in standard medium. (C) AMTN expression on nanostructured Ti surfaces with RA medium pretreatment. *p<0.01.
Abbreviations: EL, extremely low level; RA, retinoic acid.
Disclosure
The authors report no conflicts of interest in this work.
References
- PetersenPEYamamotoTImproving the oral health of older people: the approach of the WHO Global Oral Health ProgrammeCommunity Dent Oral Epidemiol2005332819215725170
- BaelumVFejerskovOTooth loss as related to dental caries and periodontal breakdown in adult TanzaniansCommunity Dent Oral Epidemiol19861463533573466765
- NiessenLCWeyantRJCauses of tooth loss in a veteran populationJ Public Health Dent198949119232642966
- CooperLFThe current and future treatment of edentulismJ Prosthodont200918211612219254301
- RavindranSGeorgeABiomimetic extracellular matrix mediated somatic stem cell differentiation: applications in dental pulp tissue regenerationFront Physiol2015611825954205
- MonteiroNYelickPCAdvances and perspectives in tooth tissue engineeringJ Tissue Eng Regen Med20171192443246127151766
- LaiWFLeeJMJungHSMolecular and engineering approaches to regenerate and repair teeth in mammalsCell Mol Life Sci20147191691170124270857
- HirayamaMOshimaMTsujiTDevelopment and prospects of organ replacement regenerative therapyCornea201332Suppl 1S13S2124104927
- XuWJiangSChenQSystemically Transplanted Bone Marrow-derived Cells Contribute to Dental Pulp Regeneration in a Chimeric Mouse ModelJ Endod201642226326826686823
- ShenJZKosmačTAdvanced Ceramics for DentistryWaltham, MAElsevier/BH2014
- Ten CateARTen Cate’s Oral HistologyOxfordElsevier2013
- NanciATen Cate’s Oral Histology: Development, Structure, and FunctionSingaporeElsevier Pte limited2013
- ZafarMSKhurshidZAlmasKOral tissue engineering progress and challengesTissue Engin Regener Med2015126387397
- FukumotoSKibaTHallBAmeloblastin is a cell adhesion molecule required for maintaining the differentiation state of ameloblastsJ Cell Biol2004167597398315583034
- ZhangYDChenZSongYQLiuCChenYPMaking a tooth: growth factors, transcription factors, and stem cellsCell Res200515530131615916718
- DalbyMJGadegaardNTareRThe control of human mesenchymal cell differentiation using nanoscale symmetry and disorderNat Mater2007612997100317891143
- ZhaoLLiuLWuZZhangYChuPKEffects of micropitted/nanotubular titania topographies on bone mesenchymal stem cell osteogenic differentiationBiomaterials20123392629264122204980
- SchwartzZLohmannCHSiskMLocal factor production by MG63 osteoblast-like cells in response to surface roughness and 1,25-(OH)2D3 is mediated via protein kinase C- and protein kinase A-dependent pathwaysBiomaterials200122773174111246968
- WangWZhaoLWuKThe role of integrin-linked kinase/β-catenin pathway in the enhanced MG63 differentiation by micro/nano-textured topographyBiomaterials201334363164023107296
- ZhaoLMeiSChuPKZhangYWuZThe influence of hierarchical hybrid micro/nano-textured titanium surface with titania nanotubes on osteoblast functionsBiomaterials201031195072508220362328
- TzaphlidouMThe role of collagen in bone structure: an image processing approachMicron2005367–859360116209926
- MeiSZhaoLWangWMaQZhangYBiomimetic titanium alloy with sparsely distributed nanotubes could enhance osteoblast functionsAdv Eng Mater2012144B166B174
- YilmazEDSchneiderGAMechanical behavior of enamel rods under micro-compressionJ Mech Behav Biomed Mater20166318319427415405
- MaQWangWChuPKConcentration- and time-dependent response of human gingival fibroblasts to fibroblast growth factor 2 immobilized on titanium dental implantsInt J Nanomedicine201271965197622619534
- MaQLZhaoLZLiuRRImproved implant osseointegration of a nanostructured titanium surface via mediation of macrophage polarizationBiomaterials201435379853986725201737
- WangWZhaoLMaQWangQChuPKZhangYThe role of the Wnt/β-catenin pathway in the effect of implant topography on MG63 differentiationBiomaterials201233327993800222889483
- ChenLSCouwenhovenRIHsuDLuoWSneadMLMaintenance of amelogenin gene expression by transformed epithelial cells of mouse enamel organArch Oral Biol199237107717781444889
- SarkarJSimanianEJTuggySYComparison of two mouse ameloblast-like cell lines for enamel-specific gene expressionFront Physiol2014527725120490
- KhangDLuJYaoCHaberstrohKMWebsterTJThe role of nanometer and sub-micron surface features on vascular and bone cell adhesion on titaniumBiomaterials200829897098318096222
- WebsterTJSchadlerLSSiegelRWBiziosRMechanisms of enhanced osteoblast adhesion on nanophase alumina involve vitronectinTissue Eng20017329130111429149
- KhangDKimSYLiu-SnyderPPalmoreGTDurbinSMWebsterTJEnhanced fibronectin adsorption on carbon nanotube/poly(carbonate) urethane: independent role of surface nano-roughness and associated surface energyBiomaterials200728324756476817706277
- GongDGrimesCAVargheseOKTitanium oxide nanotube arrays prepared by anodic oxidationJ Mater Res2001161233313334
- NelAEMädlerLVelegolDUnderstanding biophysicochemical interactions at the nano-bio interfaceNat Mater20098754355719525947
- ZafarMNajeebSKhurshidZPotential of electrospun nanofibers for biomedical and dental applicationsMaterials20169273
- Heydarkhan-HagvallSChoiCHDunnJInfluence of systematically varied nano-scale topography on cell morphology and adhesionCell Commun Adhes200714518119418163229
- YimEKDarlingEMKulangaraKGuilakFLeongKWNanotopography-induced changes in focal adhesions, cytoskeletal organization, and mechanical properties of human mesenchymal stem cellsBiomaterials20103161299130619879643
- OhSBrammerKSLiYSStem cell fate dictated solely by altered nanotube dimensionProc Natl Acad Sci U S A200910672130213519179282
- GuvendirenMBurdickJAThe control of stem cell morphology and differentiation by hydrogel surface wrinklesBiomaterials201031256511651820541257
- LaxmanBHallDEBhojaniMSNoninvasive real-time imaging of apoptosisProc Natl Acad Sci U S A20029926165511655512475931
- LeeSChoiJShinSAnalysis on migration and activation of live macrophages on transparent flat and nanostructured titaniumActa Biomater2011752337234421232636
- TrinchieriGSherACooperation of Toll-like receptor signals in innate immune defenceNat Rev Immunol20077317919017318230
- KhandwekarARhoCKModulation of cellular responses on engineered polyurethane implantsJ Biomed Mater Res A201210092211222222492665
- IvanovskiSHamletSSalviGETranscriptional profiling of osseointegration in humansClin Oral Implants Res201122437338121561479
- YangTZhangYZhengDHaoYSneadMLDuanXHigh-fluoride promoted phagocytosis-induced apoptosis in a matured ameloblast-like cell lineArch Oral Biol2015601849025260155
- YangTZhangYLiYHigh amounts of fluoride induce apoptosis/cell death in matured ameloblast-like LS8 cells by downregulating Bcl-2Arch Oral Biol20135891165117323598055
- KoleAJKnightERDeshmukhMActivation of apoptosis by cytoplasmic microinjection of cytochrome cJ Vis Exp201152pii2773
- TaitSWGreenDRMitochondrial regulation of cell deathCold Spring Harb Perspect Biol201359piia008706
- Shamas-DinABindnerSZhuWtBid undergoes multiple conformational changes at the membrane required for Bax activationJ Biol Chem201328830221112212723744079
- NieHRathbunGTuckerHSmyd1C mediates CD8 T cell death via regulation of Bcl2-mediated restriction of outer mitochondrial membrane integrityJ Cell Signal201723pii163
- HoffmanBLiebermannDAApoptotic signaling by c-MYCOncogene200827506462647218955973
- DesagherSMartinouJCMitochondria as the central control point of apoptosisTrends Cell Biol200010936937710932094
- NagaseYIwasawaMAkiyamaTAnti-apoptotic molecule Bcl-2 regulates the differentiation, activation, and survival of both osteoblasts and osteoclastsJ Biol Chem200928452366593666919846553
- MoriishiTMaruyamaZFukuyamaROverexpression of Bcl2 in osteoblasts inhibits osteoblast differentiation and induces osteocyte apoptosisPLoS One2011611e2748722114675
- OliverLHueERossignolJDistinct roles of Bcl-2 and Bcl-Xl in the apoptosis of human bone marrow mesenchymal stem cells during differentiationPLoS One201165e1982021589877
- TummersMThesleffIThe importance of signal pathway modulation in all aspects of tooth developmentJ Exp Zool B Mol Dev Evol2009312B430931919156667
- LiuGMaSZhouYSignaling pathways in dental stem cells during their maintenance and differentiationŞahinFDoğanADemirciSDental Stem CellsChamSpringer International Publishing20166992
- WuCShimoTLiuMPacificiMKoyamaESonic hedgehog functions as a mitogen during bell stage of odontogenesisConnect Tissue Res200344Suppl 1929612952180
- LiJFengJLiuYBMP-SHH signaling network controls epithelial stem cell fate via regulation of its niche in the developing toothDev Cell201533212513525865348
- MonteiroNSmithEEAngstadtSZhangWKhademhosseiniAYelickPCDental cell sheet biomimetic tooth bud modelBiomaterials201610616717927565550
- DuJWangQYangPWangXFHL2 mediates tooth development and human dental pulp cell differentiation into odontoblasts, partially by interacting with Runx2J Mol Histol201647219520226759258
- CamilleriSMcDonaldFRunx2 and dental developmentEur J Oral Sci2006114536137317026500
- DhamijaSLiuYYamadaYSneadMLKrebsbachPHCloning and characterization of the murine ameloblastin promoterJ Biol Chem199927429207382074310400709
- DassuleHRLewisPBeiMMaasRMcMahonAPSonic hedgehog regulates growth and morphogenesis of the toothDevelopment2000127224775478511044393
- HuCCFukaeMUchidaTCloning and characterization of porcine enamelin mRNAsJ Dent Res19977611172017299372788
- KrebsbachPHLeeSKMatsukiYKozakCAYamadaKMYamadaYFull-length sequence, localization, and chromosomal mapping of ameloblastin. A novel tooth-specific geneJ Biol Chem19962718443144358626794
- LlanoEPendásAMKnäuperVIdentification and structural and functional characterization of human enamelysin (MMP-20)Biochemistry1997364915101151089398237
- NelsonPSGanLFergusonCMolecular cloning and characterization of prostase, an androgen-regulated serine protease with prostate-restricted expressionProc Natl Acad Sci U S A19999663114311910077646
- SimmerJPFinchamAGMolecular mechanisms of dental enamel formationCrit Rev Oral Biol Med199562841087548623
- HuJCYamakoshiYEnamelin and autosomal-dominant amelogenesis imperfectaCrit Rev Oral Biol Med200314638739814656895
- XuLHaradaHYokohama-TamakiTMatsumotoSTanakaJTaniguchiAReuptake of extracellular amelogenin by dental epithelial cells results in increased levels of amelogenin mRNA through enhanced mRNA stabilizationJ Biol Chem200628142257226216293627
- HuJCChunYHAl HazzazziTSimmerJPEnamel formation and amelogenesis imperfectaCells Tissues Organs20071861788517627121
- BartlettJDSimmerJPProteinases in developing dental enamelCrit Rev Oral Biol Med199910442544110634581
- ShintaniSKobataMKamakuraNToyosawaSOoshimaTIdentification and characterization of matrix metalloproteinase-20 (MMP20; enamelysin) genes in reptile and amphibianGene20073921–2899717223283
- SulkalaMLarmasMSorsaTSaloTTjäderhaneLThe localization of matrix metalloproteinase-20 (MMP-20, enamelysin) in mature human teethJ Dent Res200281960360712202640
- BartlettJDRyuOHXueJSimmerJPMargolisHCEnamelysin mRNA displays a developmentally defined pattern of expression and encodes a protein which degrades amelogeninConnect Tissue Res1998391–3101109 discussion 141–14911062992
- OverallCMLimebackHIdentification and characterization of enamel proteinases isolated from developing enamel. Amelogeninolytic serine proteinases are associated with enamel maturation in pigBiochem J198825639659723223966
- SuzawaTItohNTakahashiNEstablishment of primary cultures for mouse ameloblasts as a model of their lifetimeBiochem Biophys Res Commun200634531247125316707102
- MorkmuedSLaugel-HaushalterVMathieuERetinoic acid excess impairs amelogenesis inducing enamel defectsFront Physiol2017767328111553